Medication for management of pregnancy-induced hypertension
2018-07-04
Department of Cardiology, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
INTRODUCTION
Hypertension refers to increased arterial blood pressure.Long-term hypertension can lead to coronary artery disease,stroke, heart failure, atrial fibrillation, peripheral vascular disease, vision loss, chronic kidney disease, and dementia.1,2Hypertension can be divided into primary and secondary types.3Primary hypertension accounts for 90–95% of hypertension cases.3,4Primary hypertension caused by angiogenic degenerative changes is a type of degenerative disease.5
Blood pressure (BP) measured at intervals during pregnancy> 140/90 mmHg is considered to denote PIH. The prevalence of PIH worldwide is 8–10%,6,7whereas that in China is 5.6–9.4%.8China has a two-child policy, so the maternal age may increase, which may further increase PIH prevalence.
Drugs used for the management of primary hypertension are ganglionic blockers, adrenergic neuron-blocking agents,alpha-blockers, diuretics, and beta blockers.9PIH is different from primary hypertension, but similarities exist in the treatment of these two types of hypertension. Using drugs for the management of primary hypertension as a reference, we review the selection and use of drugs for PIH management to provide evidence for its clinical treatment.
CLASSIFICATION AND DEFINITION OF PIH
According to the guideline set by the American Congress of Obstetricians and Gynecologists (ACOG) in 2013, there are four categories of hypertension during pregnancy.
The first type of hypertension during pregnancy is preeclampsia-eclampsia. In the absence of proteinuria,preeclampsia is diagnosed as hypertension in association with thrombocytopenia (platelet count < 100 000/mL), impaired liver function (increased blood levels of liver transaminases to twice the normal concentration), new development of renal insufficiency (increased serum creatinine > 1.1 mg/dL or doubling of serum creatinine in the absence of other renal disease), pulmonary edema, or new-onset cerebral/visual disturbances. The second type is chronic hypertension. This is defined as systolic blood pressure (SBP) > 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg before pregnancy or at a gestational age of 20 weeks, or if BP at 12 weekspost partumremains abnormal. The third type is chronic hypertension with superim-posed preeclampsia. This is chronic hypertension in association with preeclampsia. The final type is PIH developing after 20 weeks without proteinuria or the systemic findings mentioned above.10,11For the convenience of initiating antihypertensive medication, PIH severity is usually divided into two categories: (i) mild–moderate (SBP: 140-159 mmHg; DBP: 90–109 mmHg) and severe (SBP ≥ 160 mmHg;DBP ≥ 110 mmHg).12
PATHOPHYSIOLOGIC CHANGES IN PIH
Under physiologic conditions, the BP of pregnant women begins to decrease after pregnancy onset. Simultaneously,cardiac output increases slightly, and peripheral vascular resistance decreases significantly. Moreover, renal blood flow and the estimated glomerular filtration rate increase. These conditions peak at 12 weeks of gestational age. Peripheral vascular resistance and BP also increase slightly during pregnancy. These tendencies return to the pre-pregnancy level after 36 weeks of gestational age.13The pathologic changes of PIH are spasm of small arteries and sodium retention inthe whole body, which can result in target-organ damage and eclampsia. In addition to pathologic changes, several risk factors can induce eclampsia: nulliparity; multiple gestation;family history of preeclampsia; chronic hypertension; diabetes mellitus; renal disease; history of preeclampsia, especially if early (before 34 weeks) in a previous pregnancy; history of hemolysis; increased level of liver enzymes; HELLP syndrome in a previous pregnancy, obesity; hydatidiform mole.
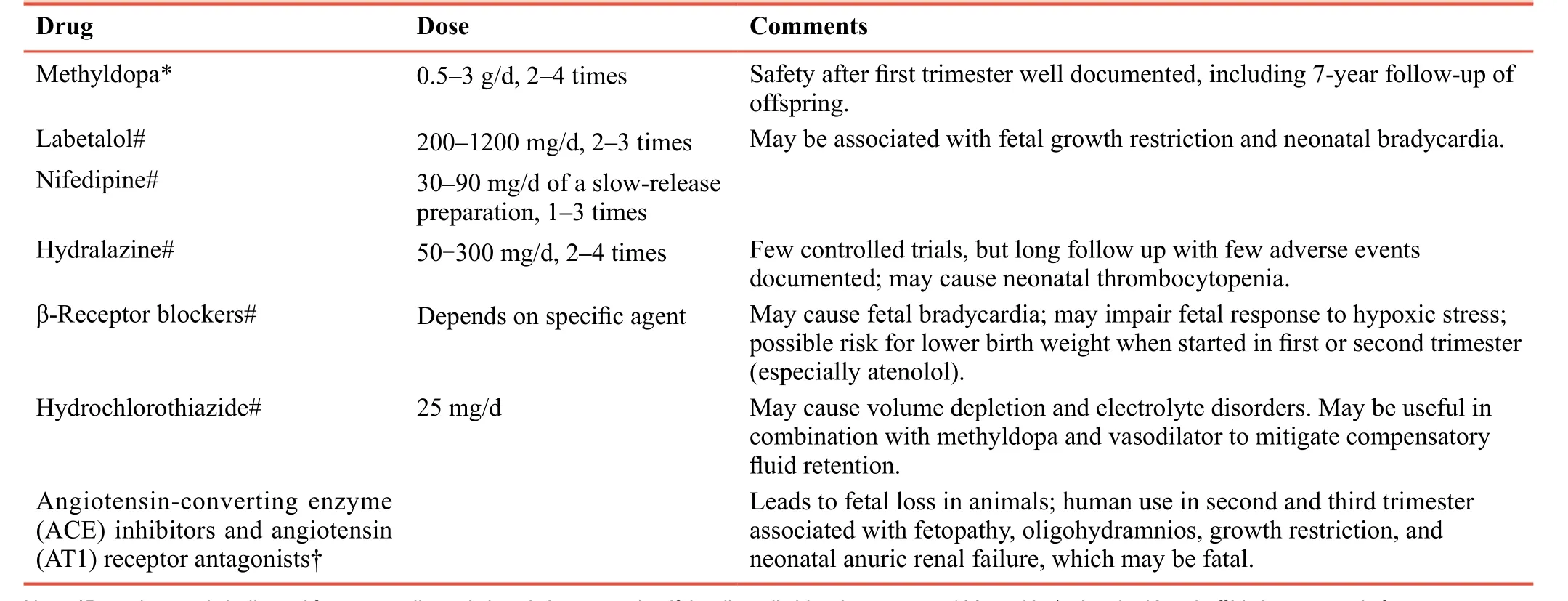
Table 1: Oral medication and doses used in pregnancy-induced hypertension
DAMAGE CAUSED BY PIH
PIH is a pregnancy complication that threatens maternal and fetal health. The risk of restriction of fetal growth and placental abruption is increased greatly in PIH patients.14In the mother,the risk of brain edema, acute heart failure, stroke, and acute renal failure is also increased due to the pathologic changes wrought by PIH. A recent large meta-analysis showed that women with a history of pre-eclampsia had approximately double the risk of ischemic heart disease, stroke and venous thrombo-embolic events 5–15 years after the pregnancy.15Also, the risk of developing hypertension is almost four-fold in women with a history of pre-eclampsia.16
NON-PHARMACOLOGIC MANAGEMENT OF PIH
In women with PIH, a normal diet without salt restriction is advised, particularly close to delivery. Salt restriction may lead to small intravascular volume. Calcium supplementation(≥ 1 g/day) is associated with a significant reduction in preeclampsia risk, particularly for women on low-calcium diets.Fish-oil supplementation and supplementation with vitamins and nutrients have no role in the prevention of hypertensive disorders.17Clinical trials have not shown a beneficial effect of vitamin D supplementation on preeclampsia prevention, but the dose, timing, and duration of supplementation should be investigated in future research.18Aerobic exercise for 30–60 minutes twice a week during pregnancy can reduce PIH risk significantly.19
PHARMACOLOGIC MANAGEMENT OF PIH
Details are shown inTable 1.
BP Control
A major benefit of BP control is to reduce the prevalence of severe hypertension and decrease the risk of maternal and fetal complications.20,21A common consensus among national and international guidelines is to start medication at BP ≥ 160/110 mmHg.21-23Guidelines from the American Heart Association/American Stroke Association suggest considering pharmacologic therapy for BP at 150–159/100–109 mmHg.22However, the European Society of Cardiology recommends treatment of BP ≥ 140/90 mmHg in women with organ damage, symptoms or superimposed PIH on chronic hypertension.11,24In patients with PIH, reducing the risk of maternal organ damage without affecting placental blood flow is important. However, there is no evidence regarding the target for BP control. In 2014, the Japanese Society of Hypertension suggested a target SBP ≤ 160 mmHg, target DBP ≤ 110 mmHg or a 15–20% decrease in the mean BP.25A recent large clinical trial, the Control of Hypertension In Pregnancy Study (CHIPS), demonstrated that women who can maintain BP at 130–140/85 mmHg have fewer episodes of severe hypertension in pregnancy.20Importantly, there were no adverse fetal effects in the lower-BP target group, which challenged a previous concern that lowering BP to “normal”might be associated with reduced fetal growth.26The incidence of preeclampsia is similar in women treated with a standard,less tightly controlled DBP (100 mmHg) or tightly controlled DBP (85 mmHg). The ACOG recommends therapy adjustment to maintain BP at 120–160/80–105 mmHg during pregnancy.10The target range is narrower in Canadian guidelines and is divided further into 130–155/80–105 mmHg for women with chronic hypertension without comorbidities, and < 140/90 mmHg if comorbidities are present.23

Table 2: Drugs for PIH treatment in emergencies
Drugs for PIH treatment
Methyldopa
The α2 adrenergic receptor agonist methyldopa is used widely as a sympatholytic drug. It is first-line treatment for PIH.27,28Serious adverse effects on maternal or fetal conditions have not been reported during 40 years of use. The recommended daily dose of methyldopa is 0.5–3.0 g in 2–4 doses.29Side-effects include sleepiness, dry mouth, general malaise, hemolytic anemia, and hepatopathy.
Hydralazine
Previously, the vasodilator hydralazine was recommended as first-line treatment for severe hypertension in pregnancy.30,31The common side-effects of this drug are headache, nausea,and vomiting. According to a recently reported metaanalysis, hydralazine is less effective than labetalol for PIH in all aspects.32The recommended daily dose is 50–300 mg administered in 3–4 doses. Side-effects include hypotension and neonatal thrombocytopenia.
Calcium-channel blockers
Calcium-channel blockers can be of two subtypes:dihydropyridine (nifedipine) and non-dihydropyridine(verapamil, diltiazem). Nifedipine is considered safe to use in pregnancy.33Calcium-channel blockers other than the longacting nifedipine should be used according to the physician’s evaluation after full explanation of the necessity for treating PIH and obtaining informed consent. Calcium-channel blockers have not been recommended in guidelines because of insufficient supporting evidence. The recommended starting dose for nifedipine is 10-20 mg (p.o., t.d.s.) with a maximum dose of 180 mg per day. The long-acting tablet formulation of nifedipine is usually dosed once daily starting at 30–60 mg and a maximum of 120 mg per day.34Recently, nimodipine(20–60 mg, p.o., b.d. or t.d.s.) and nicardipine (20–40 mg, p.o.,t.d.s.) have been recommended for PIH by guidelines in China.
Beta-blockers (β-blockers)
Labetalol can blockade α1 adrenoreceptors to cause vasodilation. It has a greater β-blocking effect than α-blocking effect (3:1 ratio). Labetalol is first-line treatment for hypertensive disease during pregnancy. It is used in widely Europe, USA and China because it is considered safe.35,36A meta-analysis demonstrated that labetalol has fewer adverse effects on maternal conditions than hydralazine.32Most β-blockers are contraindicated for pregnant women.Therefore, if administration of other β-blockers is necessary,then informed consent must be obtained after explaining the reasons of treatment.
Diuretics
Diuretics can lead to reductions in the pre-eclampsiaassociated volume of: (i) circulating plasma; (ii) placental blood flow. Therefore, diuretics should be avoided in patients with pre-eclampsia. Diuretics can be used if pulmonary edema or heart-failure signs are absent.37For patients with chronic hypertension who take diuretics before pregnancy,the effect of reduction in placental blood flow is not apparent if the drug is continued after pregnancy. A commonly used diuretic is hydrochlorothiazide at a daily dose of 12.5–25 mg.Spironolactone is not recommended because it has been found to have an anti-androgenic effect during fetal development in animal models, though it does not seem to induce adverse outcomes in small cohorts of human participants.38We do not suggest spironolactone use in pregnant women, but it can be used only if a potassium-sparing diuretic is needed.
Alpha-blockers (α-Blockers)
α-Blockers are not contraindicated for pregnant women or those who may be pregnant, but use of α-blockers in this population should be avoided. Only one study has recommended using these drugs in pregnant women with hypertension secondary to pheochromocytoma.39Phentolamine is an α1 and α2 receptor agonist recommended for use in Chinese guidelines in 2015.
Renin aldosterone system (RAS) blockers
There are three types of RAS blockers: angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers and direct inhibitors of renin. These drugs are strictly contraindicated in women who have been pregnant or are planning to become pregnant. Use of any RAS blocker can lead to teratogenicity and oligohydramnios during pregnancy.40,41
EMERGENCY TREATMENT OF PIH
Different countries have slightly different treatment strategies for PIH, but the common agents are labetalol (i.v.), hydralazine(i.v.), and nifedipine (p.o., s.l.).23,42Nitroprusside is used rarely during pregnancy because it can increase the risk of cyanide intoxication in the fetus (Table 2).
CONCLUSION
PIH increases eclampsia risk and threatens maternal and fetal health. In this review, we recommend treatment if BP≥ 150/90 mmHg using labetalol, nifedipine, or methyldopa given as first-line agentsviathe oral route. In the setting of chronic hypertension, one agent should be administered at the highest dose before combination with another agent.Hypertension emergencies due to BP > 160/110 mmHg can result in maternal stroke or eclampsia. If delivery is imminent,parenteral therapy with labetalol (i.v.), hydralazine (i.v.) or nifedipine (p.o.) is indicated.
Author contributions
Writing the manuscript: YL; supervising the study: YZ and YNJ;proofreading: WS. All authors approved the final version of this manuscript for publication.
Conflicts of interest
None declared.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
REFERENCES
1. Lackland DT, Weber MA. Global burden of cardiovascular disease and stroke: hypertension at the core.Can J Cardiol.2015;31:569-571.
2. Hernandorena I, Duron E, Vidal JS, Hanon O. Treatment options and considerations for hypertensive patients to prevent dementia.Expert Opin Pharmacother.2017;18:989-1000.
3. Poulter NR, Prabhakaran D, Caulfield M. Hypertension.Lancet.2015;386:801-812.
4. Carretero OA, Oparil S. Essential hypertension. Part I:definition and etiology.Circulation.2000;101:329-335.
5. Simon AC, Levenson J, Bouthier J, Safar ME, Avolio AP.Evidence of early degenerative changes in large arteries in human essential hypertension.Hypertension. 1985;7:675-680.
6. O'Brien E, Beevers DG, Lip GYH.ABC of Hypertension.London: BMJ Books; 2007.
7. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension,United States, 1987-2004.Am J Hypertens. 2008;21:521-526.
8. Jiang YN, Song W. Blood pressure assessment and drug selection in pregnancy-induced hypertension.Zhongguo Yixue Qianyan Zazhi: Dianzi Ban.2014;6:3-8.
9. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension:diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension.2008;51:1403-1419.
10. American College of Obstetricians and Gynecologists;Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy.Obstet Gynecol.2013;122:1122-1131.
11. European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM), et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology(ESC).Eur Heart J. 2011;32:3147-3197.
12. Committee Opinion No 652: Magnesium sulfate use in obstetrics.Obstet Gynecol.2016;127:e52-53.
13. Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy.Kidney Int.1998;54:2056-2063.
14. Nahar L, Nahar K, Hossain MI, Yasmin H, Annur BM.Placental changes in pregnancy induced hypertension and its impacts on fetal outcome.Mymensingh Med J.2015;24:9-17.
15. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Preeclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis.BMJ.2007;335:974.
16. ONTARGET Investigators, Yusuf S, Teo KK, et al. Telmisartan,ramipril, or both in patients at high risk for vascular events.N Engl J Med.2008;358:1547-1559.
17. Olsen SF, Osterdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil.Eur J Clin Nutr.2007;61:976-985.
18. Purswani JM, Gala P, Dwarkanath P, Larkin HM, Kurpad A,Mehta S. The role of vitamin D in pre-eclampsia: a systematic review.BMC Pregnancy Childbirth.2017;17:231.
19. Magro-Malosso ER, Saccone G, Di Tommaso M, Roman A,Berghella V. Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta-analysis.Acta Obstet Gynecol Scand.2017;96:921-931.
20. Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy.N Engl J Med.2015;372:407-417.
21. Molvi SN, Mir S, Rana VS, Jabeen F, Malik AR. Role of antihypertensive therapy in mild to moderate pregnancyinduced hypertension: a prospective randomized study comparing labetalol with alpha methyldopa.Arch Gynecol Obstet.2012;285:1553-1562.
22. Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association.Stroke.2014;45:1545-1588.
23. Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P;Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary.J Obstet Gynaecol Can.2014;36:416-441.
24. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC).Eur Heart J.2013;34:2159-2219.
25. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension(JSH 2014).Hypertens Res.2014;37:253-390.
26. August P. Lowering diastolic blood pressure in non-proteinuric hypertension in pregnancy is not harmful to the fetus and is associated with reduced frequency of severe maternal hypertension.Evid Based Med.2015;20:141.
27. Plouin PF, Breart G, Maillard F, Papiernik E, Relier JP.Comparison of antihypertensive efficacy and perinatal safety of labetalol and methyldopa in the treatment of hypertension in pregnancy: a randomized controlled trial.Br J Obstet Gynaecol.1988;95:868-876.
28. Redman CW, Beilin LJ, Bonnar J. Treatment of hypertension in pregnancy with methyldopa: blood pressure control and side effects.Br J Obstet Gynaecol. 1977;84:419-426.
29. Anderson GD, Carr DB. Effect of pregnancy on the pharmacokinetics of antihypertensive drugs.Clin Pharmacokinet.2009;48:159-168.
30. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy.Am J Obstet Gynecol.2000;183:S1-S22.
31. Brown MA, Hague WM, Higgins J, et al. The detection,investigation and management of hypertension in pregnancy:executive summary.Aust N Z J Obstet Gynaecol. 2000;40:133-138.
32. Magee LA, Cham C, Waterman EJ, Ohlsson A, von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis.BMJ.2003;327:955-960.
33. Clark SM, Dunn HE, Hankins GD. A review of oral labetalol and nifedipine in mild to moderate hypertension in pregnancy.Semin Perinatol.2015;39:548-555.
34. Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months.BJOG.2000;107:792-794.
35. Pickles CJ, Broughton Pipkin F, Symonds EM. A randomised placebo controlled trial of labetalol in the treatment of mild to moderate pregnancy induced hypertension.Br J Obstet Gynaecol.1992;99:964-968.
36. Pickles CJ, Symonds EM, Broughton Pipkin F. The fetal outcome in a randomized double-blind controlled trial of labetalol versus placebo in pregnancy-induced hypertension.Br J Obstet Gynaecol. 1989;96:38-43.
37. Collins R, Yusuf S, Peto R. Overview of randomised trials of diuretics in pregnancy.Br Med J (Clin Res Ed). 1985;290:17-23.
38. Riester A, Reincke M. Progress in primary aldosteronism:mineralocorticoid receptor antagonists and management of primary aldosteronism in pregnancy.Eur J Endocrinol.2015;172:R23-30.
39. Freier DT, Thompson NW. Pheochromocytoma and pregnancy:the epitome of high risk.Surgery.1993;114:1148-1152.
40. Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors.N Engl J Med.2006;354:2443-2451.
41. Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study.BMJ.2011;343:d5931.
42. Visintin C, Mugglestone MA, Almerie MQ, et al. Management of hypertensive disorders during pregnancy: summary of NICE guidance.BMJ.2010;341:c2207.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- Ranibizumab combined with photodynamic therapy for the treatment of advanced-stage exudative age-related macular degeneration: study protocol for a self-controlled trial and preliminary results
- Ranibizumab versus conbercept for wet age-related macular degeneration: protocol for a prospective cohort study
- Deep brain stimulation for the treatment of moderateto-severe Alzheimer’s disease: study protocol for a prospective self-controlled trial
- Three methods for reducing back pain in older adults with age-related osteoporotic vertebral compression fractures of the thoracolumbar spine: protocol for a non-randomized controlled trial with 2-year follow-up and preliminary results
- Rapamycin-eluting stents for unprotected left main coronary artery stenosis in older adult patients with coronary atherosclerosis: study protocol for a prospective, non-randomized, controlled trial and preliminary results
- Percutaneous transforaminal endoscopic discectomy for treatment of degenerative lumbar disc herniation in older adult patients: study protocol for a randomized controlled trial and preliminary results
