Rapamycin-eluting stents for unprotected left main coronary artery stenosis in older adult patients with coronary atherosclerosis: study protocol for a prospective, non-randomized, controlled trial and preliminary results
2018-07-04ZhiChengFangXiangZhengBoYiLiuJiXianZhaoYingYingLiao
, Zhi-Cheng Fang Xiang Zheng Bo-Yi Liu Ji-Xian Zhao, Ying-Ying Liao
1 Intensive Care Unit, Taihe Hospital (Affiliated Hospital of Hubei University of Medicine), Shiyan, Hubei Province, China
2 Department of Cardiology, Shiyan Renmin Hospital (Affiliated Hospital of Hubei University of Medicine), Shiyan, Hubei Province, China
3 Department of Gastroenterology, Shiyan Renmin Hospital (Affiliated Hospital of Hubei University of Medicine), Shiyan, Hubei Province, China
INTRODUCTION
Background
Coronary atherosclerotic heart disease (CAD) refers to myocardial ischemia and hypoxia caused by atherosclerotic narrowing or obstruction of the lumen.1-4In clinical practice,a lesion with > 50% narrowing of the left coronary artery as shown by angiography is referred to as unprotected left main coronary artery (ULMCA) stenosis. The coronary arteries consist of two main arteries: the left and right coronary arteries.The left coronary artery has two branches, the circumflex artery and the left anterior descending artery, and supplies blood to the left heart ventricle and left atrium. If the left coronary artery is narrowed, then the blood flow of the entire left coronary system will be reduced, leading to serious consequences such as fatal myocardial infarction.5
Left main coronary artery disease is classified into two subtypes: “protected” and “unprotected”. Protected left main coronary artery stenosis means that there is an unobstructed blood vessel bridge or other collateral circulations in the left coronary artery, while cases of ULMCA stenosis do not have a blood vessel bridge or collateral circulation; therefore, patients with ULMCA stenosis are prone to have a greater mortality rate and a high incidence of adverse events.6Furthermore,patients with ULMCA stenosis have a greater mortality rate and a greater incidence of adverse events 1 year after receiving stents than patients with protected left main coronary artery stenosis.7
Coronary artery bypass grafting (CABG) is often considered the gold standard therapy for ULMCA stenosis.8However,one study reported that patients with left main coronary artery lesions treated with CABG had a greater mortality rate than those treated with simple drug therapy.8The treatment of ULMCA stenosis using drug-eluting stents has recently been reported to lead to a relatively low incidence of cardiovascular events.9Thus, drug-eluting stents are a potential surrogate for CABG, and could become the new gold standard treatment for ULMCA stenosis; however, this issue remains controversial.Table 1summarizes the clinical trials published from 2015-2016 that evaluated treatments for ULMCA stenosis.10-12
Features of this study
This prospective, non-randomized, controlled trial will investigate the efficacy and long-term prognosis of rapamycin-
Strengths and limitations
Strength
• Provision of clinical evidence for screening and for the optimal therapeutic strategy for ULMCA stenosis.
Limitation
• Lack of randomized grouping and blinding.eluting stentsversusCABG in the treatment of ULMCA stenosis in older adult patients with degenerative CAD.
Study objective
Our hypothesis is that rapamycin-eluting stents will have superior safety and efficacy compared with CABG in the treatment of ULMCA stenosis in elderly patients with CAD.This study will validate this hypothesis.
METHODS/DESIGN
Study design
A prospective, non-randomized, controlled trial.
Study setting
Taihe Hospital and Shiyan Renmin Hospital, Shiyan, Hubei Province, China.
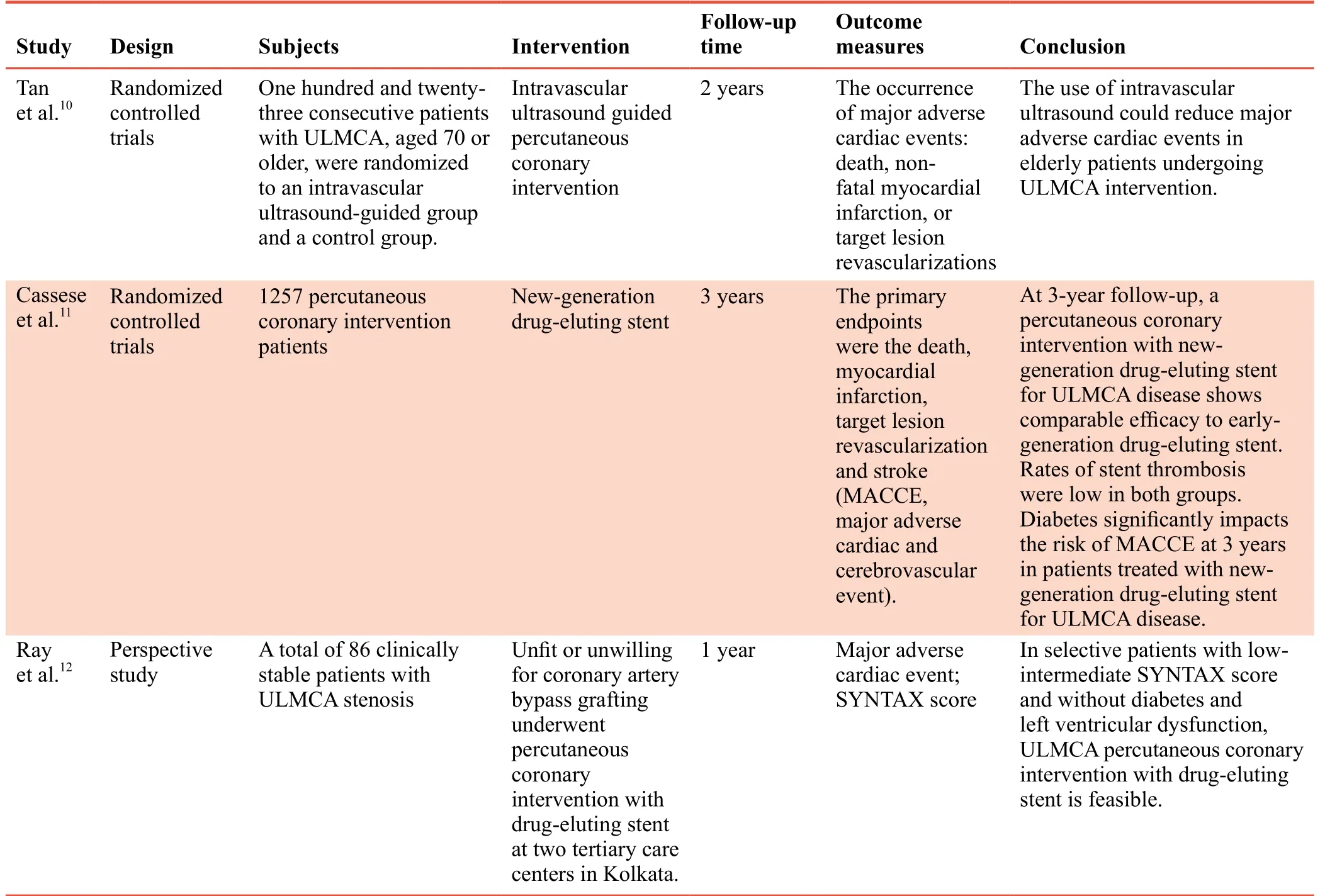
Table 1: Three most representative clinical trials published during 2015–2016 that evaluated treatments for ULMCA stenosis in patients with coronary atherosclerosis
Recruitment
A leaflet containing detailed information about the trial will be distributed to patients being treated in the clinics and wards of Taihe Hospital and Renmin Hospital, China. After being informed of the purpose of the trial and its interventions,patients interested in participation (or their close relatives) will provide written informed consent, and confirmed participants will undergo follow-up examinations (routine blood testing,routine urine testing, electrocardiography, and liver and kidney function testing) free of charge, and will receive compensation for transportation expenses.
Eligibility criteria
The target will be to recruit 224 older adult patients with degenerative CAD from the clinics and wards of the Department of Cardiology at Taihe Hospital and Renmin Hospital, China.
Inclusion criteria
Patients with all of the following criteria will be considered for inclusion:
• Degenerative CAD confirmed by imaging examination.Diagnostic criteria are myocardial damage caused by an imbalance between coronary blood flow and cardiac muscle requirements owing to changes in the coronary circulation;This occurs because of functional and/or organic changes13
• Coronary angiography shows that the lesion in the left main coronary artery is unprotected, and over 50% of the left main coronary artery is necrotic14
• Coronary angiography shows that there are more than two lesions in the main coronary arteries
• Aged 60-75 years
• Provision of written informed consent
Exclusion criteria
Patients with any one of the following conditions will be excluded:
• Left main coronary artery disease caused by factors, not coronary atherosclerosis
• Diseases such as stroke, malignant tumor, and traumatic brain injury that may influence the follow-up results
• Surgical contraindications such as severe diabetes mellitus,liver disease, and abnormal blood coagulation
• Participation in other clinical trials
Assignment and blinding
In accordance with each patient’s treatment choice and indications, the 224 included patients will be assigned to receive either Excel rapamycin-eluting stents (JW Medical Systems, Shandong Province, China) (n= 112) or CABG (n= 112). Randomization will not be used. The assessors who will perform the follow-up examinations will be blinded to the groupings.
Interventions
Stent group
Excel drug-eluting stents are made of 316 L stainless steel,and are biodegradable polymer carriers. Rapamycin-eluting stents have good histocompatibility as coronary artery stents. Rapamycin can inhibit the proliferation of vascular smooth muscle cells, prevent vascular restenosis, prevent the occurrence of advanced within-stent thrombosis, and shorten the treatment time of dual antiplatelet therapy. The degradable polymer carriers can spontaneously degrade to natural substances, and are excreted through normal processes in the forms of carbon dioxide and water.
Either the femoral artery or the radial artery will be selected for puncture in accordance with the severity of the peripheral vascular lesions. After insertion of the guide wire and other devices, the vessels that contain lesions will be pre-expanded with a balloon, and then expanded again with a high-pressure balloon so that the stents can fully adhere to the vascular wall. In addition, the vascular length of the stent coverage will be longer than the length of the lesion site, with 4 mm extra coverage at each end of the lesion site. There will be at least 3 mm overlap at the junction of stents. The accuracy of stent positioning will be confirmed by ultrasound. The patients will be administered 300 mg clopidogrel (Sanofi(Hangzhou) Pharmaceuticals Co. Ltd., China) and aspirin(Bayer Healthcare Co., Ltd., Beijing, China) starting from 3 days postoperatively. Aspirin administration will be maintained throughout the patient’s life, while clopidogrel will be administered for 1 year. Regular postoperative follow-up examinations will be necessary.
Procedure of stent intervention is shown inFigure 1.
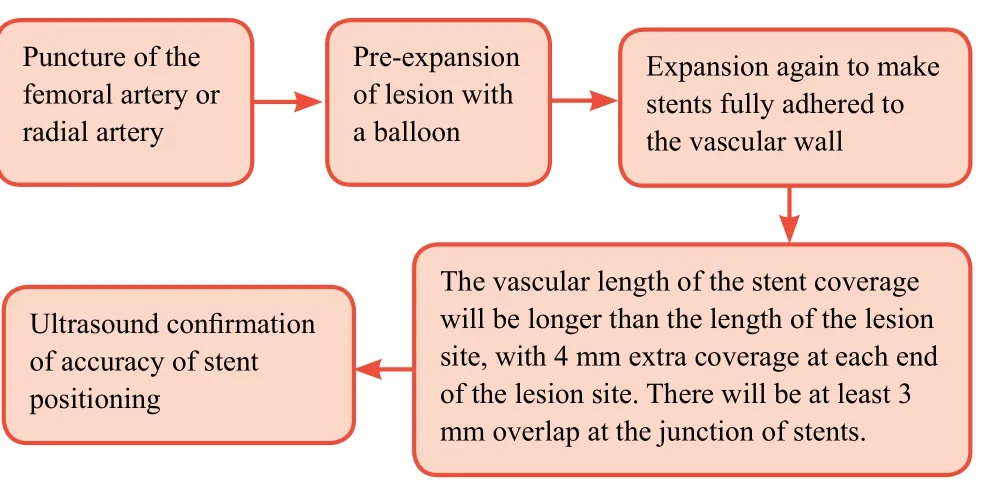
Figure 1: Schematic diagram of the stent implantation.
Coronary artery bypass grafting group
For patients scheduled to undergo CABG, clopidogrel and aspirin will not be administered for 1 week prior to surgery;low molecular weight heparin (4000 U) will be administered subcutaneously once every 12 hours during this week. After induction of general anesthesia, tracheal intubation, and commencement of ventilator-assisted breathing, CABG will be performed without cardiopulmonary bypass. Heparin will be administered intravenously to prevent thrombus formation. The left internal mammary artery will be selected as a bypass blood vessel. The diseased coronary artery will then be searched until the obstructed area or stenotic sites are found. Incisions of ≤ 5 mm long (depending on the internal diameters of the coronary artery and bridge vessel) will be made at the appropriate position in the distal coronary artery.An appropriately-sized cut will be made at the corresponding site of a pre-determined bridge vessel. Then, the bridge vessels and the diseased coronary artery will be anastomosed using 7-0 polypropylene suture. The patients will be instructed totake aspirin for the remainder of their life, and clopidogrel for 2 years. Regular postoperative follow-up examinations will be necessary.

Table 2: Timing of trial outcome measures
Procedure for coronary artery bypass grafting is shown inFigure 2.
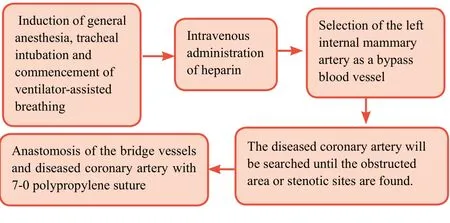
Figure 2: Schematic diagram of coronary artery bypass grafting.
Follow-ups
The included patients will be followed-upviatelephone consultations, clinic visits, and re-admission records at 9, 12,24, and 36 months postoperatively.
Outcome measures
Primary outcome measure
The rate of repeat revascularizations in the target lesion at 36 months postoperatively: in the stent group, revascularization will be performed in the area spanning across all the stents plus 4 mm proximal and distal to the stents. Revascularization will also be conducted in any new lesions in the circumflex branch and anterior descending branch.15In the CABG group, revascularization will be performed in the narrowed and obstructed bridge vessels, and in the circumflex branch and anterior descending branch.16Rate of repeat revascularizations in the target lesion = number of patients receiving repeat revascularization in the target lesion / total number of patients × 100%. Higher values indicate a greater risk of the development of cardiovascular events.
Secondary outcome measures
- The rate of repeat revascularizations in the target lesion at 9, 12, and 24 months postoperatively: the surgical method and evaluation criteria will be the same as that described in the primary outcome measure section above.
- Rate of restenosis: coronary angiography will be performed to check for the presence of in-stent restenosis. In-stent restenosis will be defined as over 50% narrowing of the expanded segment in the stent group, and ≥ 50% restenosis of the bridge vessel in the CABG group. Rate of restenosis of the diseased vessels = number of patients with restenosis of the diseased vessels / total number of patients × 100%.
- Mortality at 9, 12, 24, and 36 months postoperatively:mortality = number of dead patients / total number of patients × 100%.
- Cause of death at 9, 12, 24, and 36 months postoperatively:the causes of death will be recorded, such as myocardial infarction and major adverse cardiac and cerebrovascular events.
- Survival time at 9, 12, 24, and 36 months postoperatively:the survival time of patients in each group will be recorded.
- Angiographic appearance of diseased vessels preoperatively and at 9, 12, 24, and 36 months postoperatively: the main pathological changes of the left main coronary artery, such as stenosis and invasion, will be evaluated using coronary angiography at 9, 12, 24, and 36 months postoperatively.
Safety indicators
- Incidences of major adverse cardiac and cerebrovascular events at 9, 12, 24, and 36 months postoperatively: this will include deaths, non-fatal myocardial infarction, and cerebrovascular accidents caused by various factors. The major adverse cardiac and cerebrovascular event that occurs first will be recorded.
Schedules for primary and secondary outcome measures are shown inTable 2.
Adverse events
The adverse reactions,i.e., major adverse cardiac and cerebrovascular events, occurring during the follow-up period will be recorded. The first onset time, severity, and management of adverse events, such as myocardial infarction,cerebral ischemia, and angina will be recorded and reported to the project manager and the study’s ethics committee within 24 hours.
Trial procedure
224 older adult patients with degenerative coronary atherosclerotic cardiovascular disease presenting with ULMCA stenosis will be recruited in this study from the clinics and wards of department of cardiology of Taihe Hospital and Renmin Hospital, China. Flow chart of trial is shown inFigure 3.

Figure 3: Flow chart of trial.
Sample size
In accordance with clinical experience, we hypothesized that the rate of repeat revascularizations in the target lesion at 36 months post-surgery in the stent and coronary artery bypass grafting groups will be 5% and 20% respectively. Assumingβ= 0.1, power = 90%,α= 0.05 (two-sided), a final sample size of n = 93 per group were calculated using the PASS 11.0 software (PASS, Kaysville, UT, USA). Assuming a participant loss rate of 20%, we will require a sample size ofn= 112 per group. Therefore, a total sample size of 224 will be used in this study.
Statistical analysis
Data description
All data will be statistically processed using the SPSS 20.0 software (IBM, Armonk, NY, USA) following the intentionto-treat principle. Normally distributed measurement data will be expressed as means and standard deviations. Non-normally distributed data will be expressed as lower quartiles (q1),medians, and upper quartiles (q3). Count data and rating data will be expressed as percentages.
Selection of statistical methods
Pearson chi-square test will be used to compare the rate of repeat revascularizations in the target lesion, rate of restenosis of diseased vessel, mortality, and incidence of major adverse cardiac and cerebrovascular events at each time point between stent and coronary artery bypass grafting groups. Two samplet-test (for normally distributed data) and Mann-WhitneyUtest (non-normally distributed data) will be used to compare survival time at different time points between two groups.Kaplan-Meier method will be used for survival analysis and Log-rank test for comparison of survival curve. Multivariate survival analysis will be performed using Cox’s regression model. An inspection level ofα= 0.05 (bilateral) will be considered.
Data sets
The patients included in the final analysis are mainly the population assigned to the per protocol set.
Data collection and management
Data collection
Case report forms will be filled by the investigators accurately,completely, and on time. Written records will be transferred to an electronic format by professional staff using a double data entry strategy.
Data management
Research data will be statistically analyzed and reported by professional statisticians. A final report will be made by principle investigators and the database will be locked. All data relating to this study will be preserved by Taihe Hospital and Shiyan Renmin Hospital, China.
Monitoring
Independent Data Monitoring Committee composition
The role and responsibilities of the Independent Data Monitoring Committee relative to the investigators and ethics committee will be identified. The role and responsibilities of the Independent Data Monitoring Committee will be relative to the project steering committee, epidemiologists, statisticians,clinical trial managers.
Investigator qualification
All surgeons participating in this study have a wealth of experience in similar operations and can skillfully perform surgical procedures.
Auditing
The monitors will report the progress of the trial to the EthicsCommittee every 3 months and update the trial progress in the registration database. The monitors will visit the trial institute regularly or according to the actual situation to carry out clinical quality audit work.

Table 3: Patient baseline information

Table 4: Comparisons of waiting time for surgery, hospitalization time, and rate of complete revascularizations in the target lesion between stent and coronary artery bypass grafting groups

Table 5: Comparisons of follow-up data between stent and coronary artery bypass grafting groups at 6 months postoperatively
Compensation to patients
Patients included in the clinical trial will be followed up closely by professional medical teams without charge. They can be compensated in follow-up-related examination and registration fees.
Ethics and dissemination
This study was approved by Medical Ethics Committee,Taihe Hospital (approval No. TH0058X), and Shiyan Renmin Hospital (approval No. RM011X), China in July 2017. This study protocol will be performed in strict accordance with theDeclaration of Helsinki. The study protocol was devoloped based on the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist (Additional file 1).Written informed consent will be obtained from each patient.When the investigators discover that there are risks beyond expectations in the clinical trials, they will modify the contents of the informed consent together with the sponsors. After the approval of relevant working procedures has been achieved from the ethics committee, the affected patients or their guardians will re-sign the modified informed consent forms.Anonymized trial data will be available at www.figshare.com.
TRIAL STATUS
Preliminary results
Eighty-six older adult patients with degenerative CAD who underwent treatment of ULMCA stenosis between January 2016 and December 2017 were included in a pilot study. Of these 86 patients, 48 received stents and 38 underwent CABG.Baseline information of patients included in the two groups is shown inTable 3.
Preliminary results showed that the stent group had significantly reduced waiting time before surgery,hospitalization time, and rate of complete revascularizations in the target lesion compared with the CABG group (allP< 0.05;Table 4). At 6 months postoperatively, there were no significant differences between the two groups in mortality, incidence of myocardial infarction, rate of repeat revascularizations in the target lesion, and incidence of major adverse cardiac and cerebrovascular events (allP> 0.05;Table 5).
Design of this study has been completed at the time of submission. Patient recruitment will begin in August 2018.
DISCUSSION
Previous contributions and the existing problems
CABG was previously considered the gold standard treatment for left main coronary artery disease. With the emergence of stents, interventional therapy has been shown to exhibit certain therapeutic effects in left main coronary artery disease.Recent studies evaluating the treatment of ULMCA stenosis have shown that drug-eluting stents and CABG result in an equivalent incidence of deaths, recurrent myocardial infarction, and other adverse events.17However, there is controversy regarding the safety and efficacy of drug-eluting stentsversusCABG in the treatment of ULMCA stenosis.8,9
Novelty of this study
This prospective, non-randomized, trial with a 3-year followup duration will investigate the safety, efficacy, and long-term survival rate of rapamycin-eluting stents versus CABG in the treatment of older adult patients with ULMCA stenosis.
Limitations of this study
Because of differences in the patients’ disease conditions and treatment methods, randomization and blinding will not be used; this will influence the study results. Multi-center,prospective, randomized, controlled trials involving a larger sample size will be performed in future to resolve this issue.
Significance of this study
There are controversies regarding the choice of treatment method for ULMCA stenosis, and the clinical efficacy of the available treatment options. The results from this proposed study will evaluate rapamycin-eluting stents and CABG for the treatment of older adult patients with ULMCA stenosis to identify which of these two treatments is safer, exhibits better efficacy, has a lower rate of repeat revascularizations in the target lesion, has a lower incidence of in-stent restenosis, and leads to longer-term survival.
Additional file
Additional file 1: SPIRIT checklist.
Author contributions
Conception and design of this study: ZCF and BYL; manuscript preparation: WC; patient recruitment: XZ; data collection and analysis:XZ and JXZ. All authors approved the final version of this manuscript for publication.
Financial support
None.
Conflicts of interest
There are no conflicts of interest.
Institutional review board statement
This study protocol will be performed in strict accordance with theDeclaration of Helsinki.This study was approved by Medical Ethics Committee, Taihe Hospital (approval No. TH0058X), and Shiyan Renmin Hospital (approval No. RM011X), China in July 2017.Surgeons performing surgery will meet the qualification requirements for coronary artery bypass grafting and stent implantation.
Declaration of patient consent
The authors certify that they will obtain patient consent forms. In the form, patients will give their consent for their images and other clinical information to be reported in the journal. The patients will understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement
This study follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatisticians of Taihe Hospital and Shiyan Renmin Hospital, China.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be available. Study protocol and informed consent will be available without end date. Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal.Anonymized trial data will be available indefinitely at http://www.jsxyfy.com.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
REFERENCES
1. Wang L, Li HC, Zhou J, et al. Correlation between the GP78 gene polymorphism and coronary atherosclerotic heart disease.Hellenic J Cardiol.2017. doi: 10.1016/j.hjc.2017.02.001.
2. Shi JR, Tian CJ, Zeng Q, Guo XJ, Lu J, Gao CR. Expressions of mast cell tryptase and brain natriuretic peptide in myocardium of sudden death due to hypersensitivity and coronary atherosclerotic heart disease.Fa Yi Xue Za Zhi.2016;32:161-164.
3. Yang RH, Liu YF, Wang XJ, Liang JG, Liu JC. Correlation between high density lipoprotein and monocyte subpopulations among stable coronary atherosclerotic heart disease patients.Int J Clin Exp Med.2015;8:16969-16977.
4. Wong MC, Zhang DX, Wang HH. Rapid emergence of atherosclerosis in Asia: a systematic review of coronary atherosclerotic heart disease epidemiology and implications for prevention and control strategies.Curr Opin Lipidol.2015;26:257-269.
5. Song CP, Wang DZ, Hu HY, et al. Carotid plaque characteristics detected with 3.0T high resolution nuclear magnetic resonance imaging in patients with coronary artery disease.Zhuohua Xinxueguanbing Zazhi. 2016;44:38-42.
6. Ellis SG, Tamai H, Nobuyoshi M, et al. Contemporary percutaneous treatment of unprotected left main coronary stenoses: initial results from a multicenter registry analysis 1994-1996.Circulation.1997;96:3867-3872.
7. Kelley MP, Klugherz BD, Hashemi SM, et al. One-year clinical outcomes of protected and unprotected left main coronary artery stenting.Eur Heart J.2003;24:1554-1559.
8. Caracciolo EA, Davis KB, Sopko G, et al. Comparison of surgical and medical group survival in patients with left main equivalent coronary artery disease. Long-term CASS experience.Circulation.1995;91:2335-2344.
9. Park SJ, Kim YH, Lee BK, et al. Sirolimus-eluting stent implantation for unprotected left main coronary artery stenosis:comparison with bare metal stent implantation.J Am Coll Cardiol.2005;45:351-356.
10. Tan Q, Wang Q, Liu D, Zhang S, Zhang Y, Li Y. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly.Saudi Med J.2015;36:549-553.
11. Cassese S, Kufner S, Xhepa E, et al. Three-year efficacy and safety of new- versus early-generation drug-eluting stents for unprotected left main coronary artery disease insights from the ISAR-LEFT MAIN and ISAR-LEFT MAIN 2 trials.Clin Res Cardiol.2016;105:575-584.
12. Ray S, Mazumder A, Kumar S, et al. Angioplasty of unprotected left main coronary stenosis: real world experience of a singleoperator group from eastern India.Indian Heart J.2016;68:28-35.
13. Zhou YL, Jia DA. Review three years, add, delete and delete ten times-the birth of the Diagnostic Standard of Coronary Atherosclerotic Heart Disease.Zhongguo Weisheng Biaozhun Guanli. 2010;1:20-21.
14. Yang FF, Wang Y, Jing J, et al. Angiographic follow-up results after drug-eluting stents intervention for unprotected left main coronary artery disease.Zhonghua Laonian Xinnao Xueguan Bing Zazhi.2010;12:795-798.
15. Gao Hk, Wang Q, Li H. Clinical efficacy and safety of drug coated balloon for treating in-stent restenosis of left anterior descending complex artery lesion in coronary.Lingnan Xinxue Guanbing Zazhi.2017;23:649-652.
16. Cai JF, Wu WC, Sun YJ, et al. Comparison of different methods to revascularize the isolated left anterior descending artery disease.Zhonghua Xiongxin Xueguan Waike Zazhi.2013;29:209-211.
17. Brener SJ, Galla JM, Bryant R 3rd, Sabik JF 3rd, Ellis SG.Comparison of percutaneous versus surgical revascularization of severe unprotected left main coronary stenosis in matched patients.Am J Cardiol.2008;101:169-172.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- Wharton’s jelly derived allogeneic mesenchymal stromal cells for treatment of type 1 diabetes: study protocol for a double-blinded, randomized, parallel,placebo-controlled trial
- Fenestration and debridement combined with percutaneous minimally invasive fibula implantation in the treatment of senile degenerative osteonecrosis of the femoral head: a study protocol for a nonrandomized, controlled, clinical trial
- Percutaneous transforaminal endoscopic discectomy for treatment of degenerative lumbar disc herniation in older adult patients: study protocol for a randomized controlled trial and preliminary results
- Three methods for reducing back pain in older adults with age-related osteoporotic vertebral compression fractures of the thoracolumbar spine: protocol for a non-randomized controlled trial with 2-year follow-up and preliminary results
- Deep brain stimulation for the treatment of moderateto-severe Alzheimer’s disease: study protocol for a prospective self-controlled trial
- Ranibizumab versus conbercept for wet age-related macular degeneration: protocol for a prospective cohort study
