Early mortality and safety after transcatheter aortic valve replacement using the SAPIEN 3 in nonagenarians
2018-08-17EijiIchimotoAdamArnofskyMichaelWildermanRichardGoldweitJosephDeGregorio
Eiji Ichimoto, Adam Arnofsky, Michael Wilderman, Richard Goldweit, Joseph De Gregorio
Early mortality and safety after transcatheter aortic valve replacement using the SAPIEN 3 in nonagenarians
Eiji Ichimoto1, Adam Arnofsky2, Michael Wilderman3, Richard Goldweit4, Joseph De Gregorio1
1Department of Invasive Cardiology, Englewood Hospital and Medical Center, Englewood, NJ, United States2Department of Cardiothoracic Surgery, Englewood Hospital and Medical Center, Englewood, NJ, United States3Department of Vascular Surgery, Englewood Hospital and Medical Center, Englewood, NJ, United States4Department of Interventional Cardiology, Englewood Hospital and Medical Center, Englewood, NJ, United States
Transcatheter aortic valve replacement (TAVR) has been performed for many elderly patients with severe aortic stenosis (AS). The SAPIEN 3 is one of the latest balloon-expandable prosthesis. This study aimed to investigate the early clinical outcomes after TAVR using the SAPIEN 3 in nonagenarians.A total of 97 consecutive patients underwent TAVR for severe AS between December 2015 and December 2016. Of these, 85 consecutive patients who underwent TAVR using the SAPIEN 3 were included. According to the age, patients were classified into age ≥ 90 years group (17 patients) or age < 90 years group (68 patients). The clinical outcomes including all-cause mortality and composite endpoint of early safety at 30 days were evaluated.The Society of Thoracic Surgeons score in age ≥ 90 years group was higher than age < 90 years group (12.3 ± 6.1%. 8.5 ± 5.1%,< 0.01). There was no significant difference in 30-day mortality between the two groups. However, the life-threatening bleeding and major vascular complications in age ≥ 90 years group were greater than age < 90 years group (11.8%. 1.5%,= 0.04 and 11.8%. 1.5%,= 0.04, respectively). The composite endpoint of early safety at 30 days was similar between the two groups. Multivariate logistic regression analysis showed that prior myocardial infarction was an independent predictor of the composite endpoint of early safety (odds ratio: 4.76, 95% confidence interval: 1.02–22.21,=0.047).The early mortality and safety after TAVR using the SAPIEN 3 in nonagenarians were similar and acceptable despite of higher operative risk.
J Geriatr Cardiol 2018; 15: 387393. doi:10.11909/j.issn.1671-5411.2018.06.002
Nonagenarians; SAPIEN 3; Transcatheter aortic valve replacement;
1 Introduction
The number of patients with severe aortic stenosis (AS) has been increasing, which significantly reduces quality of life and survival in the elderly.[1]However, the elderly patients with severe symptomatic valve disease are not referred to surgery because of significant multiple comorbidities or advanced age.[2]Transcatheter aortic valve replacement (TAVR) has emerged as a viable treatment option for patients with severe AS who are inoperable or at high surgical risk, prolonging survival and improving quality of life in the majority of patients.[3]Previous study has reported outcomes of TAVR in the very elderly and higher short- time mortality rate.[4]The development of novel transcatheter heart valves and further iterations of delivery systems and prosthesis have contributed to decrease in complications rates in TAVR.[5]The Edwards SAPIEN 3 Transcatheter Heart Valve (Edwards lifesciences, Irvine, CA) is one of the latest development balloon-expandable prosthesis. The SAPIEN 3 can diminish vascular complication and paravalvular regurgitation.[6]However, there is little information about the early clinical outcomes after TAVR using the SAPIEN 3 in nonagenarians. Thus this study investigated the early clinical outcomes after TAVR using the SAPIEN 3 in nonagenarians.
2 Methods
2.1 Patients
Between December 2015 and December 2016, a total of 97 consecutive patients underwent TAVR for severe AS at Englewood Hospital and Medical Center. Symptomatic severe AS was defined as having an aortic valve area (AVA) ≤1.0 cm2, aortic valve peak aortic velocity (Vmax) ≥ 4 m/s, and mean gradient ≥ 40 mmHg on transthoracic echocardiogram and cardiac catheterization, with symptoms of external dyspnea or decreased exercise tolerance, angina, or syncope.[7]Patients were at high risk for surgical valve replacement indicated by the Society of Thoracic Surgeons score. Of these, 85 consecutive patients (87.6%) who underwent TAVR using the SAPIEN 3 were included in this study. Nonagenarians were defined as patients age ≥ 90 years at the time of the procedure. According to the age, patients were classified into age ≥ 90 years group (17 patients) or age < 90 years group (68 patients). This study was approved by the local council on human research.
2.2 Procedure
Eligibility for TAVR was established by the local heart team including interventional cardiologists and cardiothoracic surgeons. Each patient underwent extensive preoperative evaluation to assess risk factors. Based on preoperative imaging, including CT scans and echocardiography, annulus size was measured for the appropriate size of valve. The access approach was determined by CT scans based on patient anatomy, comorbidities, and physician discretion.
TAVR was performed under general anesthesia and received fluoroscopy and transesophageal echocardiography (TEE) for procedural guidance. Device positioning was based on supra-annular aortography. Prosthesis position and function were evaluated by TEE. After the procedure, aortography was performed to verify the absence of coronary ostial obstruction and assess the degree of aortic regurgitation. Vascular access site closure was achieved by two Proglide devices (Abbott Vascular, Abbott Park, IL). Aortography was performed to detect ilio-femoral complications. And then all patients were further evaluated postprocedural echocardiographic findings after TAVR by TTE.
Three commercially available valves were used at the same period in this study. The SAPIEN 3 and SAPIEN XT (Edwards lifesciences, Irvine, CA) are balloon-expandable prostheses and deployed under rapid pacing. The SAPIEN XT was used in the valve-in-valve cases for prior surgical aortic valve replacement. The CoreValve (Medtronic, Minneapolis, MN) is a self-expandable prosthesis. The CoreValve was preferred when high risks for annular rupture or repeated rapid pacing were anticipated. The CoreValve was used in 10 cases. Patients who were used the SAPIEN XT and CoreValve were excluded to eliminate the differences of prosthesis in this study.
Postprocedural antiplatelet therapy for patients without indication for oral anticoagulation consisted of low-dose of aspirin and clopidogrel 75 mg daily.
2.3 Clinical outcome
Baseline demographic and clinical data along with in-hospital outcomes were obtained by review of the medical records and procedural reports. Clinical follow-up data were obtained from outpatient record reviews. Mortality, myocardial infarction, stroke and transient ischemic attack, bleeding, acute kidney injury, vascular complication were defined according to the Valve Academic Research Consortium-2 (VARC-2).[8]The composite endpoint of early safety at 30 days included all-cause death, all stroke, life-threatening bleeding, acute kidney injury stage 2 or 3, coronary artery obstruction requiring intervention, major vascular complication and valve-related dysfunction requiring repeat procedure.
2.4 Statistical analysis
Statistical analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC). Continuous variables are expressed as mean ± SD and categorical variables as frequency (%). Continuous variables were compared using Student’stest or analysis of variance. Categorical variables were compared with chi-square statistics or Fisher’s exact test. Multivariate logistic regression analysis was used to identify independent predictors of the composite endpoint of early safety at 30 days. Univariate analysis of factors affecting the composite endpoint of early safety was performed using factors in Tables 1–3. Variables with< 0.20 in univariate analysis were included in the multivariate analysis.< 0.05 was considered significant.
3 Results
Baseline clinical characteristics are presented in Table 1. Weight, body mass index and body surface area in age ≥ 90 years group were lower than in age < 90 years group. Patients aged ≥ 90 years with past history of coronary artery disease and prior myocardial infarction were less than patients age < 90 years with those. On the other hand, the Society of Thoracic Surgeons score in age ≥ 90 years group was significantly higher than age < 90 years group (12.3 ± 6.1%. 8.5 ± 5.1%,< 0.01). There was no significant difference in prevalence of New York Heart Association class III or IV between the two groups.
The echocardiographic and angiographic characteristics before TAVR are listed in Table 2. AVA and mean aortic valve gradient, left ventricular ejection fraction on transthoracic echocardiogram and cardiac catheterization before TAVR were similar between the two groups. The preva- lence of coronary artery disease in age ≥ 90 years group was lower than age < 90 years group.

Table 1. Baseline characteristics.
Data are presented as mean ± SD or(%).

Table 2. Baseline echocardiographic and angiographic characteristics.
Data are presented as mean ± SD or(%).

Table 3. Procedural and postprocedural echocardiographic characteristics.
Data are presented as mean ± SD or(%). NA: not applicable.
The procedural and postprocedural echocardiographic characteristics are presented in Table 3. The SAPIEN 3 could be implanted in all patients by transfemoral approach. There was no significant difference in procedural characteristics and valve size with the two groups. Furthermore, there was no significant difference in mean aortic valve gradient and the presence of aortic regurgitation moderate or severe after TAVR between the two groups.
The postprocedural clinical outcomes at 30 days after TAVR defined by VARC-2 were shown in Table 4. There was no significant difference in 30-day mortality between the two groups (0. 4.4%,= 0.38). On the other hand, the life-threatening bleeding and major vascular complications in age ≥ 90 years group were greater than age < 90 years group (11.8%. 1.5%,= 0.04 and 11.8%. 1.5%,= 0.04, respectively). However, the composite endpoint of early safety at 30 days was similar between the two groups (17.6%. 10.3%,= 0.40) (Figure 1).
Table 5 presents the results of multivariate logistic regression analysis. Prior myocardial infarction was an independent predictor of the composite endpoint of early safety at 30 days.

Table 4. Postprocedural outcomes at 30 days.
Data are presented as mean ± SD or(%). NA: not applicable.
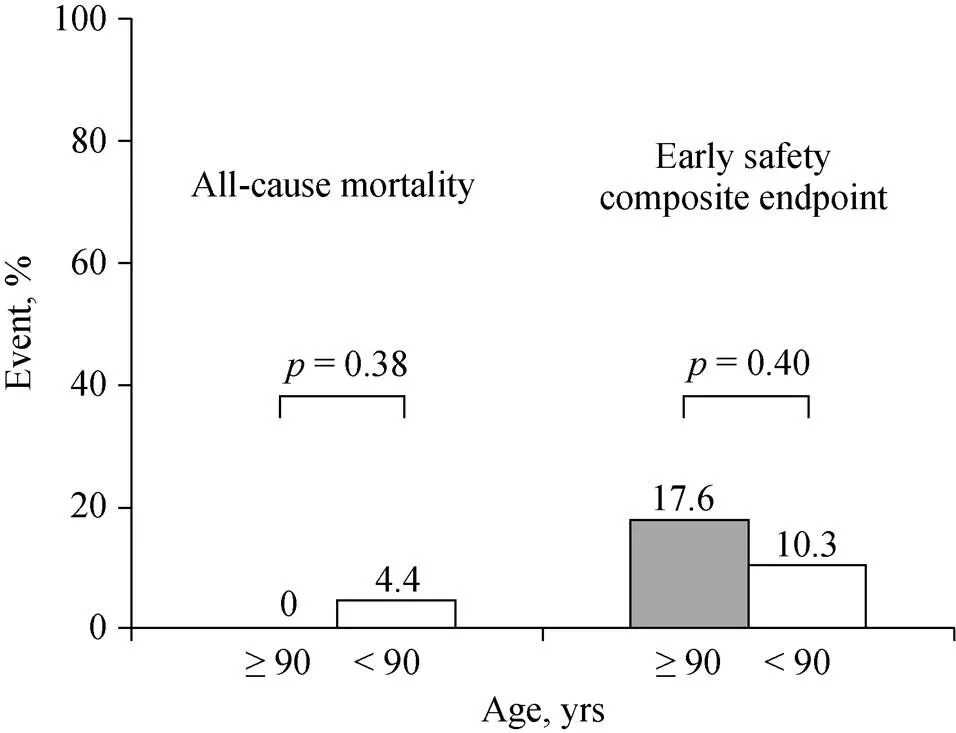
Figure 1. Comparison of all-cause mortality and composite endpoint of early safety at 30 days between patients aged ≥90 years and < 90 years. The composite endpoint of early safety at 30 days defined by the Valve Academic Research Consortium-2 includes all-cause death, all stroke, life-threatening bleeding, acute kidney injury stage 2 or 3, coronary artery obstruction requiring intervention, major vascular complication and valve-related dysfunction requiring repeat procedure.
4 Discussion
The present study investigated the early clinical outcomes after TAVR using the SAPIEN 3 in nonagenarians. The Society of Thoracic Surgeons score in nonagenarians was significantly higher than younger patients. There was no significant difference in 30-day mortality between nonagenarians and others. However, the life-threatening bleeding and major vascular complications in patients aged ≥ 90 years was greater than patients aged < 90 years. The composite endpoint of early safety at 30 days including all-cause death, all stroke, life-threatening bleeding, acute kidney injury stage 2 or 3, coronary artery obstruction requiring intervention, major vascular complication and valve-related dysfunction requiring repeat procedure was similar between nonagenarians and others.
As the prevalence of severe symptomatic AS increases with age, the need for treatment of nonagenarians with this disease has become more frequent.[9]Previous study has shown that surgical aortic valve replacement can be safe in octogenarians.[10]However, nonagenarians are more frail and have more comorbid conditions compared with younger cohorts, and surgical aortic valve replacement might been precluded in nonagenarians.[11]Previous trials demonstrated that TAVR significantly reduced mortality compared to medical therapy and was comparable to surgical aortic valve replacement among high-risk patients.[12,13]
Yamamoto,.[14]previously demonstrated that no statistically significant difference was found between TAVR patients aged ≥ 90 years and aged < 90 years with respect to the 30-day mortality rates (15%. 6%,= 0.22). On the other hand, in the insights from the Society of Thoracic Surgeons/American College of Cardiology TVT (Transcatheter Valve Therapy), a 30-day mortality rate of 8.8% after TAVR in nonagenarians, and the mortality rate for nonagenarians remained higher than that of 5.9% observed in younger patients.[4]In the present study, a 30-day mortality rate was 0 after TAVR using the SAPIEN 3 in nonagenarians although the Society of Thoracic Surgeons score was high.
The SAPIEN 3 is the improved generation of balloon-expandable prosthesis and has been designed to address the problem of paravalvular aortic regurgitation and to deliver with a lower profile.[15]In the Placement of Aortic Transcatheter Valves (PARTNER) trial, paravalvular aortic regurgitation was more frequent after TAVR than after surgery and moderate or severe paravalvular aortic regurgitation was seen in 11.8% of patients implanted with the previous balloon-expandable prosthesis.[12]Patients with moderate or severe paravalvular aortic regurgitation have the lower short-term survival than those with trivial or mild paravalvular aortic regurgitation.[16,17]In this study, moderate or severe paravalvular aortic regurgitation was shown in 3.5% of all patients after TAVR using the SAPIEN 3. There was no significant difference in moderate or severe paravalvular aortic regurgitation between nonagenarians and others (0. 4.4%;= 0.38).
Increased age might heighten the risk of particular postprocedural complications.[4]In the PARTNER trial for high risk patients, the TAVR cohort had higher rates of 30-day complications compared with surgical patients, with rates of major vascular events (11.0%. 3.2%,< 0.001).[13]In the present study, major vascular complications were demon- strated in 3.5% of all patients after TAVR using SAPIEN 3. The major vascular complications in age ≥ 90 years group were greater than age < 90 years group although there was no significant difference in minor vascular complications between the two groups. The SAPIEN 3 could reduce vascular complications, but more attention to major vascular complications was necessary for TAVR procedure in nonagenarians.
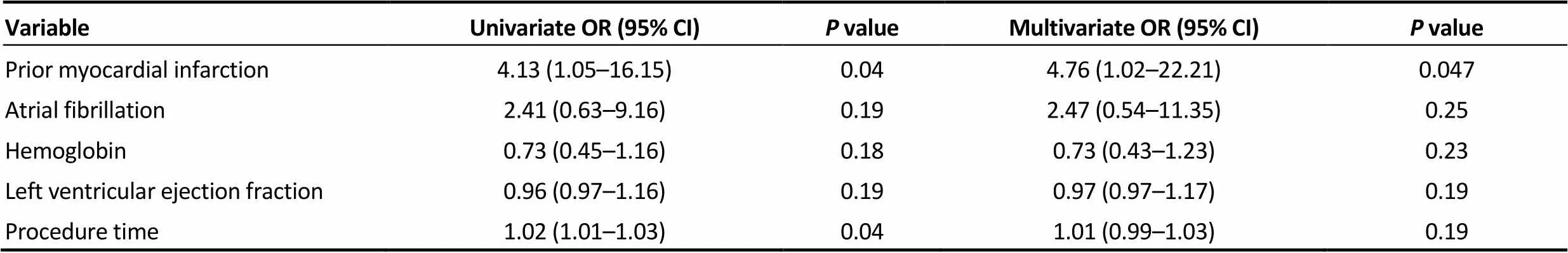
Table 5. Predictors of composite endpoint of early safety.
In this study, the life-threatening bleeding in patients aged ≥ 90 years was greater than those aged < 90 years. Previous study reported that life-threatening and major bleeding after TAVR occurred in approximately 15% and 20% of TAVR procedures.[18]Toggweiler,.[19]showed that marked reductions in bleeding and vascular complications could be achieved with careful patient selection and advanced interventional techniques. On the other hand, despite lower body mass index in age ≥ 90 years group than age < 90 years group, the antiplatelet therapy was similar between the two groups in the present study. Thus, the reduction of antiplatelet medication might be important to reduce the risk of bleeding in nonagenarians of lower body mass index.
Previous study indicated that New York Heart Association class and logistic EuroSCORE were independent predictive factors of cumulative 30-day mortality in octogenarians and nonagenarians.[20]In the present study, prior myocardial infarction was an independent predictor of the composite endpoint of early safety at 30 days. This might be associated with cardiac dysfunction and prior antiplatelet therapy. The necessity for proper antiplatelet therapy after TAVR according to baseline characteristics was considered.
The early mortality and safety after TAVR using the SAPIEN 3 in nonagenarians was evaluated in this study. Despite higher operative risk of TAVR in patients aged ≥ 90 years, the composite endpoint of early safety at 30 days was similar between nonagenarians and younger populations. A possible explanation for this observation might be the advancement of TAVR in regard to valve design improvement, increased operator experience and improved procedural pre-planning. The use of the SAPIEN 3 for TAVR might be useful in nonagenarians.
There are some limitations in the present study. The sample size is small and this is a single-center nonrandomized study. In this study, the use of a self-expandable prosthesis was not included to eliminate the differences of prosthesis. However, there might be a selection bias between devices. This was a retrospective study with a small number of patients. This might be associated with no significant difference in all-cause mortality between the two groups. In the present study, the assessment of quality of life such as the Kansas City Cardiomyopathy Questionnaire before and after TAVR was not evaluated.[21]This study was included only early clinical outcomes. Further studies with a larger number of patients and longer follow-up are required to evaluate usefulness of TAVR using the SAPIEN 3 in nonagenarians to predict the clinical events.
In conclusions, the early mortality after TAVR using the SAPIEN 3 in nonagenarians was similar in younger population despite of higher operative risk. The life-threatening bleeding and major vascular complications after TAVR were greater in nonagenarians. However, the early safety after TAVR using the SAPIEN 3 in nonagenarians was similar and acceptable.
Acknowledgements
The authors have no conflicts of interest to disclose.
1 Kodali SK, Kodali SK, Williams MR,; PARTNER trial investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement.2012; 366: 1686–1695.
2 Iung B, Baron G, Butchart EG,. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on valvular heart disease.2003; 24: 1231–1243.
3 Makkar RR, Fontana GP, Jilaihawi H,; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis.2012; 366: 1696–1704.
4 Arsalan M, Szerlip M, Vemulapalli S,. Should Transcatheter Aortic Valve Replacement Be Performed in Nonagenarians? Insights From the STS/ACC TVT Registry.2016; 67: 1387–1395.
5 Barbanti M, Binder RK, Freeman M,. Impact of low-profile sheaths on vascular complications during transfemoral transcatheter aortic valve replacement.2013; 9: 929–935.
6 Binder RK, Rodés-Cabau J, Wood DA,. Transcatheter aortic valve replacement with the SAPIEN 3: a new balloon-expandable transcatheter heart valve.2013; 6: 293–300.
7 Nishimura RA, Otto CM, Bonow RO,; ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines.2014; 129: e521-e643.
8 Kappetein AP, Head SJ, Généreux P,. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document.2012; 33: 2403–2418.
9 Mack MC, Szerlip M, Herbert MA,. Outcomes of treatment of nonagenarians with severe aortic stenosis.2015; 100: 74–80.
10 Craver JM, Puskas JD, Weintraub WW,. 601 octogenarians undergoing cardiac surgery: outcome and comparison with younger age groups.1999; 67: 1104–1110.
11 Speziale G, Nasso G, Barattoni MC,. Operative and middle-term results of cardiac surgery in nonagenarians: a bridge toward routine practice.2010; 121: 208–213.
12 Leon MB, Smith CR, Mack M,; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery.2010; 363: 1597–1607.
13 Smith CR, Leon MB, Mack MJ,; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients.2011; 364: 2187–2198.
14 Yamamoto M, Meguro K, Mouillet G,. Comparison of effectiveness and safety of transcatheter aortic valve implantation in patients aged ≥ 90 years versus < 90 years.2012; 110: 1156–1163.
15 Binder RK, Stortecky S, Heg D,. Procedural results and clinical outcomes of transcatheter aortic valve implantation in Switzerland: an observational cohort study of Sapien 3 versus Sapien XT transcatheter heart valves.2015; 8: e002653.
16 Sinning JM, Vasa-Nicotera M, Chin D,. Evaluation and management of paravalvular aortic regurgitation after transcatheter aortic valve replacement.2013; 62: 11–20.
17 Jerez-Valero M, Urena M, Webb JG,. Clinical impact of aortic regurgitation after transcatheter aortic valve replacement: insights into the degree and acuteness of presentation.2014; 7: 1022–1032.
18 Généreux P, Head SJ, Van Mieghem NM,. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta- analysis of 3,519 patients from 16 studies.2012; 59: 2317–2326.
19 Toggweiler S, Gurvitch R, Leipsic J,. Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure.2012; 59: 113–118.
20 Yamamoto M, Mouillet G, Meguro K,; FRANCE-2 Registry Investigators. Clinical results of transcatheter aortic valve implantation in octogenarians and nonagenarians: insights from the FRANCE-2 registry.2014; 97: 29–36.
21 Arnold SV, Arnold SV, Spertus JA,. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis.2013; 6: 61–67.
Eiji Ichimoto, MD, Department of Invasive Cardiology, Englewood Hospital and Medical Center, 350 Engle Street, Englewood, NJ 07631, United States. E-mail: e.ichimoto@nifty.com
February 1, 2018
April 21, 2018
April 23, 2018
June 28, 2018
杂志排行
Journal of Geriatric Cardiology的其它文章
- “Malignant” right coronary artery presenting as an ST-segment elevation myocardial infarction—a case report
- Influenza vaccination in acute coronary syndromes patients in Thailand: the cost-effectiveness analysis of the prevention for cardiovascular events and pneumonia
- The trend of change in catheter ablation versus antiarrhythmic drugs for the management of atrial fibrillation over time: a meta-analysis and meta-regression
- Depression and chronic heart failure in the elderly: an intriguing relationship
- CIED implantation in elderly patients: a single-center experience
- Use of the reported Edmonton frail scale in the assessment of patients for transcatheter aortic valve replacement: a possible selection tool in very high-risk patients?
