Adsorption Removal of Arsenic from Aqueous Solutions by Chemically Modified Wheat Bran
2018-07-25YAOShuhuaMAXichunWANGBaiyiSHIZhongliang
YAO Shu-hua, MA Xi-chun, WANG Bai-yi, SHI Zhong-liang
(Shenyang University of Chemical Technology, Shenyang 110142, China)
Abstract: Contamination of water with arsenic has received attention as a global problem in recent years.In this study,wheat bran was modified by chemicals and utilized as a low-cost adsorbent to remove As(Ⅴ) from aqueous solutions.Batch experiments were carried out to study the effect of various experimental parameters such as initial solution pH,contact time,initial As(Ⅴ) concentration,adsorbent dosage and co-existing anionic component on As(Ⅴ) adsorption,and the optimal condition was selected.The results show that the removal percentage of As(Ⅴ) could be over 95 % in the conditions of adsorbent dosage 5.0 g/L,initial solution pH 4.0~8.0,and contact time 1 h.Under the experimental conditions,phosphate and silicate causes greater decrease in arsenate removal percentage among the anions,and sulfate has almost no effect on the adsorption of arsenate.Kinetic studies show that the As(Ⅴ) adsorption process obeyed pseudo-second-order rate equation.The applicability of the Langmuir and Freundlich models for the data was tested.Both the models adequately describe the experimental data of the adsorption of As(Ⅴ).The maximum adsorption capacity calculated from the Langmuir model is 22.65 mg/g for As(Ⅴ).The study has shown that the effectiveness of modified wheat bran in the removal of As(Ⅴ) from aqueous solutions.
Key words: arsenic; adsorption; wheat bran; chemical modification
Arsenic (As) is a toxic element and is classified as a Group Ⅰ human carcinogen[1].Arsenic contamination in groundwater is widely recognized as a global health problem.Elevated levels of arsenic can be present in the environment as a result of mineral weathering and dissolution[2],geothermal sources[3],microbial activities[4],and anthropogenic activities[2,5-6].The toxicity,mobility,and bioavailability of arsenic are highly dependent on its oxidation state and chemical speciation[7].The most prevalent species of dissolved As in the environment are arsenate[As(Ⅴ)] and arsenite[As(Ⅲ)].As(Ⅴ) is the major arsenic species under oxidizing conditions,while As(Ⅲ) is the dominant arsenic species under reducing conditions.Inorganic species of arsenic[As(Ⅲ) and As(Ⅴ)] represent a potential threat to the environment,human health,and animal health due to their carcinogenic and other effects.Permanent arsenic intake can lead to chronic intoxication,and prolonged arsenic exposure can damage the central nervous system,liver,and skin and results in the appearance of diverse types of cancers,such as hyperkeratosis,lung,skin,and prostate cancers[8-9].
Arsenic contamination has aroused attention due to groundwater levels in many parts of the world at much higher concentrations than the maximum contaminant level (MCL) of 10 μg/L for arsenic in drinking water recommended by the World Health Organization (WHO)[10].Over 137 million people in more than 70 countries are affected by a high level of arsenic in drinking water,including Bangladesh,Argentina,Mexico,Chile,China,Hungary,Thailand,the United States,New Zealand,South Africa and India[11-13].Therefore,an effective arsenic removal technology is thus highly desirable to provide safe drinking water to the affected people.Several methods have evolved over the years on the removal of arsenic present in water and wastewater.These are chemical precipitations,conventional coagulation,reverse osmosis,ion exchange and adsorption.One of which,adsorption method,is simple and cost-effective,thus has been widely used[14-15].Activated carbon is the most popular and widely used adsorbent but it is expensive and its cost increase with the quality.In addition its regeneration with refractory technique results in a 10 %~15 % loss of the adsorbent and its uptake capacity.Thus,there has been intensive research exploring the potential of alternative low-cost materials as sorbents for arsenic.For this purpose in recent years,various biological and industrial by-products have been investigated intensively for their ability to remove arsenic from aqueous solution as they can be obtained readily and are in great abundance,such as seed extract[15],chitosan[16],natural siderite[17],fly ash[18],zeolite[19]and laterite soil[20]have the potential of being used as alternative adsorbent for the removal of arsenic from aqueous solutions.Among these materials,some biosorbents showed extraordinary properties for arsenic removal.Nevertheless,some biosorbents display low adsorption selectivity and capacity toward arsenic.Adsorption capacity and selectivity for specific environmental pollutants can be tailored by modifying the pore structure and surface chemical properties of adsorbents[21].So,modifications of biosorbents through chemical functionalization can enhance it affinity for certain contaminants.
The bran of wheat is the shell of the wheat seed and contains most nutrients of wheat.This bran is usually removed in the processing of wheat into flour.It is environmentally friendly and is nutritious to the plants.Therefore the use of wheat bran to eliminate pollution from water and wastewater is interesting.There are a few reports of heavy metal adsorption by wheat bran,as a by-product of a flour factory,such as Cr(Ⅵ)[22],Pb(Ⅱ)[23],Cu(Ⅱ) and Cd(Ⅱ)[24].This adsorbent was effective and had high capacity for heavy metal adsorption.So the purpose of the present study was,a) to investigate the adsorption of As(Ⅴ) on chemically modified wheat bran,b) to study the effect of different parameters such as contact time,initial pH,adsorbent dosage and initial As(Ⅴ) concentration on adsorption process,c) to find optimum adsorption isotherm as well as the rate of adsorption kinetics.
1 Experimental Section
1.1 Chemicals and Reagents
All the chemicals used in the study were of analytical grade.All the solutions in the study were prepared using de-ionized water.All glassware was cleaned by rinsing with hydroxylamine hydrochloride,soaking in 10 % HCl,and rinsing with de-ionized water.
As(Ⅴ) stock solution (1 000 mg·L-1) was prepared by dissolving dehydrated sodium arsenate(NaAsO3) in the de-ionized water.Dissolution of NaAsO3also includes addition of HCl.Further working solutions were freshly prepared from stock solution for each experimental run.
1.2 Preparation of Adsorbent
The wheat bran used in this study is a by-product of a flour factory in Shenyang,China.The wheat bran was washed thoroughly with water to ensure the removal of dust and ash.It was then sieved to 50 mesh size by passing the milled material through standard steel sieves to remove any large non-wheat bran solids.
Base modified wheat bran:Base modification was carried out by treating 5.0 g of wheat bran with 100 mL of 0.1 mol/L sodium hydroxide (NaOH) shaken at 200 r/min for 1 h at room temperature [(20±1) ℃].Then it was filtered and washed with de-ionized water until neutral and then dried in oven at 50 ℃ for 24 h to produce base treated wheat bran (labeled as BWB).
Hydrochloric acid modified wheat bran:The modification was carried out by stirring 5.0 g of wheat bran in 100 mL of 1.0 mol/L hydrochloric acid (HCl) at room temperature [(20±1) ℃] for 1 h.Then it was filtered and washed with de-ionized water until neutral and then dried in oven at 50 ℃ for 24 h to produce hydrochloric acid modified wheat bran (labeled as AWB).
Citric acid modified wheat bran:Wheat bran (5.0 g) was treated with 40 mL of 1.2 mol/L citric acid.The mixture was placed in the oven at 50 ℃ for 24 h and subsequently heated at 120 ℃ for 2 h.The reaction product was washed with de-ionized water until neutral and dried overnight in the oven at 50 ℃ to produce citric acid treated wheat bran (labeled as CAWB).
Ethylenediamine modified wheat bran:Modification was carried out by treating 5.0 g ACF with 0.1 mol ethylenediamine.The reaction mixture was kept in a water bath at 80 ℃ for 2 h with intermittent stirring and then washed with de-ionized water until neutral,dried overnight in the oven at 50 ℃ (labeled as EAWB).
1.3 Characterization
The morphology of modified wheat bran was determined by scanning electron microscopy (SEM,Philips XL-30).Infrared spectra were obtained to characterize the principal groups.
1.4 Batch Adsorption Experiments
Batch technique was used to investigate As(Ⅴ) adsorption,which was examined via kinetic studies and adsorption isotherms,together with the effect of some operating parameters.All the batch experiments were carried out in duplicate and the results given are the means with a relative standard deviation of less than 5 %.Control experiments without sorbent was carried out to ascertain that the sorption was by the adsorbent and not the wall of the container.
The adsorption capacities of modified wheat bran were determined by batch adsorption isotherms at room temperature [(20±1) ℃] in aqueous solution.In several glass vials,100 mL of solution containing various As(Ⅴ) concentrations (50,100,150,200,250 mg/L) were contacted with 5.0 g/L of adsorbent.The vials were placed in a water bath at 20 ℃ and shaken at 180 r/min for approximately 24 h to ensure equilibrium was reached,and the pH was adjusted by adding 0.1 mol/L NaOH or HNO3until it remained constant (±0.10).After filtration through a 0.22 μm membrane filter,the As(Ⅴ) concentration of the filtered solutions was analyzed with an atomic fluorescence spectrometer (AFS) (PS Analytical Ltd.,Kent,UK) coupled with a hydride generator.Arsenic concentration was determined by treating the solution with a reducing agent containing 50 g/L thiourea and 50 g/L ascorbic acid prior to hydride generation and AFS measurement,using a solution containing 15 g/L KBH4and 3 g/L NaOH as reducing solution and 1 mol/L HCl as carrier solution.
The adsorption kinetic study was performed for As(Ⅴ) in solution at pH 6.0 and room temperature [(20±1) ℃].Several glass vials were used to hold 50 mL As(Ⅴ) solution of known initial concentration (2,5,and 10 mg/L) and 5.0 g/L of adsorbent,and shaken at 180 r/min for a duration ranging from 0 to 240 min.At certain period of time,each vial was removed from the shaker,and the solution was then filtered through 0.22 micron filter paper.The filtrates were analyzed for residual As(Ⅴ) concentration.To determine the effects of different parameters on As(Ⅴ) adsorption,experiments were performed at various initial pH,ranging between 2 and 11.Initial concentration of 10 mg/L of As(Ⅴ) and adsorbent dosage 5.0 g/L were employed.The effects of adsorbent dosage and contact time were conducted.
2 Results and Discussion
2.1 Characterization of Adsorbents
Fig.1 shows the SEM image of the ethylenediamine modified wheat bran.It clearly indicated the surface texture and different levels of porosity of the material.The specific surface area of the adsorbent calculated by the BET method is 256.2 m2/g.

Fig.1 SEM image of ethylenediamine modified wheat bran
The FTIR spectra of the ethylenediamine modified wheat bran before and after the adsorption of As(Ⅴ) were shown in Fig.2.It could be seen that the IR spectra showed five intense bands,around 3 400,2 930,1 700,1 450 and 1 030 cm-1.The broad band around 3 400 cm-1was attributed to the surface hydroxyl groups and chemisorbed water.The bands at 2 930 and 1 700~1 650 cm-1were assigned to C—H stretches of methylene groups on the surface and to ketonic and aldehydic C==O stretching frequencies,and to amino groups,respectively.Small peaks observed at 1 465~1 400 cm-1are attributed to carboxylate groups.At around 1 030 cm-1the band can be assigned to phosphate and silicate groups.Therefore,the FTIR spectra of the modified wheat bran indicated the presence of ionisable functional groups able to bind with MB,and showed the influence of pH on the deprotonation of functional groups.After the adsorption of As(Ⅴ),the intensity of peaks became weak.

(a) before adsorption of As(Ⅴ) (b) after adsorption of As(Ⅴ)Fig.2 FTIR spectra of ethylenediamine modified wheat brans
2.2 Effect of Chemical Modification on Adsorption Process
The comparison result on the uptake of As(Ⅴ) by unmodified and chemically modified wheat bran was shown in Fig.3 (experimental conditions employed:initial As(Ⅴ) concentration 10.0 mg/L,adsorbent dosage 5.0 g/L,solution pH 6.0,adsorption time 1 h,agitation speed 180 r/min).From the results,it appears that the removal rate of As(Ⅴ) is in the order:EAWB (95.7 %)>AWB(85.5 %)>BWB(81.6 %)>CAWB(77.3 %)>WB(72.2%).It can be seen that the adsorption of As(Ⅴ) was enhanced when chemically modified wheat bran was used as the adsorbent.Among the modified samples,the best performance was attributed to EAWB.It could be calculated that the adsorption capacity of EAWB is about 1.3 times higher than that of unmodified wheat bran.Modification with ethylenediamine would probably enhance the introduction of amine groups which are responsible for the adsorption of anionic species onto the wheat bran.The addition of amine groups at the surface of the impregnated wheat bran led to an increase in As(Ⅴ) removal because of the high affinity of the amine functional group for As(Ⅴ).Due to the higher adsorption efficiency of EAWB,subsequent studies were carried out using EAWB.

Fig.3 Effect of chemical modification on the adsorption of As(Ⅴ)
2.3 Effect of Initial As(Ⅴ) Concentration on Adsorption Process
Initial concentration is one of the effective factors on adsorption efficiency.The effect of initial As(Ⅴ) concentrations (10,30,50,75,100 and 150 mg/L) on adsorption percentage of As(Ⅴ) was shown in Fig.4 (experimental conditions employed:adsorbent dosage 5.0 g/L,solution pH 6.0,adsorption time 1 h,agitation speed 180 r/min).It can be seen that the As(Ⅴ) removal rate decreased with the increase in initial As(Ⅴ) concentration,the percentage adsorption of As(Ⅴ) on EAWB decreased from 96.8 % to 43.7 % as the initial As(Ⅴ) concentration was increased from 10 mg/L to 150 mg/L.At lower As(Ⅴ) concentrations,the ratio of the available adsorption sites of adsorbent to the initial number of molecules of As(Ⅴ) is large and subsequently the fractional adsorption becomes independent of initial concentration.However,at higher concentrations,the available sites of adsorption become fewer,and hence the percentage removal of As(Ⅴ) which depends upon the initial concentration,decreases[25].

Fig.4 Effect of initial As(Ⅴ) concentration on the adsorption of As(Ⅴ)
2.4 Effect of Adsorbent Dosage on Adsorption Process
The effect of adsorbent dosage on percentage adsorption of As(Ⅴ) was shown in Fig.5 (experimental conditions employed:initial As(Ⅴ) concentration 10.0 mg/L,solution pH 6.0,adsorption time 1 h,agitation speed 180 r/min).The increase in adsorbent dosage from 1.0 g/L to 5.0 g/L resulted in an increase from 35.6 % to 95.8 % in adsorption of As(Ⅴ).This may be due to the availability of more and more adsorption sites for As(Ⅴ) adsorption during the adsorption reaction.A further increase in adsorbent dosage (>5 g/L) did not cause significant improvement in As(Ⅴ) adsorption.This may be due to the adsorption of almost all As(Ⅴ) to the adsorbent and the establishment of equilibrium between the As(Ⅴ) molecules adsorbed to the adsorbent and those remaining unadsorbed in the solution[26].The rate of adsorption depends upon the uncovered surface available for adsorption.Initially,as the whole surface was uncovered,the rate of adsorption was very high.As the surface was covered increasingly,the rate of adsorption decreased.Ultimately,a stage was reached when there was no more adsorption with the further addition of adsorbent,and at that time,equilibrium was achieved.The results of this study are in accordance with obtained findings by other researchers[8,15,27].Thus 5.0 g/L of modified wheat bran was chosen for next study.

Fig.5 Effect of adsorbent dosage on the adsorption of As(Ⅴ)
2.5 Effect of Initial Solution pH on Adsorption Process
The solution pH is one of the most critical parameters in the adsorption of pollutants from aqueous solutions since it strictly depends upon the nature of adsorbate and adsorbent.In order to determine the preferred pH for adsorption of As(Ⅴ) over EAWB,the uptake of As(Ⅴ) as a function of solution pH was studied.Fig.6 depicts the effect of solution pH (2.0~11.0) on adsorption of As(Ⅴ) onto EAWB (experimental conditions employed:initial As(Ⅴ) concentration 10.0 mg/L,adsorbent dosage 5.0 g/L,adsorption time 1 h,agitation speed 180 r/min).

Fig.6 Effect of solution pH on the adsorption of As(Ⅴ)
It is evident that the percentage of As(Ⅴ) removal strongly depended on the media pH.Furthermore,it can be noticed that the maximum adsorption capacities of adsorbent for As(Ⅴ) occurred at pH 4.0~8.0.Nevertheless,the highest removal efficiency has taken place at pH 6.0(95.8 %) which was chosen as an optimum pH condition for further experiments.In addition,after adsorption,the pH of solution was slightly relevated.One reason for the change of pH may be the ion exchange process.
2.6 Effect of Contact Time on Adsorption Process
Contact time is one of the effective factors in batch adsorption process.The effect of contact time on As(Ⅴ) adsorption efficiency was shown in Fig.7 (experimental conditions employed:initial As(Ⅴ) concentration 10.0 mg/L,adsorbent dosage 5.0 g/L,solution pH 6.0,agitation speed 180 r/min).As it is shown,the removal efficiency of As(Ⅴ) onto EAWB significantly increase during the initial adsorption stage (0~40 min) and then continue to increase at a relatively slow speed with contact time until a state of equilibrium is attained after 60 min.There was no significant change in As(Ⅴ) removal rates after 1 h up to 3 h.Based on these results,1 h was taken as the time in adsorption experiments.Generally the removal rate of sorbate is rapid initially,but it gradually decreases with time until it reaches equilibrium.This phenomenon can be attributed to the fact that a large number of vacant surface sites are available for adsorption at the initial stage,and after a lapse of time,the remaining vacant surface sites are difficult to be occupied due to repulsive forces between the solute molecules on the solid and bulk phases.Similar findings were reported by other researchers[8,28].

Fig.7 Effect of contact time on the adsorption of As(Ⅴ)
2.7 Effect of Co-Existing Anionic Componenton on Adsorption Process
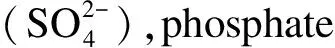

Fig.8 Effect of co-existing anionic components on the adsorption of As(Ⅴ)

2.8 Adsorption Isotherms
The adsorption isotherm indicates how the adsorption molecules distribute between the liquid phase and the solid phase when the adsorption process reaches an equilibrium state.Langmuir and Freundlich isotherm equations are the most widely used models to describe the experimental data of adsorption isotherms.The adsorption isotherm of As(Ⅴ) obtained for EAWB was shown in Fig.9 (experimental conditions employed:adsorbent dosage 5.0 g/L,adsorption time 24 h,agitation speed 180 r/min).These isotherms represent the adsorption behavior of As(Ⅴ) on the adsorbent as a function of increasing aqueous As(Ⅴ) concentration for a contact time of 24 h.The isotherm showed that the adsorption capacity increased with increasing equilibrium concentration of As(Ⅴ).
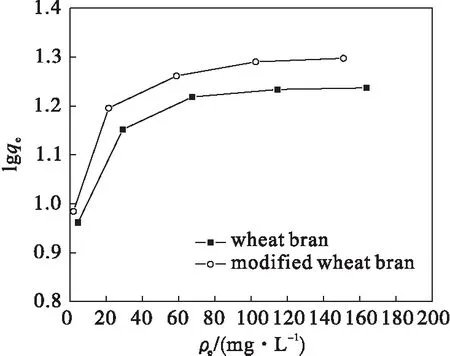
Fig.9 Adsorption isotherms for As(Ⅴ) by wheat bran
The results of As(Ⅴ) adsorption on EAWB (Fig.9) were analyzed using Langmuir model to evaluate parameters associated to the adsorption behavior.The linear form of Langmuir equation at a given temperature is represented by:
qe=qm·b·ρe/(1+b·ρe)
(1)
whereρeis the aqueous phase As(Ⅴ) equilibrium concentration (mg/L),qeis the amount of As(Ⅴ) sorbet onto 1 g of the considered adsorbent (mg/g),bis the adsorption constant (L/mg) related to the energy of adsorption and represents the affinity between the adsorbent and adsorbate,qmis the maximum adsorption capacity (mg/g).
Equation (1) can be rearranged to obtain:
ρe/qe=1/(b·qm)+ρe/qm
(2)
The adsorption data fit to the linear form of the Langmuir equation can obtained by plotting ofρe/qeagainstρe.This linear plot was employed to obtain the values ofqmandb(Table 1) from the slope and intercept of the plot.It could be seen that bothqmandbremain the higher for As(Ⅴ) adsorption onto modified wheat bran.This implies that modified wheat bran has a higher adsorption of As(Ⅴ) than unmodified wheat bran.High value ofbwas reflected in the steep initial slope of an adsorption isotherm,indicating desirable high affinity.Therefore,modified wheat bran performed well in As(Ⅴ) adsorption.
The Freundlich isotherm model was also used to analyze the results of As(Ⅴ) adsorption on EAWB (Fig.9).The Freundlich model can be expressed by the following equation:
qe=kf·ρe1/n
(3)
wherekfandnare constants related to the adsorption capacity and affinity,respectively.The equation is conveniently used in the linear form by taking the logarithm of both sides as:
lgqe=lgkf+(1/n)lgρe
(4)
The adsorption data fit to the linear form of the Freundlich equation can be obtained by plotting of lgqeagainst lgρe.The Freundlich isotherm parameters obtained for the adsorption of As(Ⅴ) on wheat bran were listed in Table 1.The data showed that thekfconstant is higher for modified wheat bran than that for unmodified wheat bran,1/nvalue for modified wheat bran is smaller than that for unmodified wheat bran.These imply more favorable adsorption of As(Ⅴ) on modified wheat bran,and the affinity between modified wheat bran and As(Ⅴ) molecules was also higher[30].From Table 1,the Langmuir adsorption isotherm yielded better fit as indicated by the higherR2values compared to the Freundlich adsorption isotherm model.

Table 1 The parameters of Langmuir and Freudlich equation
2.9 Kinetic Study
In order to obtain the adsorption kinetic information of As(Ⅴ) on EAWB,the change of As(Ⅴ) concentration with adsorption time was recorded for an initial concentration of 2,5,10 mg/L.Fig.10 shows the adsorption percentage of As(Ⅴ) on EAWB (experimental conditions employed:solution pH 6.0,adsorbent dosage 5.0 g/L,agitation speed 180 r/min).Obviously,the adsorption is a rapid process,and the equilibrium is reached at 60 min for all three concentrations.For longer periods,adsorption trend seems to remain constant.

Fig.10 Adsorption kinetics of As(Ⅴ) by modified wheat bran
In order to investigate the mechanism of As(Ⅴ) adsorption on EAWB,the pseudo-second-order rate equation model was applied to the kinetic data.The pseudo-second-order kinetic equation could be derived as[31]:
dqt/dt=k2(qe-qt)2
(5)
Separating the variables in equation (5) gives:
-d(qe-qt)/(qe-qt)2=k2·dt
(6)
Integrating both sides for the boundary conditionst=0 tot=tandqt=0 toqt=qtgives the integrated rate law for a pseudo-second-order reaction:
1/(qe-qt)=1/qe+k2·t
(7)
Equation (7) can be rearranged to obtain:
(8)
The kinetic constant,k2,can be determined by plotting oft/qtagainstt.
The kinetic experimental data of As(Ⅴ) on the composite adsorbent was simulated by pseudo-second-order rate equation (8).The results were listed in Table 2.

Table 2 Kinetic parameters for As(Ⅴ) adsorption by modified wheat bran
Remarkably,the kinetic data could be described well by the pseudo-second-order kinetic equation which was based on the assumption that the rate limiting step may be chemical sorption or chemisorptions involving valency forces through sharing or exchange of electron between adsorbent and adsorbate[30].It could also be seen that the values of the pseudo-second-order rate constant decreased with increasing the initial As(Ⅴ) concentrations.
3 Conclusions
Chemically modified wheat bran was utilized as adsorbent to remove As(Ⅴ) from aqueous solution by adsorption.The modification of wheat bran by chemicals significantly improved its adsorption capacity,the adsorption capacity of wheat bran modified by ethylenediamine for As(Ⅴ) is about 1.3 times higher than that of unmodified wheat bran,its equilibrium adsorption capacity is 22.65 mg/g for As(Ⅴ),and made this material a suitable adsorbent to remove As(Ⅴ) from aqueous solutions.The amount of As(Ⅴ) adsorbed was found to vary with initial solution pH and contact time.The overall adsorption rate was illustrated by the pseudo-second-order kinetic model.The equilibrium data obtained from this study was well presented by Langmuir model.As wheat bran is readily available in great abundance in China,it can be considered as an attractive alternative to the more expensive technologies used in wastewater treatment containing arsenic.
