羟甲基功能化吡唑金属羰基化合物的合成及催化性质
2018-08-01甘贤雪唐良富
李 松 甘贤雪 唐良富*,
(1南开大学化学学院,元素有机化学国家重点实验室,天津 300071)
(2宜宾学院化学与化工学院,宜宾 644007)
0 Introduction
Itiswell-known thathydrogen bondsplay important roles in the self-assembly of metal complexes to form supramolecular architectures[1-2].Organometallic building blocks can also aggregate into supramolecular structures through hydrogen-bonding interactions[3-4].Metal carbonyls as hydrogen bond acceptors in organometalliccrystalsengineering have been observed in several systems,in which these metal carbonyl derivatives show interesting one to threedimensional supramolecular structures[5-8].One the other hand,derivatives of pyrazoles have been used extensively in bioinorganic,coordination chemistry and organometallic fields because of their versatile coordination behavior towards main group and transition metals[9-11].Among pyrazole derivatives,hydroxymethyl functionalized pyrazole is an excellent candidate forthe construction ofsupramolecular architectures,since it not only has multiple coordination modes but also can form regular hydrogen bonding by functioning as both a hydrogen-bonding donor and acceptor[12-13].The group 6 metal carbonyl complexes are of great interest to scientists since they are widely applied in electron beam induced deposition technique as well as employed as catalysts in various organic synthesis[14-15].In this paper,we report the reaction of hydroxymethyl functionalized pyrazoles(L)with group 6 metal carbonyls,which yields a series of LW(CO)5and LM(CO)4(M=Mo or W)derivatives with organometallic supramolecular structures through O-H…O,N-H…O and O-H…OC-M hydrogen-bonding interactions,and the preliminary catalytic activity of these corresponding complexes in the cyclotrimerization reaction of phenylacetylene.
1 Experimental
Solvents were dried and freshly distilled prior to use according to standard procedures.All reactions were carried out under an atmosphere of argon.NMR spectra were recorded on a Bruker 400 spectrometer using DMSO-d6as solvent,and the chemical shifts were reported with respect to the reference(internal SiMe4for1H and13C NMR spectra).IR spectra were recorded as KBr pellets on a Tensor 27 spectrometer.Elemental analyses were carried out on an Elementar Vario EL analyzer.Bis(3-hydroxymethyl-5-methylpyrazol-1-yl)methane was prepared by the published method[16].All the other chemicals were analytical reagents and used as received.
1.1 Syntheses of 1 and 2
3(5)-Hydroxymethyl-5(3)-methylpyrazole(0.112 g,1 mmol)was added to a solution of W(CO)5THF in THF,prepared in situ by the irradiation of a solution of W(CO)6(0.359 g,1 mmol)in THF(60 mL)for 8 h.The mixture was stirred and heated at reflux for 4 h.After the reaction was completed,the solvent was removed under a reduced pressure,and the residue was isolated by column chromatography on silica using ethyl acetate/hexane (1∶2,V/V)as the eluent to give 1 and 2 as yellow solids.
Complex 1:Yield:12%.1H NMR:δ 2.28(s,3H,CH3),4.46(d,J=5.6 Hz,2H,CH2),5.43(t,J=5.6 Hz,1H,OH),6.20 (s,1H,H4of pyrazole),13.28 (s,1H,NH).13C NMR:δ 16.4(CH3),54.5(CH2),105.6(C4of pyrazole),148.3,153.3 (C3and C5of pyrazole),198.2(4 CO),202.5(CO).IR(cm-1): ν(OH)3 208;ν(NH)3 150;ν(CO)2 073,1 918(br),1 879.Anal.Calcd.for C10H8N2O6W(%):C 27.55,H 1.85,N 6.42;Found(%):C 27.69,H 1.78,N 6.65.
Complex 2:Yield:33%.1H NMR:δ 2.29(s,3H,CH3),4.47(s,2H,CH2),5.45(s,1H,OH),6.21(s,1H,H4of pyrazole),13.29 (s,1H,NH).13C NMR:δ 15.9(CH3),54.0(CH2),105.1(C4of pyrazole),147.8,152.8(C3and C5of pyrazole),197.7(4 CO),202.1(CO).IR(cm-1):ν(OH)3 234;ν(NH)3 162;ν(CO)2 073,1 984,1 915,1 847.Anal.Calcd.for C10H8N2O6W(%):C 27.55,H 1.85,N 6.42;Found(%):C 27.62,H 1.94,N 6.29.
1.2 Synthesis of 3
The solution of 4-hydroxymethylpyrazole(49 mg,0.5 mmol)and W(CO)6(180 mg,0.5 mmol)in THF(30 mL)was irradiated with a 300 W high-pressure mercury lamp for 8 h at room temperature.After the reaction was completed,the solvent was removed under a reduced pressure,and the residue was isolated by column chromatography on silica using ethyl acetate/hexane(1∶1,V/V)as the eluent to give 3 as a yellow solid.Yield:125 mg(60%).1H NMR:δ 4.37(d,J=5.3 Hz,2H,CH2),5.01(t,J=5.3 Hz,1H,OH),7.82(s,1H)and 7.86(s,1H)(H3and H5of pyrazole),13.64(s,1H,NH).The signals at 5.01 and 13.64 disappeared when D2O was added.13C NMR:δ 54.0(CH2),124.5(C4of pyrazole),131.1,146.4 (C3and C5of pyrazole),198.3(4CO),202.8(CO).IR(cm-1):ν(OH)3 182;ν(NH)3 137;ν(CO)2 074,1 968(sh),1 909,1 859.Anal.Calcd.for C9H6N2O6W(%):C 25.62,H 1.43,N 6.64;Found(%):C 25.28,H 1.25,N 6.38.
1.3 Synthesis of 4
Complex 4 was similarly obtained using 3,5-dimethyl-4-hydroxymethylpyrazole instead of 4-hydroxymethylpyrazole as above-mentioned synthesis of 3.Yield:57%.1H NMR:δ 2.23(s,3H,CH3),2.26(s,3H,CH3),4.25(s,2H,CH2),4.69(s,br,1H,OH),12.93(s,1H,NH).13C NMR:δ 8.9(CH3),14.0(CH3),52.3(CH2),117.8(C4of pyrazole),141.2,151.9(C3and C5of pyrazole),197.7(4 CO),202.1(CO).IR(cm-1):ν(OH)3 199;ν(NH)3 159;ν(CO)2 072,1 969(sh),1 908,1 888.Anal.Calcd.for C11H10N2O6W(%):C 29.36,H 2.24,N 6.22;Found(%):C 29.31,H 2.31,N 6.24.
1.4 Synthesis of 5
Bis(3-hydroxymethyl-5-methylpyrazol-1-yl)methane(0.118 g,0.5 mmol)was added to a solution of Mo(CO)5THF in THF,prepared in situ by the irradiation of a solution of Mo(CO)6(0.132 g,0.5 mmol)in THF(60 mL)for 8 h.The mixture was stirred and heated at reflux for 4 h.After the reaction was completed,the solvent was removed under a reduced pressure,and the residue was purified by column chromatography on silica using acetone/hexane(2∶3,V/V)as the eluent to give 5 as a yellow solid.Yield:0.13 g(57%).1H NMR:δ 2.48(s,6H,CH3),4.67(d,J=5.2 Hz,4H,CH2),5.49(t,J=5.2 Hz,2H,OH),6.17(s,br,1H,CH2N),6.30(s,2H,H4of pyrazole),6.51(s,br,1H,CH2N).13C NMR:δ 10.8(CH3),57.1(CH2),58.3(CH2),105.5(C4of pyrazole),142.4,157.9 (C3and C5of pyrazole),220.1(CO).IR(cm-1):ν(OH)3 393;ν(CO)2 024,1 921,1 843.Anal.Calcd.for C15H16MoN4O6(%):C 40.55,H 3.63,N 12.61;Found(%):C 40.18,H 3.42,N 12.87.
1.5 Synthesis of 6
Complex 6 was similarly obtained using W(CO)6instead of Mo(CO)6as above-mentioned synthesis of 5.Yield:53%.1H NMR:δ 2.50(s,6H,CH3),4.63(s,2H,CH2),4.72(s,2H,CH2),5.56(s,2H,OH),6.14(d,J=13.1 Hz,1H,CH2N),6.34 (s,2H,H4of pyrazole),6.55(d,J=13.1 Hz,1H,CH2N).13C NMR:δ 11.4(CH3),58.4(CH2),59.6(CH2),106.1(C4of pyrazole),143.0,158.7(C3and C5of pyrazole),211.9 (CO).IR(cm-1):ν(OH)3 393;ν(CO)2 016,1 908,1 840.Anal.Calcd.for C15H16N4O6W(%):C 33.86,H 3.03,N 10.53;Found(%):C 33.92,H 3.25,N 10.37.
1.6 Catalyzed cyclotrimerization of phenylacetylene
Phenylacetylene(0.12 mL,1 mmol)and complexes 1~6(x%,molar ratio)were charged in the reaction tube with 5 mL of toluene.After the reaction mixture was stirred at reflux for 9 h,the volatile materials were removed under reduced pressure.The residuals were purified by column chromatography on silica using CH2Cl2/hexane(1∶10,V/V)as the eluent to give the products,which were analyzed by1H NMR[17].
1.7 Crystal structure determination
Green-yellow crystals of 1~3 suitable for X-ray analysis were grown by slow diffusion of hexane into their CH2Cl2solutions at-18℃.While crystals of 6 were obtained through slow diffusion of hexane into the acetone solution.All intensity data were collected on a Rigaku Saturn CCD detector using Mo Kα radiation (λ=0.071 073 nm)at-160 ℃.Semi-empirical absorption corrections were applied using the Crystalclear program[18].The O(6)atom in 3 was disordered over two sites,with the occupancy factor of 0.5.The complex 6 crystallized with one acetone and one water molecules in the asymmetric unit.The structures were solved by direct methods and difference Fourier map using SHELXS of the SHELXTL package and refined with SHELXL[19]by full-matrix least-squares on F2.All non-hydrogen atoms were refined anisotropically.Hydrogen atoms were added geometrically and refined with riding model position parameters.A summary of the fundamental crystal data for these complexes is listed in Table 1.
CCDC:1835473,1;1835474,2;1835475,3;1835476,6.
2 Results and discussion
2.1 Syntheses of complexes 1~6
Reaction of 3(5)-hydroxymethyl-5(3)-methylpyrazole with W(CO)5THF at refluxing THF or the direct photochemical reactions of 4-hydroxymethylpyrazoles with W(CO)6at room temperature yielded complexes 1~4(Scheme 1).Complexes 5 and 6 were also obtained through the similar reactions of bis(3-hydroxymethyl-5-methylpyrazol-1-yl)methanewith M(CO)5THF(M=Mo or W)in moderate yields.Complexes 1~6 have been characterized by spectroscopic methods.Complexes 1~4 displayed similar IR spectra.These four complexes showed two characteristic absorption peaks for the OH(3 199~3 234 cm-1)and N-H stretching bands(3 137~3 162 cm-1).A ν(C≡O)band at ca.2 073 cm-1corresponding to the A1eqmode for the pseudo C4vmetal center in the M(CO)5fragment[20]was observed in these four complexes,consistent with monodentate pyrazole complexes.The IR spectra of complexes 5 and 6 were different from those of 1~4.Four carbonyl absorption peaks in the range of 1 840~2 024 cm-1were observed for 5 and 6,matching a typical cistetracarbonyl arrangement[21].The NMR spectra of 1~6 also support the suggested structures.For example,the13C NMR spectra of 1~4 showed two carbonyl carbon signals with ca.a 1∶4 intensity ratio,corresponding to a monosubstituted pentacarbonyl species.In addition,the protons of the methylene bridge of 5 and 6 displayed an AB system in their1H NMRspectra,indicating that the inversion of the boat conformation of six-membered metallacycle(crystal structure of 6)was hindered possibly due to the repulsion among ligands.
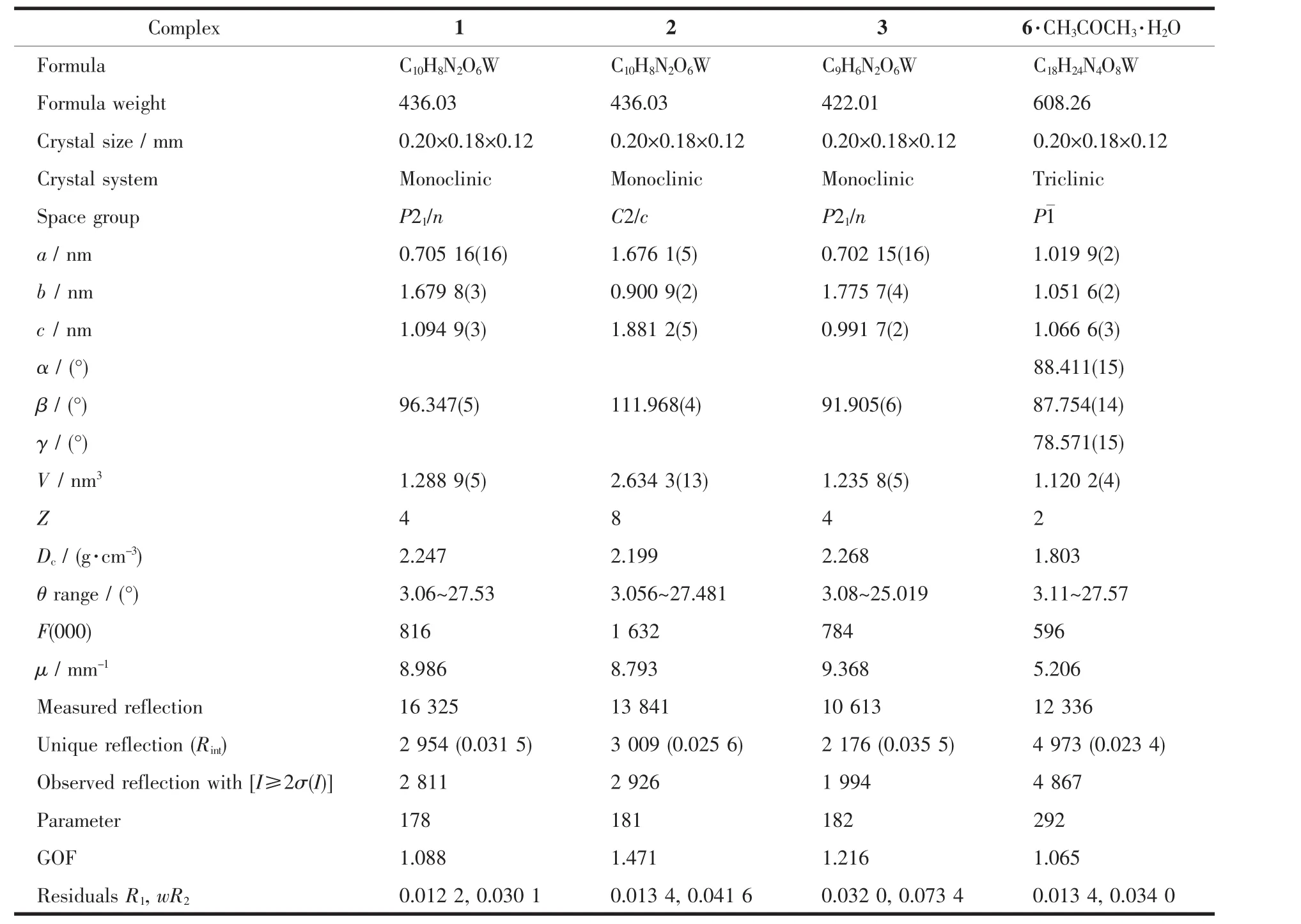
Table 1 Crystallographic data and refinement parameters for complexes 1~3 and 6
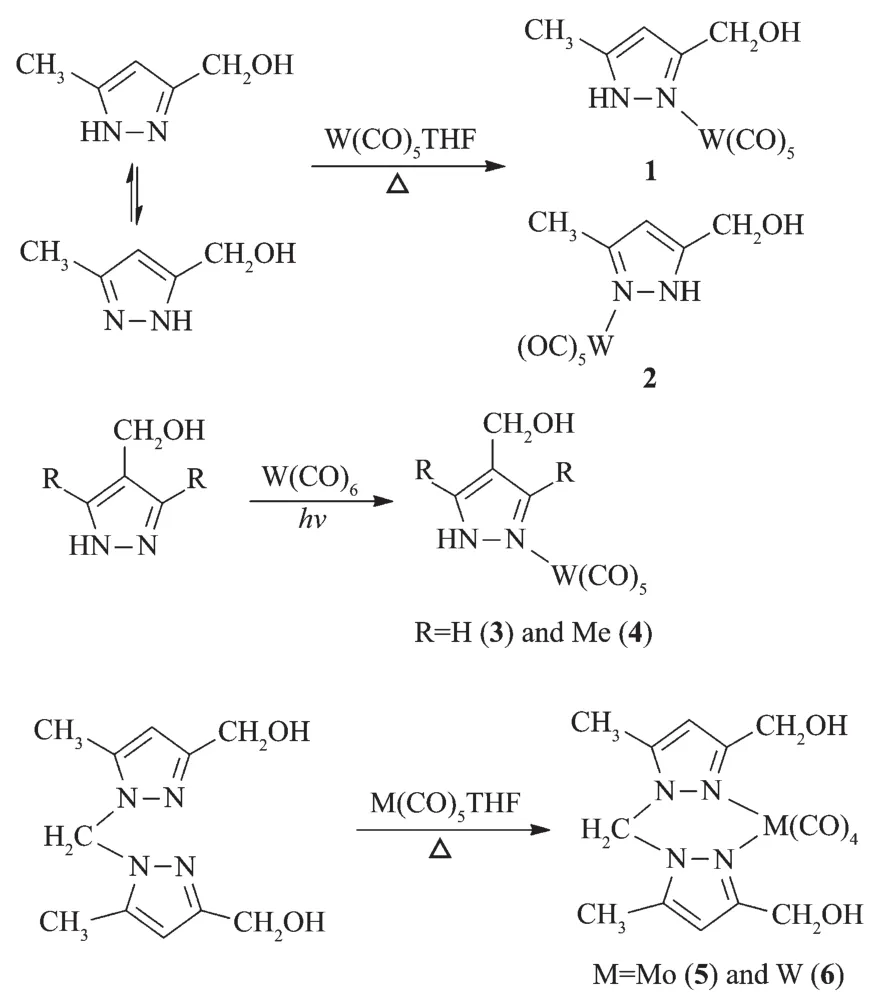
Scheme 1 Syntheses of complexes 1~6
2.2 Crystal structures of complexes 1~3 and 6
The structures of complexes 1~3 and 6 were further confirmed by X-ray crystallography,and are shown in Fig.1~4,respectively.The selected bond distances and angles are listed in Table 2.Fig.1~3 show that hydroxymethylpyrazoles coordinate to the metal center in a monodentate fashion in 1~3,causing them to possess a similar pentacarbonyl tungsten fragment,as shown by their NMR spectra.Complexes 1~3 also share some analogous structural parameters,such as similar W-N bond distance and N-W-C angle.The W-N distances in 1~3(0.223 7~0.226 4 nm)are similartothosereported in otherpentacarbonyl tungsten(0)complexes with azole ligands,such as 0.225 6(4)nm in CH2(3,5-Me2Pz)(Bt-SnPh3)W(CO)5[22].Fig.4 shows that bis(3-hydroxymethyl-5-methylpyrazol-1-yl)methane acts as a chelating κ2-[N,N]bidentate ligand to the tungsten atom in 6,yielding a sixmembered metallacycle with a boat conformation.The W-N bond distances(0.226 4(2)and 0.226 9(2)nm)are similar to those in 1~3,and comparable to those reported for other tetracarbonyl tungsten(0)derivatives with chelating bidentate pyrazolyl groups[23].Additionally,two cis-carbonyls(C(2)O(2)and C(5)O(5)in 1,C(1)O(1)and C(4)O(4)in 2 and 3,as well as C(1)O(1)and C(3)O(3)in 6)are markedly distorted in these four complexes,as evidenced by the corresponding nonlinear C-W-C and W-C-O angles (Table 2),indicating the presence of steric repulsion between the ligand and these carbonyls.
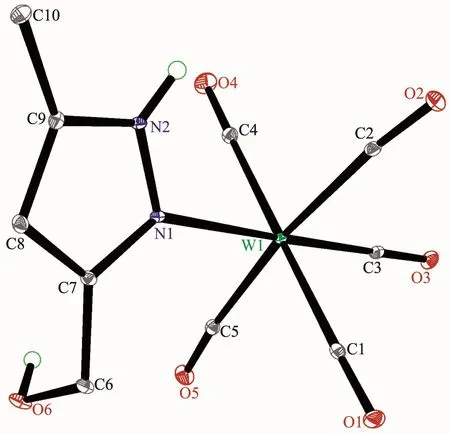
Fig.1 Molecular structure of 1 with 30%probability displacement ellipsoids
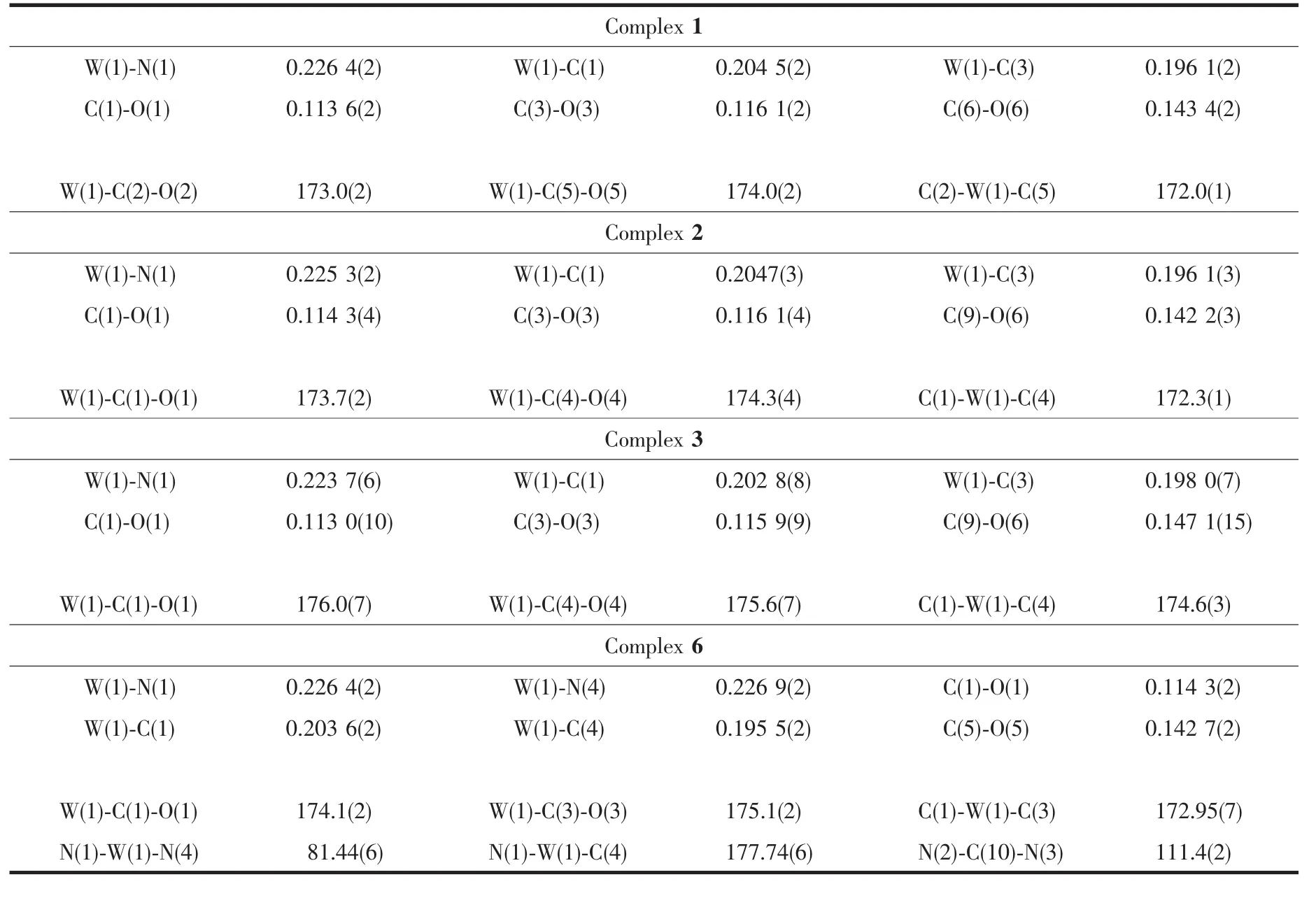
Table 2 Selected bond distances(nm)and angles(°)for complexes 1~3 and 6
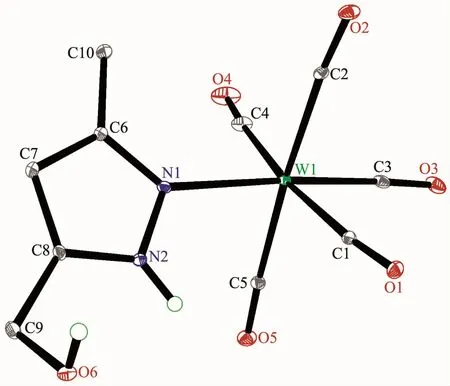
Fig.2 Molecular structure of 2 with 30%probability displacement ellipsoids
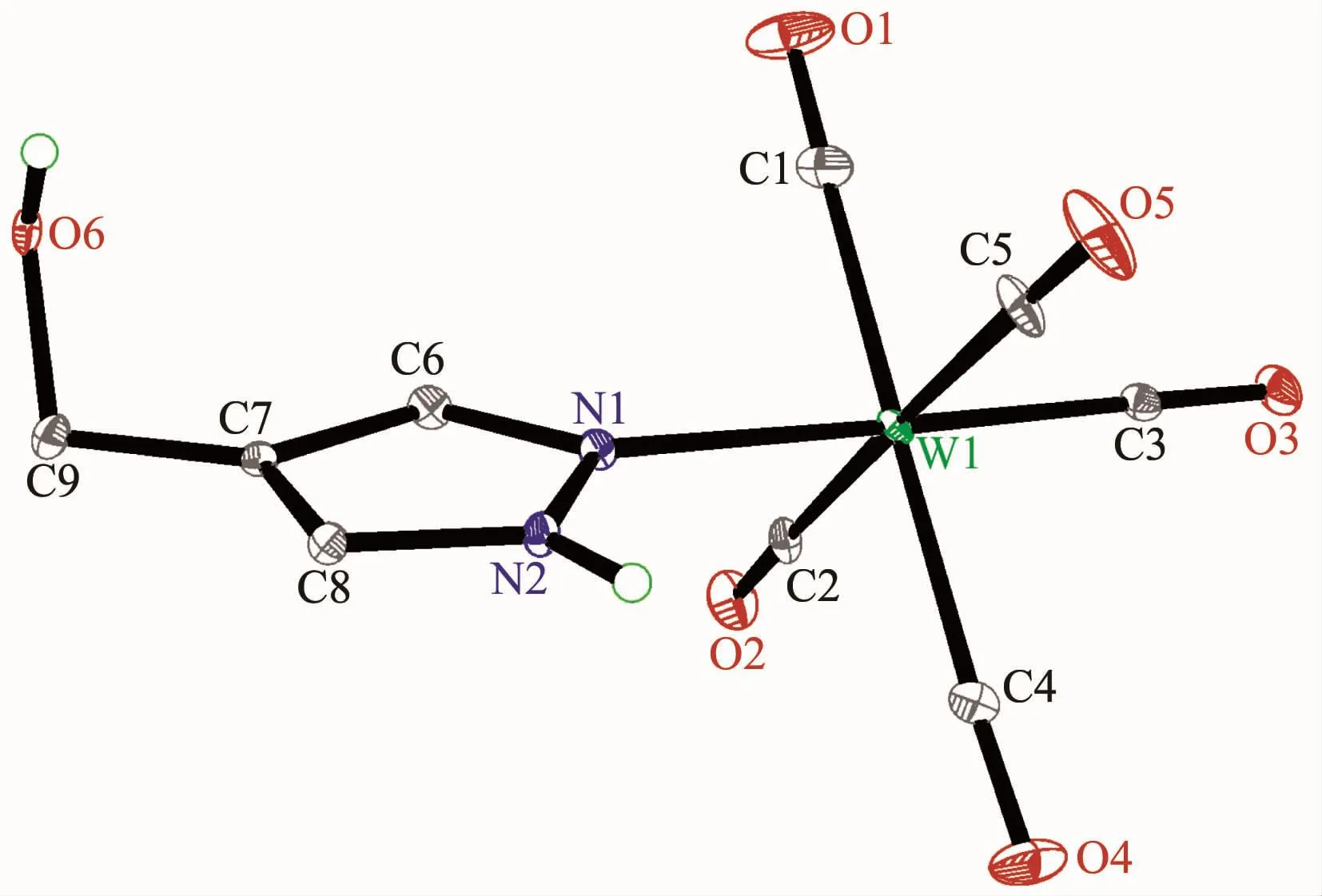
Fig.3 Molecular structure of 3 with 30%probability displacement ellipsoids
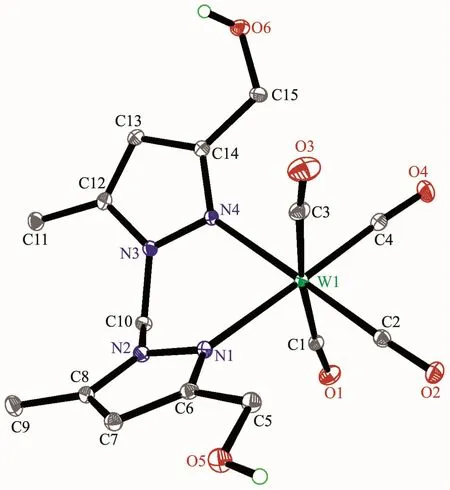
Fig.4 Molecular structure of 6 with 30%probability displacement ellipsoids
It is noteworthy that although complexes 1~3 have a similar molecular skeleton,they show significantly different supramolecular structures(Fig.5~7)owing to the different substitutional position of hydroxymethyl on the pyrazolyl ring.For example,Fig.5 shows that complex 1 aggregates into a 2D supramolecular network through O-H…O(carbonyl)and N-H…O(hydroxyl)hydrogen bonds,and Fig.6 illustrates that complex 2 only extends into a 1D supramolecular double chain.Moreover,Fig.7 shows that the metal carbonyls in 3 do not participate in the hydrogen bonding interactions,and this molecule is only linked into a onedimensional chain via intermolecular N-H…O(hyd-roxyl)hydrogen bonds.In addition,Fig.8 shows that complex 6 forms a 1D supramolecular double chain with macrocyclic units through crystallization water molecule,and also no hydrogen bond is observed between the metal carbonyls with hydroxyl protons.
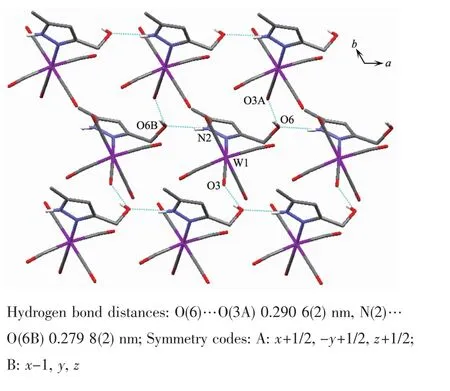
Fig.5 Two dimensional supramolecular structure of 1
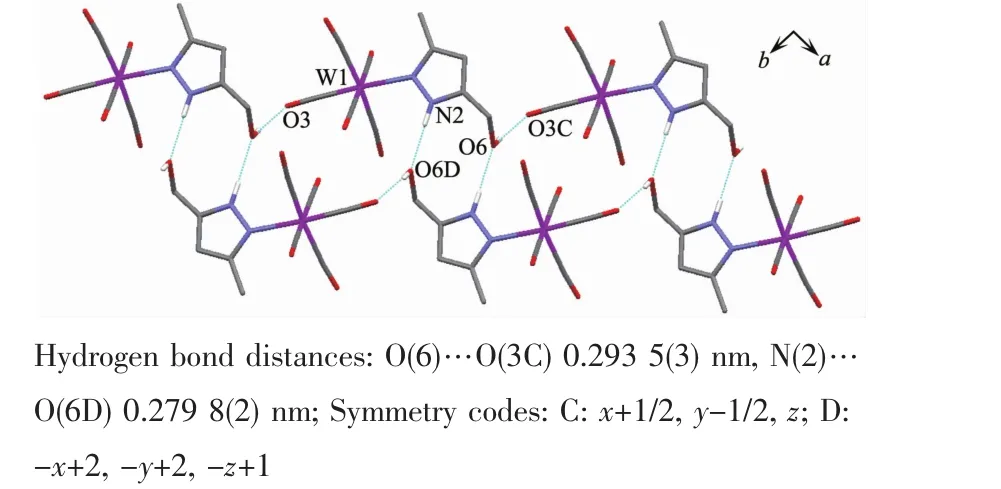
Fig.6 One dimensional supramolecular structure of 2
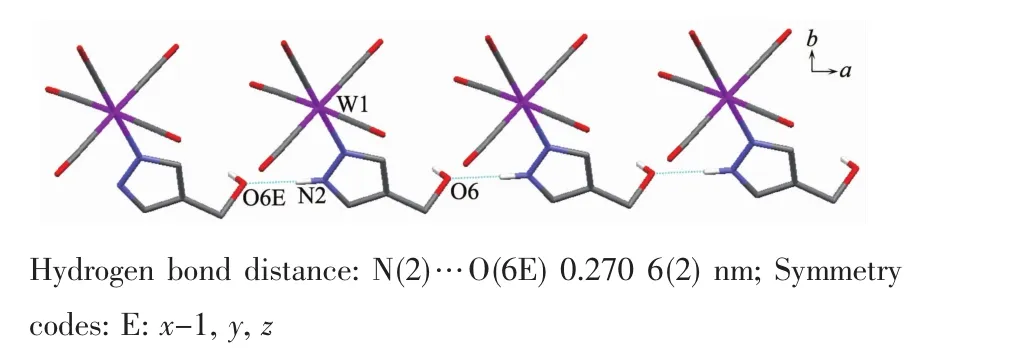
Fig.7 One dimensional supramolecular structure of 3
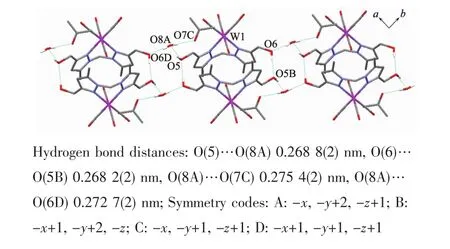
Fig.8 One dimensional supramolecular structure of 6·CH3COCH
2.3 Catalytic activity of complexes 1~6
The transition metal-catalyzed cyclotrimerization reaction of alkynes has been widely used to prepare various polysubstituted benzene derivatives in recent years[24].Molybdenum carbonyl and its derivatives have exhibited efficient catalytic activity in the cyclotrimerization of alkynes[25-26],but it seems that phenylacetylene tends to form polyphenylacetylene when tungsten carbonyl was used as the catalyst[27].Herein,our preliminary studies showed that all these molybdenum and tungsten carbonyl derivatives exhibited effective catalytic activity in the cyclotrimerization reaction of phenylacetylene(Table 3).Two isomers were obtained in moderate yields when 15%(n/n)of complex was used as the catalyst.1,3,5-Trisubstituted benzene is the major product,similar to the result of the cyclotrimerization of phenylacetylene catalyzed by molybdenum carbonyl[26].The ratio of isomers needs to be further improved in future work.
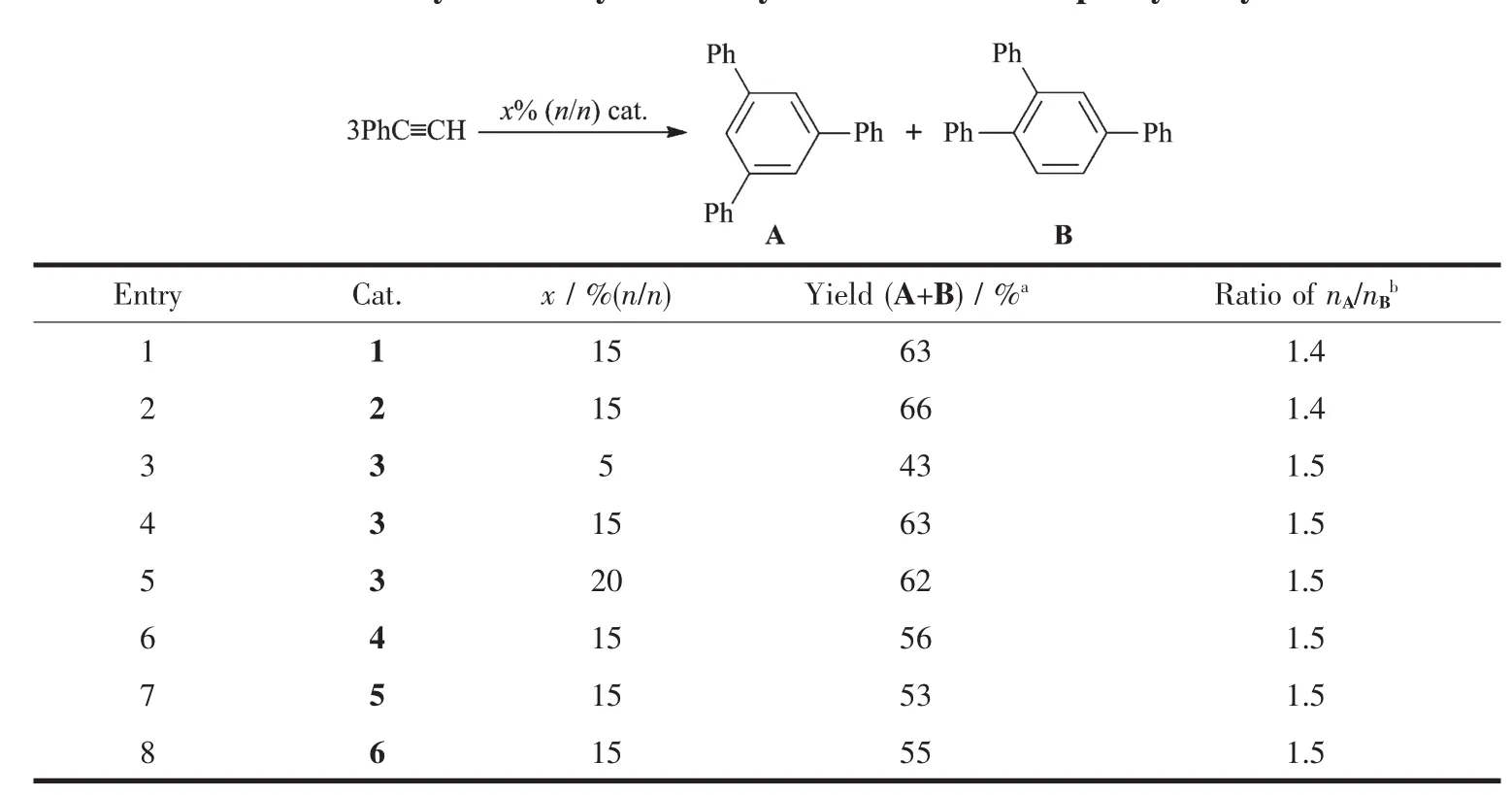
Table 3 Catalytic activity for the cyclotrimerization of phenylacetylene
3 Conclusions
In summary,a series of tungsten and molybdenum carbonyl derivatives with hydroxymethyl functionalized pyrazoles have been synthesized.These complexes show significantly different supramolecular structures owing to the different substitutional position of hydroxymethyl on the pyrazolyl ring.Preliminary catalytic studies prove that all these complexes exhibit moderate catalytic activity in the cyclotrimerization reaction of phenylacetylene.
