Ranibizumab combined with photodynamic therapy for the treatment of advanced-stage exudative age-related macular degeneration: study protocol for a self-controlled trial and preliminary results
2018-07-04
Department of Ophthalmology, Affiliated Hospital of Qinghai University, Xining, Qinghai Province, China
INTRODUCTION
Research background
Age-related macular degeneration (AMD) is a disease that causes vision loss in the center of the visual field, and approximately 6.3 million people globally suffer from this disease.1Patients with AMD usually have no obvious symptoms at the early stage of the disease; however, as the disease progresses,some patients experience a gradual loss of vision that may affect one or both eyes. Although complete blindness does not usually occur, the loss of central vision has a serious impact on daily activities.2
There are two types of AMD, atrophic and exudative, based on different pathologies. Atrophic AMD causes a slow decline in visual acuity, visual deformation, atrophy of the retinal pigment epithelium in the macular area, disappearance of the central concave reflex, vitreous hernia, and fluorescent or weak light in the macular area with no fluorescent leakage,as assessed by fluorescence angiography. Exudative AMD is characterized by sudden acuity loss, shallow subretinal neovascularization with dark red hemorrhage involving the posterior pole, or even with massive vitreous hemorrhage in severe cases, and fluorescence leakage or shielding shown by fluorescence angiography.3In the advanced stage of exudative AMD, degeneration of the macular area is detected. Because of the decreased phagocytic capacity of the retinal pigment epithelium, the non-digested rod outer segment of the visual cell becomes a residual body that resides in the cytoplasm of the basal cell, is excreted outside the cell and eventually deposited on the Bruch membrane, to form a drusen.3,4Advanced exudative AMD occurs predominantly in subjects over 45 years of age and the prevalence rate increases with age. It is one of the most important causes of blindness in the elderly worldwide.Anti-vascular endothelial growth factor (VEGF) monoclonal antibodies, bevacizumab and ranibizumab, can be used to treat exudative AMD.5,6Laser coagulation therapy can remove drusen, but does not damage choroidal neovascularization.7,8Practice guidelines by the American Academy of Ophthalmology, however, do not recommend this method for macular degeneration. In photodynamic therapy, specific photosensitizers are circulated into the retina and excited by 689 nm laser irradiation, thereby destroying abnormal neovascularization.9Surgical methods, such as resection of subretinal neovascular membranes, macular transposition, and retinal transplantation,are also used for the treatment of exudative AMD. In recent years, the combined use of ranibizumab and photodynamic therapy (PDT) has become the main method for the treatment of exudative AMD (Table 1).
Features of the study
Previous studies have mostly concerned the combined use of ranibizumab and PDT in patients with early stage of exudative AMD,10-12but not enough emphasis has been placed on the advanced stage of exudative AMD, which can cause severe visual impairment in the elderly.
Main objective
The authors intend to investigate changes in vision recovery,retinal thickness, and leakage of choroidal neovascular lesions before and after treatment with ranibizumab plus PDT
Strengths and limitations
• The study will focus on the advanced stage of exudative AMD, which has received limited attention in the past. Visual improvement will be the main outcome measure.
• There is no randomized assignment in this trial.in patients with advanced exudative AMD, and to explore the therapeutic efficacy of the combined therapy.
METHODS/DESIGN
Study design
This prospective self-controlled clinical trial will be completed at the Department of Ophthalmology, Affiliated Hospital of Qinghai University, China. The study population will comprise 113 patients with advanced exudative AMD who meet the inclusion and exclusion criteria and will be subjected to PDT combined with intravitreal injection of ranibizumab. The primary outcome measure is the improvement rate of visual acuity at 12 months after treatment. Secondary outcome measures include monthly logarithmic visual acuity chart scores,retinal thickness, leakage of choroidal neovascular lesions, and adverse events at 1-12 months after treatment.
Recruitment
Recruitment will be performed by advertising for patients using posters displayed at the Department of Ophthalmology and at the Admissions office of Affiliated Hospital of QinghaiUniversity, China. Interested patients or their relatives can contact the principal investigator by telephone, email, or WeChat.All subjects will receive the specifications of the clinical trial and will provide written informed consent to participate in the study. Each subject will be explicitly informed of the detailed information regarding the clinical trial, and can withdraw from the study at any time. Withdrawal from the trial will not influence a patient’s follow-up treatment.
Table 1: Combined use of ranibizumab and photodynamic therapy for exudative age-related macular degeneration in clinical practice
Study participants
All subjects will be recruited from the Department of Ophthalmology, Affiliated Hospital of Qinghai University, China.
Inclusion criteria
Patients will be required to meet all of the following conditions to be included in the trial:
• Presence of choroidal neovascular lesion leakage shown by both fluorescein fundus angiography (FFA) and indocyanine green angiography4
• Presence of macular retinal edema in both eyes shown by optical coherence tomography12
• Visual acuityof 0.1-0.3
• Retinal thickness of 216.8-496.2 μm
• Aged 55-80 years, both sexes
• Provision of written informed consent by participants or their legal guardians
Exclusion criteria
Patients presenting with any of the following conditions will be excluded from the trial:
• With polypoid choroidal vasculopathy
• With systemic or local surgical contraindications
• Having other eye diseases that involve or may involve vision, such as glaucoma, amblyopia, and diabetic retinopathy
• Receiving intraocular surgery on the affected eye within 2 months
• Pregnant or lactating women
Withdrawal criteria
Patients will be withdrawn from the study if they fulfill any of the following criteria:
• During the trial, subject has comorbidities or complications affecting efficacy assessment or onset of diseases affecting the outcome
• Inability to complete the follow-up
Trial progress
The design of the trial was completed in December 2017, and the study protocol was approved by the Ethics Committee of the Affiliated Hospital of Qinghai University (approval No.QHY001Y) in January 2018. The trial was registered with the Chinese Clinical Trial Registry in March 2018. Participant recruitment was started in March 2018, and data analysis will be completed by December 2020.
Interventions
• PDT apparatus was purchased from Zeiss, Germany, and the PDT photosensitizer, benzoporphyrin derivative monoacid,was purchased from Novartis (Northwest Branch), China.PDT is characterized by the activation of photosensitizers under light irradiation to produce oxygen free radicals and singlet oxygen, triggering choroidal neovascular vasoconstriction, thrombosis, atrophy and necrosis. The PTD parameters will be: light energy 50 J/cm2, illumination time 83 seconds,13and light emissivity 600 mW/cm2. The PDT treatment will be performed twice with an interval of 1 week.
• At 48-72 hours after PDT, surgery will be conducted under topical anesthesia by injection of lidocaine hydrochloride(Beijing Zizhu Pharmaceutical Co., Beijing, China). After rinsing the conjunctival sac, 0.5 mg of ranibizumab in a 0.05 mL volume (Novartis AG, Swiss; Import Drug License No. JS20100025; specification 10 mg/mL, 0.2 mL per bottle) will be injected using a No. 30 needle piercing the pars plana into the vitreous cavity of the affected eye. This injection will be performed once a month. Disinfection will be achieved through the use of antibiotic ointment followed by gauze bandaging. Three days before and 3 days after injection of ranibizumab, 0.5% levofloxacin ophthalmic solution (Noden Plant, Santen Pharmaceutical Co., Ltd.,Japan; Chinese Drug Approval No. J20100046) will be dropped into the eye, six times per day.
• If signs of retinal edema are found in the affected eye after the first combination therapy, intravitreal injection of ranibizumab will be performed again (Figure 1).

Figure 1: Combined use of ranibizumab and photodynamic therapy for the treatment of advanced-stage exudative age-related macular degeneration.
Outcome measures
Primary outcome measure
The primary outcome measure is the improvement rate of visual acuity at 12 months after treatment. Improvement of visual acuity will be defined as an increase of ≥ 2 readable lines in the logarithmic visual acuity chart at 12 months after treatment. The improvement rate of visual acuity is expressed as the percentage of improved patients relative to the total number of patients. The logarithmic visual acuity chart, also known as the 5-point logarithmic vision chart. The chart comprises five grades with 14 readable lines.14
Secondary outcome measures
• Logarithmic visual acuity scales will be assessed monthly at 1-12 months after treatment.
• Retinal thickness will be assessed monthly at 1-12 months after treatment by optical coherence tomography. Ranibizumab is mainly used to improve macular edema in AMD patients.5,6Therefore, it is necessary to use optical coherence tomography to compare macular morphological changes before and after treatment. It is very important to evaluate the efficacy of ranibizumab as well as to guide the administration frequency and dosage of the drug. Optical coherence tomography equipment was purchased from Beijing Topcon Medical Devices Co., China.
• Leakage of choroidal neovascular lesions will be assessed monthly at 1-12 months after treatment. This assessment will be done by fluorescein angiography and indocyanine green angiography.
• During the trial, all adverse events, including ocular and systemic events, will be recorded. An adverse event refers to any undesired post-treatment sign, symptom, or temporary illness associated with the medications and devices used,regardless of whether there is a causal relationship with the treatment. The following events will be regarded as serious adverse events: (1) within 7 days after treatment,a decrease of ≥ 4 readable lines of the visual acuity chart.For these patients, color fundus photographs and fluorescein fundus angiography examinations will be performed within 7 days and 12 weeks after visual loss. Moreover, these patients cannot continue to participate in the trial unless their vision returns to pre-treatment or better levels. (2)Any other ocular adverse events, including non-perfusion of retinal arterioles or venules, or extensive retinal capillary insufficiency and vitreous hemorrhage, will be considered serious adverse events.
Flow chart and outcome assessment of the trial are shown inFigure 2andTable 2, respectively.
Sample size
Based on our preliminary results, it is hypothesized that the improvement rate of visual acuity at 12 months post-treatment is 90%. Takingβ= 0.2, power = 80%, andα= 0.05 (two-sided),a final effective sample size ofn= 94 was calculated using PASS 11.0 software (PASS, Kaysville, UT, USA). Assuming a dropout rate of 20%, a minimum of 113 subjects per group is required.
Statistical analysis
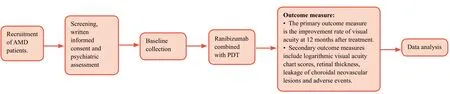
Figure 2: Flow chart of the trial.

Table 2: Timing of outcome measurement
SPSS 14.0 statistical software (SPSS, Chicago, IL, USA) will be used to analyze the retinal thickness, logarithmic visual acuity, and choroidal neovascular lesion leakage in AMD patients before and after treatment. Measurement data will be described as mean values, and numerical data as a percentage. A pairedt-test will be used to compare retinal thickness, logarithmic visual acuity, and choroidal neovascular lesion leakage, and a value ofP< 0.05 will be considered significant.
Data collection and management
Prospective data will be collected for all enrolled patients,including physical examinations, laboratory data, perioperative clinical data, and complications, but personal information, such as names, will not be collected. During data collection and management, patients are only coded to protect their privacy.
All collected data will be recorded in case report form,and entered into an electronic data capture system (Beijing Microsignalstar Technology Development Co., Ltd., Beijing,China). All data collected before data analysis will be processed confidentially, and the final data set will be managed by the Department of Ophthalmology of the Affiliated Hospital of Qinghai University in China. Collected data will be locked and saved at least 5 years after the trial is completed.
Audits
All researchers involved in clinical practice will have abundant clinical experience for ophthalmology treatments, all the evaluators will be professionally trained and have extensive clinical evaluation experience. The researchers must receive professional training and follow uniform data recording methods and judgment standards. Data analysis will be completed by statisticians.
During the clinical trial, sponsor inspectors will conduct regular periodic visits to the research center to ensure that the trial is performed in strict adherence to all aspects of the research program. In addition, the original data will be checked to ensure that the contents of the case report forms are correct and complete.
Compensation
Patients included in this trial will receive a transportation allowance and have their follow-up examination fees waived.The researchers will provide insurance coverage for all subjects, cover the costs of treatment and financial compensation for subjects suffering damage or death associated with the trial.
Ethics and dissemination
This study was approved by the Ethics Committee of the Affiliated Hospital of Qinghai University (approval No.QHY001Y), and this trial will follow the relevant laws and regulations of theDeclaration of Helsinki. All the patients and their families will voluntarily participate in the trial, and will be fully informed of the study protocol and experimental process.Written informed consent will be provided with the premise of fully understanding the treatment plan. This manuscript was prepared and modified according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)guidelines (Additional file 1). The results of this trial will be disseminated through presentations at scientific meetings or by publications in peer-reviewed journals. Anonymized trial data will be published at www.figshare.com.
PRELIMINARY RESULTS
At 12 months after treatment, the visual acuity of 43 affected eyes was improved by ≥ 2 readable lines as compared with the baseline. Optical coherence tomography showed that the mean retinal thickness decreased by 111.21 μm after treatment (P<0.05,vs.before treatment). Fundus fluorescein angiography and indocyanine green angiography showed leakage of choroidal neovascular lesions after treatment. Leakage was completely stopped in 10 eyes (24%), reduced in 26 eyes(60%), and did not change or was increased in four eyes (9%).New lesions occurred in three eyes (7%).
DISCUSSION
Past contributionsand existing problems in the field
Laser photocoagulation and infrared laser transpupillary thermotherapy are also main treatments for AMD, and they are both directed against choroidal neovascularization.7,8,15There have also been clinical trials for AMD involving stem cell therapy and gene-targeted therapy, but the results are not conclusive.16Both ranibizumab and photodynamic therapy have a very good therapeutic effect on AMD. Ranibizumab is mainly responsible for improvement of macular edema in AMD,5,6while PDT is effective at destroying choroidal neovascularization.9 Improved treatment of advanced-stage exudative AMD can be achieved using a combination of ranibizumab and PDT.
Novelty of this study
The trial will focus on advanced exudative AMD, and the primary outcome measure is to observe the improvement in vision, which is the main concern of patients. Moreover, the study will recruit at least 113 patients who meet the inclusion criteria.
Limitation of this study
This study is a self-controlled trial without randomization,which will affect the accuracy of the results. Further investigation with improved experimental design will be required.
Significance of this study
Findings from this trial will provide experimental evidence for the combined use of ranibizumab and PDT in the treatment of advanced-stage exudative AMD.
Additional file
Additional file 1: SPIRIT checklist.
Author contributions
Design of the trial: PY. Patient recruitment, screening and data collection: QW. Trial implementation and follow-up: LLL, PY. Drafting the manuscript: PY. All authors approved the final version of this manuscript for publication.
Conflicts of interest
Although ranibizumab and photodynamic therapy device will be used in the study, the authors declare that the trial will be conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support
None.
Institutional review board statement
This study was approved by the Ethics Committee of the Affiliated Hospital of Qinghai University (approval No. QHY001Y). The study will be performed in accordance with the relevant laws and regulations of theDeclaration of Helsinki, and the hospital’s relevant ethical principles.
Declaration of patient consent
The authors certify that they will obtain patient consent forms. In the form, patients will give their consent for their images and other clinical information to be reported in the journal. The patients will understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement
This study follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of the Affiliated Hospital of Qinghai University, China.
Copyright transfer agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices)will be in particular shared. Study protocol and informed consent form will be promulgated within 6 months after the completion of the trial. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
REFERENCES
1. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015.Lancet. 2016;388:1545-1602.
2. Singh N, Srinivasan S, Muralidharan V, Roy R, V J, Raman R. Prevention of age-related macular degeneration.Asia Pac J Ophthalmol (Phila). 2017;6:520-526.
3. Liu JJ, Liu ZY, Peng Q. Recent advances in anti-vascular endothelial growth factor for exudative age-related macular degeneration.Yanke Xinjinzhan. 2015;35:84-88.
4. Clinical Guidelines and Clinical Pathway Development Committee for Chinese Aged-Related Macular Degeneration,Fundus Disease Group of Ophthalmology Branch of Chinese Medical Association. Clinical pathway of age-related macular degeneration in China.Zhonghua Yandibing Zazhi.2013;29:343-355.
5. Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial.Lancet. 2013;382:1258-1267.
6. Moja L, Lucenteforte E, Kwag KH, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular agerelated macular degeneration.Cochrane Database Syst Rev.2014:Cd011230.
7. Virgili G, Michelessi M, Parodi MB, Bacherini D, Evans JR.Laser treatment of drusen to prevent progression to advanced age-related macular degeneration.Cochrane Database Syst Rev.2015:CD006537.
8. Virgili G, Bini A. Laser photocoagulation for neovascular agerelated macular degeneration.Cochrane Database Syst Rev.2007:CD004763.
9. Nakai S, Honda S, Matsumiya W, Miki A, Nakamura M.ARMS2 variants may predict the 3-year outcome of photodynamic therapy for wet age-related macular degeneration.Mol Vis. 2017;23:514-519.
10. Hatz K, Schneider U, Henrich PB, Braun B, Sacu S, Prunte C.Ranibizumab plus verteporfin photodynamic therapy in neovascular age-related macular degeneration: 12 months of retreatment and vision outcomes from a randomized study.Ophthalmologica. 2015;233:66-73.
11. Krebs I, Vecsei Marlovits V, Bodenstorfer J, et al. Comparison of Ranibizumab monotherapy versus combination of Ranibizumab with photodynamic therapy with neovascular age-related macular degeneration.Acta Ophthalmol. 2013;91:e178-183.
12. Antoszyk AN, Tuomi L, Chung CY, Singh A. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results.Am J Ophthalmol. 2008;145:862-874.
13. Barbazetto I, Burdan A, Bressler NM, et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment--TAP and VIP report No. 2.Arch Ophthalmol.2003;121:1253-1268.
14. Niu TR. The 5-grade notation of the standard logarithmic visual acuity chart.Yan Shiguang Xue Zazhi. 2005;7:217-219.
15. Wu MX, Zheng Z, Zhou XY. New research progress on the epidemiology of age-related macular degeneration.Guoji Yanke Zazhi. 2015;15:223-227.
16. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies.Lancet.2015;385:509-516.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- Wharton’s jelly derived allogeneic mesenchymal stromal cells for treatment of type 1 diabetes: study protocol for a double-blinded, randomized, parallel,placebo-controlled trial
- Fenestration and debridement combined with percutaneous minimally invasive fibula implantation in the treatment of senile degenerative osteonecrosis of the femoral head: a study protocol for a nonrandomized, controlled, clinical trial
- Percutaneous transforaminal endoscopic discectomy for treatment of degenerative lumbar disc herniation in older adult patients: study protocol for a randomized controlled trial and preliminary results
- Rapamycin-eluting stents for unprotected left main coronary artery stenosis in older adult patients with coronary atherosclerosis: study protocol for a prospective, non-randomized, controlled trial and preliminary results
- Three methods for reducing back pain in older adults with age-related osteoporotic vertebral compression fractures of the thoracolumbar spine: protocol for a non-randomized controlled trial with 2-year follow-up and preliminary results
- Deep brain stimulation for the treatment of moderateto-severe Alzheimer’s disease: study protocol for a prospective self-controlled trial
