利用邻-碳硼烷可变的C-C键实现发光分子从ACQ到AIE转变
2018-08-01燕森博芦昌盛
陈 伟 燕森博 燕 红*, 芦昌盛*,,3
(1南京大学化学化工学院,南京 210023)
(2河海大学力学与材料学院,南京 210000)
(3南京大学国家级化学实验教学示范中心,南京 210023)
0 Introduction
Usually,bright luminescence from luminophores in solutions is absent[1-3]upon aggregation.Such aggregation-caused quenching (ACQ)effect is triggered by intermolecular interactions[4]such as π…π stacking or excimers,further causing a detrimental effect on luminous efficiency[5-7],thus badly fulfill the needs of various applications.In stark contrast to ACQ,another unique photophysical phenomenon termed as aggregation-induced emission (AIE)established by Tang[8]shows excellent photophysical properties.In the past 20 years,AIE has become a significant principle to construct luminophores[9-11]in material science and biological technology.Generally,luminophores possessing AIE properties can be accessed via the restriction of intramolecular motion(RIM)[12],which blocks the non-radiativepathwayand produceseffective luminescence in aggregates.Therefore,direct transformation of conventional ACQ luminophores to AIE luminogens(AIEgens)will be a best choice to obtain AIEgens by embedding sufficient intramolecular motions[13].From this point of view,AIEgens can be generated by modifying luminophores with well-known AIE archetypes[14-15]or in the other way replacing parts ofAIE molecules with classical luminophores[16-17].Despite these pioneering tactics and enormous AIE-active compounds in hand,some systems[18]remain to suffer from complicated molecular design and tedious synthetic procedures to cater for the requirements of various high-tech applications.Therefore,it is to be the theme to develop a novel strategy to realize efficient AIEgens.
Recently,luminophores containing o-carborane unit for photoelectric functional materials[19-20]have drawn much attention.The unique properties of ocarborane[21],such as polarizable σ-aromatic character and electron-withdrawing ability through substitution at the carbon cites,make excellent properties with satisfactory stability and fantastic luminous efficiency.For example,Kang et al.[22]revealed that CT(charge transfer)emissions in o-carborane-based luminophores originate from the orbital overlap between the chromophores and the carboranyl σ(C-C)orbitals.Chujo et al.[23]reported that the AIE properties of a variety of o-carborane-based conjugated systems were caused by intramolecular charge transfer from the π-conjugated groups to o-carborane.Lee et al.[24]indicated by experiments that the phosphorescence efficiency of iridium complexes could be manipulated by the substituents at carbon cites in o-carborane,and the carboranyl C-C bond vibration in the excited state would be responsible for phosphorescence efficiency.Our group[25]has previously demonstrated the luminescent efficiency of o-carborane-based iridium complexes exhibit solventor media-dependence,which is closely related to dielectric constant of media.It can be concluded that the variable C-C bond of o-carborane might be a useful tool to tune the photophysical properties of luminophores.
Besides aforementioned results, theoretical calculations[26]also uncovered that the C-C bond lengths of o-carborane could vary substantially with the nature of the carbon substituents.It should be anticipated that the vibrating motion of the C-C bond in ocarborane can provide a radiationless channel by modifying the substituents(such as alkyl and phenyl)[27],which resultin weak luminescence in solution.However,the cage structure can suppress both the π…π stacking and the C-C bond vibration in aggregate state[28-29],thus giving rise to enhanced emissions with AIE properties.Such characteristic of the C-C bond in o-carborane could be the new strategy to realize transformation from ACQ luminophores to AIEgens.Herein,in order to confirm this idea,phenyl anthracene undergoing ACQ properties was designed with o-carborane cage bearing different substituents.Photophysical properties indicated that these compounds undergo the similar emission behavior in solution.However,the luminophores only with the substituents ethyl and phenyl in o-carborane show AIE properties.This observation can be ascribed to the elongation of C-C bond that dissipate the energy in excited state,and to the more rigid packing structures in crystalline state[30],leading to the transformation from ACQ luminophores to AIEgens.
1 Experimental
1.1 General
Allstarting materials were purchased from commercial sources and were used directly without further purification.Reactions were carried out under an argon atmosphere using standard Schlenk procedure.The1H,13C,11B NMR spectra were measured on DRX-400 at room temperature with CDCl3as a deuterated reagent.Mass spectra were tested with a Bruker Daltonics AutoflexⅡMALDI TOF MS spectrometer and a Micromass GC-TOF for EI-MS(70 eV)spectrometer.Infrared spectra were performed on a Nicolet NEXUS870 FT-IR.UV-Vis absorption spectra were recorded with Shimadzu UV-2550 spectrophotometers.PL spectra were recorded on aHitachiF-4600 fluorescence spectrophotometer.
1.2 Synthesis
All of the o-carborane-functionalized compounds(Scheme 1)were synthesized using o-carborane substituted halide as a starting material through the Suzuki reaction[31](Supporting Information,Scheme S1).All the newly synthesized compounds are stable to air and light.These compounds were carefully characterized by NMR,MS and IR.Besides,the crystal structures of all the o-carborane-based compounds were solved via X-ray crystallography(Fig.S13).

Scheme 1 Illustration of o-carborane-functionalized anthracene derivatives
1-(4-bromophenyl)-2-ethyl-o-carborane (1-et):A DMF solution of 1-(4-bromophenyl)-o-carborane(299 mg,1.0 mmol)was treated with sodium hydride(90.0 mg,2.4 mmol)at-20℃.After stirring for 1 h,an excess amount of C2H5I (1.5 equiv,1.5 mmol)was added into the above mixture.The reaction was stirred for another 4 h.After quenching the reaction mixture by saturated ammonium chloride solution (20.0 mL),the aqueous layer was extracted with diethyl ether(three times).Combined organic layers were filtered,dried over MgSO4,and concentrated under reduced pressure.Further purification was performed by column chromatography on silica.Elution with n-hexane gave white solid with a yield of 50%(160.3 mg).1H NMR(400 MHz,chloroform-d):δ 7.55~7.46(m,4H),3.25~1.70(br,10H,B-H),1.88~1.82(m,2H),0.98(t,J=7.6 Hz,3H).13C NMR(101 MHz,chloroform-d):δ 132.64,132.11,129.99,125.52,83.24,28.71,13.85.11B NMR(128 MHz,chloroform-d):δ-2.92(1B),-4.10(1B),-10.47(8B).Anal.Calcd.for C10H19B10Br(%):C,36.70;H,5.85.Found(%):C,37.47;H,5.46.IR(KBr,cm-1):2 583(B-H).EI-MS(m/z):327.23(M+,100.00%).
o-et-an:A mixture of 1-et(100.0 mg,0.31 mmol)and 9-anthraceneboronic acid (81.4 mg,0.37 mmol),Pd(P(Ph)3)4(23.1 mg,0.02 mmol),K2CO3(84.5 mg,0.62 mmol)in THF (20.0 mL)was refluxed for 48 h under argon.After cooling down to r.t.,water(40.0 mL)was added.Then,the resulting mixture was extracted with CH2Cl2(3×30 mL)and the combined organic layers were dried on MgSO4.Removal of the solvents in vacuum gave a residue,which was subjected to column chromatography on silica gel.Elution with n-hexane gave white solid with a yield of 36%(47.4 mg).1H NMR(400 MHz,chloroform-d):δ 8.54(s,1H),8.07(d,J=8.4 Hz,2H),7.84(d,J=8.4 Hz,2H),7.55~7.46(m,4H),7.40~7.31(m,4H),3.25~1.70(br,10H,B-H),2.06(q,J=7.5 Hz,2H),1.12(t,J=7.6 Hz,3H).13C NMR (101 MHz,chloroform-d):δ 141.55,134.86,131.82,131.31,131.19,130.12,129.89,128.57,127.35,126.15,125.86,125.29,83.44,28.91,14.02.11B NMR(128 MHz,chloroform-d):δ-3.09(1B),-4.17(1B),-9.69(4B),-10.60(4B).Anal.Calcd.for C24H28B10(%):C,67.89;H,6.65.Found(%):C,67.55;H,6.32.IR(KB,cm-1):2563(B-H).MALDI-TOF(m/z):424.56(M+).
ph-o-an was synthesized by the same procedure as that of o-et-an.White solid,58.6 mg,with a yield of 40%.1H NMR (400 MHz,chloroform-d):δ 8.47(s,1H),8.02(d,J=8.5 Hz,2H),7.64~7.59(m,2H),7.55~7.51(m,2H),7.47~7.42(m,2H),7.36(m,1H),7.32~7.26(m,4H),7.24~7.17(m,4H),3.25~1.70(br,10H,B-H).13C NMR (101 MHz,chloroform-d):δ 141.06,131.18,131.06,130.84,130.80,130.06,129.74,128.44,128.25,127.08,126.12,125.56,125.16.11B NMR(128 MHz,chloroform-d):δ-2.22(3B),-9.26(3B),-10.31(4B).Anal.Calcd.for C28H28B10(%):C,71.15;H,5.97.Found(%):70.88;H,5.53.IR(KBr,cm-1):2 593(BH).MALDI-TOF(m/z):472.53(M+).
o-me-an was synthesized by the same procedure as that of o-et-an.White solid,38.1 mg,with a yield of 30%.1H NMR (400 MHz,chloroform-d):δ 8.52(s,1H),8.05(d,J=8.4 Hz,2H),7.76(d,J=8.3 Hz,1H),7.59(d,J=8.8 Hz,2H),7.50~7.45(m,2H),7.41~7.36(m,2H),7.29 (dd,J=8.3,1.7 Hz,1H),7.21 (s,1H),4.69(s,1H),3.25~1.70(br,10H,B-H),2.66(s,3H).13C NMR (101 MHz,CDCl3):δ 140.16,136.44,134.99,134.79,131.34,131.27,131.16,129.91,129.70,128.49,127.41,127.15,126.32,125.73,125.24,78.09,77.35,77.03,76.71,59.89,23.37.11B NMR (128 MHz,chloroform-d):δ-2.70(2B),-8.66(4B),-11.09(2B),-13.11(2B).Anal.Calcd.for C23H26B10(%):C,67.28;H,6.38.Found(%):67.21;H,6.08.IR(KBr,cm-1):2 571(B-H).MALDI-TOF(m/z):410.54(M+).
1.3 X-ray crystal structure determination
X-ray diffraction data were collected on a Bruker Smart CCD Apex DUO diffractometer with graphite monochromated Mo Kα radiation(λ=0.071 073 nm)using the ω-2θ scan mode.Data were corrected for Lorenz and polarization effects.The structure was solved by direct methods and refined on F2by fullmatrix least-squares methods using SHELXTL-2016.All calculations and molecular graphics were carried out on a computer using the SHELX-2016 program package and Diamond.All non-hydrogen atoms were refined anisotropically.Allhydrogen atoms were generated geometrically and refined isotropically using the riding model.Crystaldata,data collection parameters and the results of the analyses of o-et-an,ph-o-an and o-me-an are listed in Table 1.
CCDC:1834185,o-me-an;1834186,ph-o-an;1838538,o-et-an.
2 Results and discussion
2.1 Photophysical studies
To check whether o-carboranyl unit can tune the emission behavior in comparison to the model compound (Scheme 1),photophysical properties of all the compounds were investigated.The absorptionspectra were measured in THF solution(Fig.S15).However,no apparent change is observed[32].All the compounds exhibit two bands due to π-π*transitions[33]:one is the sharp absorption band at ca.260 nm,and the other shapes as three weak bands from ca.340 to 400 nm.Thus,the introduction of o-carborane shows very little effect upon the anthracene unit in absorption measurements,which were proven by DFT(density functional theory)calculations (Fig.S24).Besides,all o-carborane-based compounds exhibit similar absorption spectra compared to that of the model compound in non-polar solvents,such as n-hexane and toluene(Fig.S16 and S17).Thus,the polarity of solvent does not change the ground state energy of these luminophores.
Photoluminescence(PL)spectra were then investigated in THF solution.Compounds o-an[31],o-me-an,and o-et-an display the similar PL spectra as that of ph-an,whereas the control compound is much more emissive(Fig.1).This could be ascribed to the variable C-C bond in o-carboranyl unit which is involved in the excited state to dissipate energy.Such variation of luminous efficiency is in accordance with reported results[30].To get in-depth insights,the excited state structures for all the compounds have been investigated.During emission process,the larger C-C bond length variation in o-carborane was observed(Table S2).This adequately indicates that the C-C bond variation leads to a non-radiative decay from the excited states[34-35].In addition,PL spectra in lower polarity solvent as toluene were studied,which exhibit dual emissions (Fig.2)around 410 and 600 nm,attributed to LE(locally-excited)emission from phenyl anthracene and ICT(intramolecular charge transfer)emission from anthracene to o-carborane,respectively.This reveals that o-carborane can be the electron acceptor to induce intramolecular charge transfer[36-37].However,compound ph-o-an presents only CT emission.Theoretical calculations show that the highest occupied molecularorbitals (HOMOs)ofthese compounds locate in the anthracenyl units,whereas the lowest unoccupied molecular orbitals(LUMOs)extend on the C-C bonds of o-carboranes in the cases of o-an,o-me-an and o-et-an (Fig.S25).However,LUMO of ph-o-an completely concentrates on the ocarborane cage.Hence only CT transition is formed to produce the solely low energy CT emission.Besides,these o-carborane-based compounds show a higher luminous efficiency in toluene than that in THF(Fig.1 and 2),the same as the results previously reported[38].This could be interpreted that the C-C bond in ocarborane is sensitive to polarity of solvent.Increasing the polarity of solvent could induce enhanced C-C bond length variation[39],further making the adverse effect to the luminous efficiency.Therefore,the C-C bond in o-carborane can be a lever to tune the excited state properties of chromophore,which serves as a non-radiative inducer for the excitons to decay.
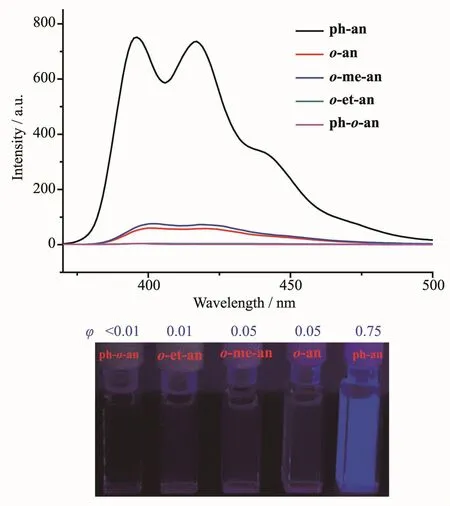
Fig.1 PL spectra(above)and luminescence photographs(below)of o-carborane-based luminophores in THF

Fig.2 Normalized PL spectra(above)and luminescence photographs(below)of o-carborane-based luminophores in toluene
2.2 AIE properties and X-ray analysis
To address whether this “flexible” C-C bond can lead to AIE properties,PL spectra in THF/H2O with different water fractions were measured for all the compounds(Fig.S20~S23).In addition,to quantify the changes of emission efficiency in solution and in aggregate state,α value is defined as follows:α=φa/φs,where φaand φsrepresent the aggregation-and solutionluminous intensity,respectively.Model compound phan shows evident ACQ phenomenon with a small α value:0.14,suggesting the non-ignorable fluorescence quenching effect[40]from the anthracenyl unit.However,those o-carborane-modified compounds exhibit totally different emission behaviors.Typical AIE effect was found in o-et-an and ph-o-an with α values:5.18 and 23.49,respectively.They were both virtually nonemissive in THF. As the fraction ofwateris increased (less than 70%),no notable enhancement was detected.However,a sharp intensified emission was observed when the fraction of water reached 70%.PL spectra of o-et-an reached its summit when the water fraction is 99%(Fig.3),whereas it is 80%in the case of ph-o-an.A further increase of water fraction to 90% leads to a decrease for ph-o-an in the emission intensity.Under this condition,ph-o-an might conglomerate into much larger particles and precipitate,thus causing the emission intensity to decline which is similar to the reported results[41-43].Thus,o-et-an and ph-o-an are AIE active.On the contrary,in the cases of o-me-an and o-an,ACQ effects were observed with α values:0.60 and 0.80,respectively.Compound o-me-an shows emission in pure THF as strong as that observed in THF/water solution until the water fraction reaches 70%(Fig.3).Above this fraction,a dramatic decline in emission intensity was recorded.In addition,noapparent change in luminous intensity has occurred for o-an.Upon the water fraction up to 99%,low luminous intensity appears.

Fig.3 PL spectral changes of o-et-an and o-me-an in THF/H2O with same concentration at room temperature
To unravel the roles that o-carborane has played,X-ray crystallographic analysis was carried out(Fig.S14).All the o-carborane-based luminophores display“face to face” (o-carborane to anthracene)packing patterns[44]without π…π stacking interactions.With careful investigation,compound o-an shows strong Ccage-H…π interaction.Such intermolecular interactions can′t fully eliminate C-C bond vibration and rotation of o-carborane in solid state,even in the case of o-me-an where sterically hindered methyl group is attached to the benzene ring(Fig.4).On the other hand,the intermolecular B-H…π interaction was observed for compounds ph-o-an and o-et-an (Fig.4 and S14).Besides,the o-carborane substituents(ethyl or phenyl)can effectively suppress both the C-C bond vibration and the rotation of o-carborane to ensure rigid packing structures,thus leading to a profound AIE effect.

Fig.4 Crystal packing structures of o-an and o-et-an
3 Conclusions
In conclusion,our approach to realize ACQ luminophores to AIEgens transformation has been proposed by the engineering of variable C-C bond of o-carborane cores in the fluorescent molecules.A series of o-carborane-based anthracene derivatives were synthesized.Photophysical properties studies demonstrate the C-C bond vibration as a main non-radiative decay in solution.However,with physical constraints via intermolecular interactions in aggregate state,such non-radiative decay channel can be inhibited and lead to AIE phenomenon.Therefore,the variable C-C bond in o-carborane could be an effective tool to construct AIEgens,and some potential applications are in process based on these molecules.
Supporting information is available at http://www.wjhxxb.cn
