A newfixation and reconstruction method versus arthroscopic reconstruction for treating avulsion fracture at the tibial insertion of the knee posterior cruciate ligament: study protocol for a nonrandomized controlled trial and preliminary results
2018-06-20GuangdongChenYangZhangYongjianNiHongmeiDuTongjunCaoZhonglinShan
Guang-dong Chen , Yang Zhang, Yong-jian Ni Hong-mei Du Tong-jun Cao Zhong-lin Shan
1 Third Department of Orthopedics, Cangzhou Central Hospital, Cangzhou, Hebei Province, China
2 Department of Joint Surgery, Xinjiang Military General Hospital, Urumqi, Xinjiang Uygur Autonomous Region, China
INTRODUCTION
Research background
Avulsion fractures at the tibial insertion of the knee posterior cruciate ligament (PCL) can lead to backward movement and instability of the knee joint.1-5Surgical treatments include open reduction and arthroscopic reduction followed by internalfixation. Arthroscopic surgery can minimize surgical trauma to the largest degree, but it provides relatively weakfixation,and patients cannot perform functional exercise during early stages.6,7However, early functional exercise can promote rapid recovery of the knee function. Therefore, open reduction is strongly recommended. In previous reports, steel wire, highstrength sutures, hollow screws, absorbable screws, and rivets were used tofix avulsion fractures at the tibial insertion of the PCL.8-11Comminuted bone blocks are often notfixedfirmly using the above-mentioned sutures or screws. In addition,long-term externalfixation is required and the time required for knee function recovery is prolonged. We reviewed three clinical trials on the repair of knee PCL injury and their relevant contents are shown in Table 1.12-14

Strengths and limitations
Novelty of this study
We designed a new inverted “L”-shaped incision in the popliteal fossa through which bone plates are inserted tofix the crushed bones and reconstruct PCL tension, facilitating knee function recovery.
Main objective
In this study, we will compare the therapeutic effects of thisnewfixation and reconstruction method, and arthroscopic reconstruction for avulsion fractures at the tibial insertion of the knee PCL.
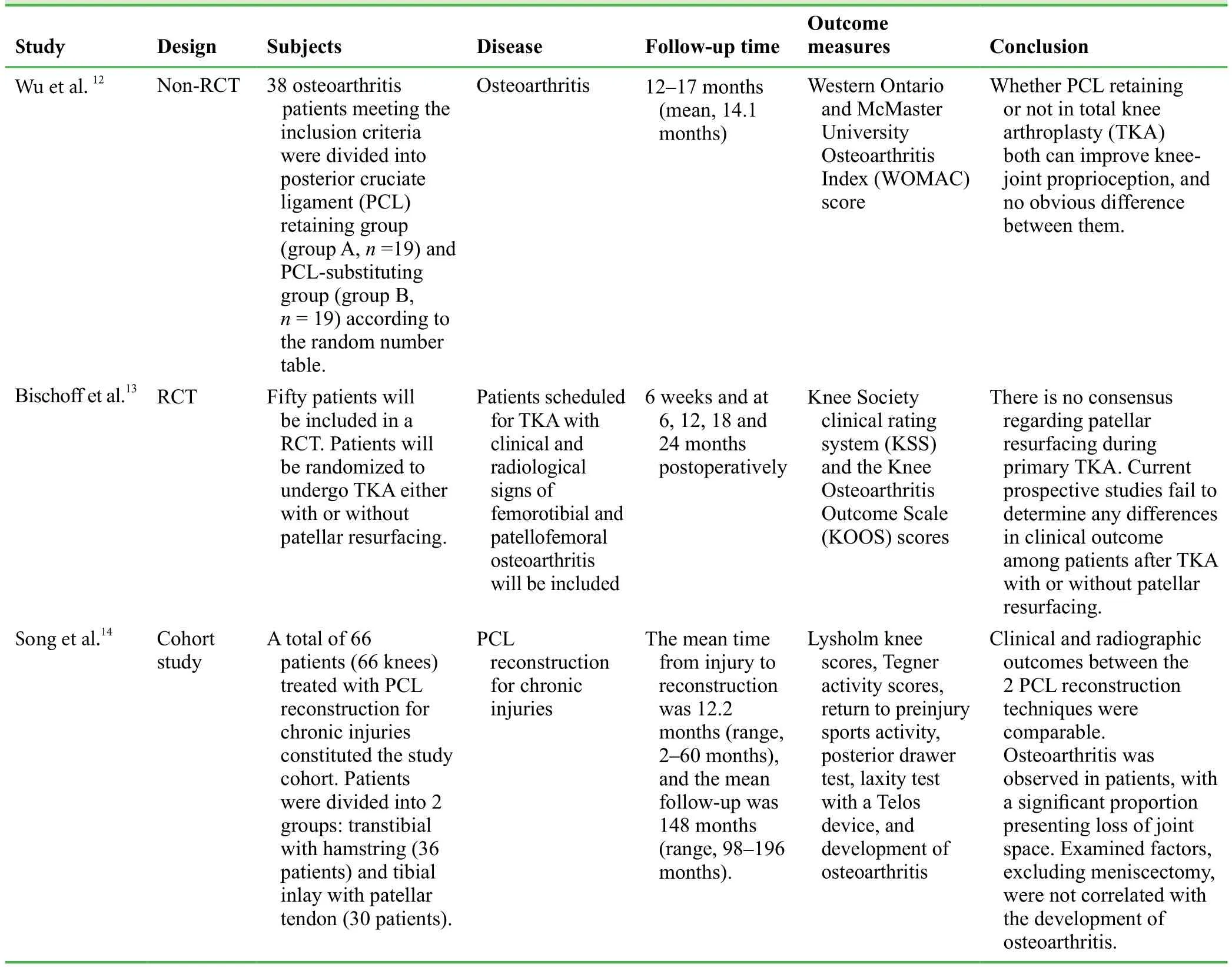
Table 1: Three clinical trials regarding repair of knee posterior cruciate ligament reported during 2013–2014
METHODS/DESIGN
Study design
A prospective, single-center, non-controlled, interventional trial.
Study setting
Department of Orthopedics, Cangzhou Central Hospital, Hebei
Province, China.
Recruitment
Recruitment will be performed using a leaflet to advertise for patients at the Cangzhou Central Hospital of China. Leaflets would provide detailed information regarding the clinical trial.After being informed of the trial and its related interventions,patients interested in participation or their close relatives willfill-in the necessary paperwork, and confirmed participants will be grouped according to the eligibility criteria.
Patient selection
Inclusion criteria
Patients meeting all of the following conditions will be considered for inclusion:
• Patients with avulsion fracture at the tibial insertion of the knee PCL
• Limited kneeflexion and extension abilities, posterior drawer test (+)
• Presence of joint trauma
• Age 35–58 years
• Provision of written informed consent
Exclusion criteria
Participants with one or more of the following conditions will be excluded from this study:
• Patients with old fracture(s)
• Patients with fracture complicated by 2nddegree or mores vere medial collateral ligament injury
• Patients with anterior cruciate ligament injury
• Patients with injury to the posterolateral corner of the knee
Grouping and blinding
Prior to surgery, the two therapeutic regimens will be explained in detail to patients. Surgical methods will be chosen by patients and their family members, and patients will be grouped in the arthroscopic reconstruction or the new method groups accordingly. Randomization and blinded grouping will not be used.
Interventions
In both groups, patients will undergo surgery under continuous epidural anesthesia and assistance by a tourniquet.
(1) Pre-operative preparations: All included patients will undergo routine X-ray, CT, and MRI examinations. Substantial ligament injury and the accompanying meniscal injury will be screened according to MRIfindings.
(2) Intervention in the arthroscopic reconstruction group:Anteromedial and anterolateral space of the knee joint will be examined through the anteromedial and anterolateral approaches. Posteromedial and posterolateral approaches will be established to be used as observation and working channels. Arthroscopic examination of the fracture will be performed. After removal of fracture stumps, a probe will be inserted through the established posterolateral approach of the knee joint to reset bone fragments. Guide pins will be introduced via the working channel andfixed. PCL will be strengthened under the assistance of a PCL femoral sight located at fracture stumps. Two Kirschner wires will be drilled via the fracture stumps. No. 5 ETHIBOND EXCEL polyester sutures will be introduced into the knee joint through the onside channel, passed through the base of the tibial insertion of PCL to cross the channel on the other side, andfinally exited out of the knee joint inflexion 90° position. The tibia will be pulled forward. The No. 5 ETHIBOND EXCEL polyester suture will be ligated right before the tibial tubercle.
(3) Intervention in the new method group: After successful anesthesia, the patient will be placed in the prone position.A median inverted “L”-shaped incision will be made in the direction of medial dermatoglyph in the popliteal fossa along the upper border of the gastrocnemius muscle. The target skin will be cut open and the deep fascia will be longitudinally incised. Next, loose tissue between the semitendinosus and medial gastrocnemius will be separated to expose the posterior capsule. In the articular capsule of the knee joint, PCL with avulsion or more severe injury will be reconstructed using tendon sutures. PCL tightening by proper traction can easily reduce bone fragments at the tibial insertion of PCL.The bone plates used for reconstruction will be placed in the median upper border of the posterior tibial plateau andfixed with cancellous bone screws. The woven suture will befixed in the hole of the self-made internalfixation device (a national patent patent number: ZL 2016 2 0025670.3). PCL tension will be examined again and knee stability will be examined in the positions offlexion and extension (Figure 1).
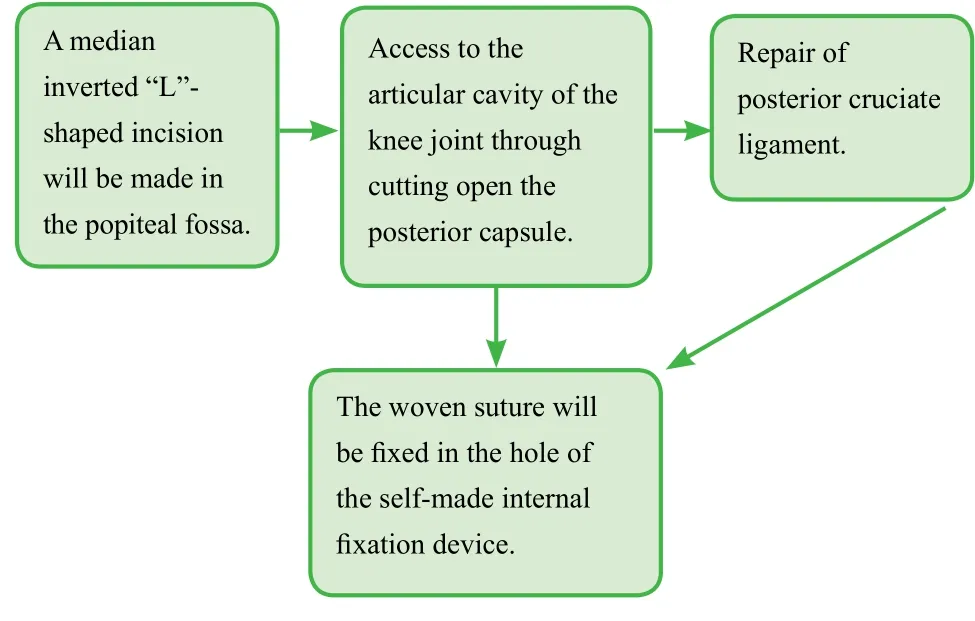
Figure 1: Schematic diagram of newfixation reconstruction surgery.
(4) Postoperative management
Arthroscopic reconstruction group: All patients will undergo early passive knee extension exercise. After surgery,each patient will wear a brace for 6 weeks. The knee in the position of extension will befixed for 2 weeks to perform knee function exercise. Partial body weight bearing exercises will begin 5 weeks after surgery and full body weight bearing exercises will begin at 7 weeks after surgery.
New method group: On the day of surgery, when anesthesia disappears, toe plantarflexion and extension on the affected side will be performed. On day 2 after surgery, subsequent to removal of the wound drainage device, the affected limb will be allowed to perform non-weight bearing walk with the help of walking sticks for quadriceps’ functional exercise.Passive kneeflexion exercises will be performed 1 week later.Active knee exercises under the protection will start at 3 or 4 weeks after surgery. At postoperative 6 weeks, patients will be allowed full weight bearing exercises if X-rayfindings show satisfactory bone fracture healing and good internalfixation position.
Criteria for termination or modification of interventions assigned to subjects
If the investigatorsfind that the risks outweigh the potential benefit, or conclude that the risk is sufficient to undermine the safety and efficacy of the surgery, they will inform the subjects that the clinical trial will be suspended or terminated,and ensure that the subjects receive appropriate treatment and follow-up. In addition, the investigators will inform the sponsor and ethics committee, by providing a detailed written explanation regarding suspension or termination of the clinical trial.
Outcome measures
Primary outcome measure
• Excellent and good rate of knee function recovery at 12 months after surgery, as evaluated by the Lysholm Knee Scoring Scale score (referred to as Lysholm score below).The Lysholm Knee Scoring Scale includes 8 items with a total score of 0–100 points. A score of 95 or higher is considered excellent, 94–85 good, 84–65 fair, and a score of 65 or lower poor. The rate of excellent and good Lysholm scores will be calculated as: number of patients who scored≥ 85 /total number of patients ×100%. A higher score will indicate better knee function recovery.
Secondary outcome measures
• Excellent and good rate of knee function recovery as evaluated by the Lysholm score before surgery, and 6 weeks and 6 months after surgery. The scoring criteria will be the same as above.
• Lysholm scores before surgery, 6 weeks, 6 months and 12 months after surgery. The scoring criteria will be the same as above.
• Hospital for Special Surgery (HSS) knee scores before surgery, 6 weeks, 6 months and 12 months after surgery:This score will be used to summarize symptoms and clinical signs: pain (30 points), function (22 points),muscle strength (10 points),flexion deformity (10 points),instability (10 points), range of motion (18 points). The overall scores will be graded as excellent (≥ 85 points),good (70–84 points), fair (60–69 points) and poor (< 59 points).15Higher scores would indicate better knee function recovery.
• Visual Analogue Scale (VAS) scores before surgery, 6 weeks, 6 months and 12 months after surgery: The VAS score ranges from 0–10 points. Higher scores indicate more severe neck pain in the patient.16
• Posterior drawer test negative rate: The posterior drawer test will be used to examine the PCL. Patients will lie in the supine position with knees bent at 90º, both hands placed behind the knee joint, and thumbs placed on the extensor side. The proximal calf will be repeatedly pushed and pulled. If the tibia moves backwards on the femur, a partially or completely fragmented PCL will be considered.If the tibia does not move on the femur, no fragmentation of PCL will be considered. Posterior drawer test negative rate will be calculated as: number of patients with negative results of posterior drawer test/total number of patients× 100%. Higher values would suggest better recovery of the PCL structure.
• X-ray morphology of the knee before surgery, at 6 weeks,6 months and 12 months after surgery: X-ray morphology of the knee in the anterio-posterior view will be examined,which will be used to determine the extent of repair of theinjured bone and ligament.

Table 2: Timing of primary and secondary outcome measures
• Incidence of adverse events 6 weeks, 6 months and 12 months after surgery: Adverse events include incision pain, infection, knee pain, peripheral nerve injury, and deep venous thrombosis. Incidence of adverse events =number of patients having adverse events/total number of patients ×100%.
Timing of outcome assessment
The scheme of primary and secondary outcome measures is shown in Table 2.
Adverse events
Any medical abnormalities that occur between the period starting from the provision of signed informed consent and recruitment to the end of the observation period, regardless of whether they are directly associated with the surgical treatment or not, will be considered as adverse events. During the study period, adverse events (including name, start and end time, severity, relationship with known events, and treatment measures) will be accurately recorded. Judgment and treatment of severe adverse events: any medical events including death, prolonged hospitalization, re-hospitalization,fetal diseases, permanent defects in body structure or body function, and medical treatment or surgical intervention to avoid permanent defects in body structure or body function,will be considered as severe adverse events. During followup, the start time and type of severe adverse events as well as the treatment measures will be recorded in detail. After occurrence of the adverse event, relevant information will be reported to the principal investigator and the institutional review board within 24 hours.
Trial procedure
One hundred and eighty patients (knees) with avulsion fracture at the tibial insertion of the knee PCL will be assigned to two groups as per the respective treatment method: arthroscopic reconstruction group (n = 90) and new method group (n = 90). The trial procedure is shown in Figure 2.
Sample size
In accordance with previous reports17,18and our experience,we hypothesized that the excellent and good rates of knee function recovery at 12 months after surgery as evaluated by Lysholm score would be 80% and 90%, in the arthroscopic reconstruction and new method groups, respectively. Assuming β = 0.1, power = 90%, and α = 0.05 (two-sided), afinal effective sample size of n = 75 was calculated using the PASS 11.0 software (PASS, Kaysville, UT, USA). Assuming a participant loss rate of 20%, we would require 90 participants per group.
Statistical analysis
Data description

Figure 2: Flow chart of the study.
All data will be statistically processed using the SPSS 19.0 software (IBM, New York, NY, USA), following the intention-to-treat principle. Normally distributed measurement data will be expressed as means and standard deviations.Non-normally distributed data will be expressed as lower quartiles (q1), medians, and upper quartiles (q3). Count data will be expressed as percentages.
Selection of statistical methods
Pearson chi-square test will be used to compare the excellent and good rates of knee function recovery as evaluated by the Lysholm score, Posterior drawer test negative rate, and incidence of adverse events between the arthroscopic reconstruction and new method groups. Kruskal-Wallis H test will be used to compare the above indices between different time points in each group. Two-sample t-test (normally distributed data) or Mann-Whitney U test (non-normally distributed data) will be used to compare the HSS knee scores and VAS scores between the two groups. Repeated measures analysis of variance will be used for comparison of the above-mentioned indices between different time points in the same group. An inspection level of α = 0.05(unilateral) will be considered.
Data sets
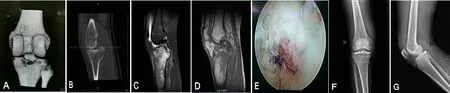
Figure 3: lmages of a 38-year-old male patient with avulsion fracture at the tibial insertion of the left knee posterior cruciate ligament before and after arthroscopic surgical treatment.

Figure 4: lmages of a 46-year-old male patient with avulsion fracture at the tibial insertion of the left knee posterior cruciate ligament complicated by contralateral femoral shaft fracture and multiple limb fractures.
Included subjects will consist of populations assigned to each protocol set. These will be subjects who meet the inclusion and exclusion criteria, complete the study without major protocol deviations, provide effective baseline efficacy and tight compliance, and complete the case report form.
Data collection and management
Data collection
Case report forms will befilled by the investigators accurately,completely, and on time. Written records including demographic information, disease classification, accompanying diseases, and adverse events will be transferred to an electronic format by professional staff using a double data entry strategy.
Data management
Only the project manager will have the right to inquire about the database, which will be locked by the project manager themselves. All research materials related to this trial will be preserved by the Cangzhou Central Hospital, China. Research data will be monitored and managed by an Independent Data Monitoring Committee throughout the clinical research process to ensure scientific integrity and rigor, as well as obtain true and complete data. Two staff members will be responsible for transcribing the required information on results. Data will be recorded and checked by investigators and the database will be locked.
Data monitoring
Independent Data Monitoring Committee composition
The role and responsibilities of the Independent Data Monitoring Committee relative to the investigators and ethics committee will be identified. The role and responsibilities of the Independent Data Monitoring Committee will be relative to the project steering committee, statisticians, data managers,inspectors, and sponsors.
Investigator qualification
All surgeons participating in this study have a wealth of orthopedic surgery and arthroscopy experience. The surgery will be performed by orthopedic chief surgeons and senior associated chief surgeons.
Auditing
All data will be checked for its accuracy and completeness as well as consistency with original records. All corrected or commented mistakes will be signed and dated by the investigators. Each subject’s dose change, treatment change,combined medication, intermittent disease, loss of follow up,and missed examinations will be confirmed and recorded.The dropout and lost to follow up subjects will be recorded in the Case Report Form.
Compensation to study participants
Patients included in this program will receive complimentary study protocol-related laboratory and imaging examinations during the follow-up period, and will also receive a transportation allowance.
Ethics and dissemination
The study design has been approved by the Medical Ethics Committee of Cangzhou Central Hospital of China (approvalNo. 2017-120-01). This study will be performed in strict accordance with the principles outlined in the Declaration of Helsinki formulated by the World Medical Association.Participants will provide signed informed consent prior to participation in the study. If and when any unexpected risk occurs during the clinical trial, informed consent-related content will be modified in accordance with the sponsor.After receiving agreement by the ethics committee, informed consent will be obtained again by the involved subjects or their guardians. Results will be disseminated through presentations at scientific meetings and/or by publication in a peerreviewed journal. Anonymized trial data will be published at www.figshare.com.
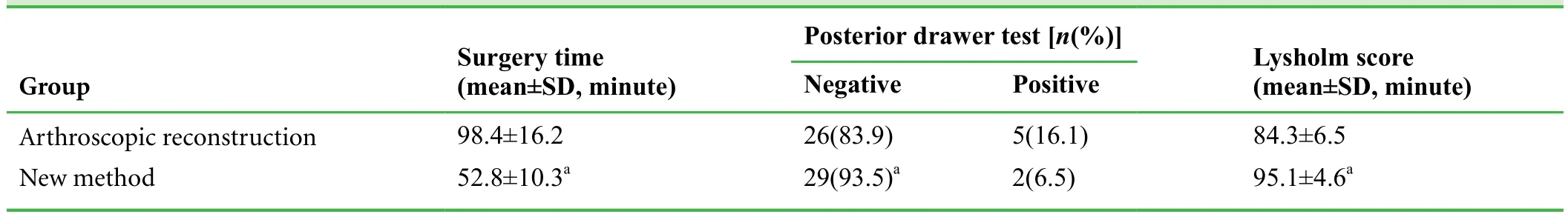
Table 3: Follow-up results of arthroscopic reconstruction and new method groups at 3 months after surgery (n = 31/group)
PRELIMINARY RESULTS
Results of a preliminary study involving 62 patients with avulsion fracture at the tibial insertion of the knee posterior cruciate ligament who underwent the above described treatment protocol revealed that the time for surgery in the new method group was shorter than that in the arthroscopic reconstruction group (P < 0.05; Table 3). The included 62 patients were followed up for an average time of 18.2(range 12–24 months) months. Poor reduction was found in 8 out of 31 patients in the arthroscopic reconstruction group and in 2 out of 31 patients in the new method group.Follow-up results showed that in the new method group,1 patient felt mild soreness in the rear of knee joint duringflexion and extension activities, but this did not affect activities of normal life. Images of a typical case are shown in Figures 3 and 4.
At 3 months after surgery, the posterior drawer test negative rate and Lysholm score in the new method group were significantly higher than those in the arthroscopic reconstruction group (P < 0.05; Table 3).
DISCUSSION
Past contributions and existing problems of others in the field of research
Surgical methods for the treatment of avulsion fracture at the tibial insertion of the knee posterior cruciate ligament mainly include open reduction with internalfixation and arthroscopic reduction. Each method has its advantages and shortcomings.Arthroscopic reduction is relatively minimally invasive. It canfind and treat other articular cavity disorders, but the tibial insertion of the knee posterior cruciate ligament is located in the posterior tibial plateau; therefore, arthroscopic reduction for the treatment of avulsion fracture at the tibial insertion of the knee posterior cruciate ligament is quite challenging.19Arthroscopic surgery has shortcomings including complex operation, longer operative time, and inaccurate fracture reduction.20A few scholars consider that a part of the tibial insertion of the posterior cruciate ligament is located outside the articular capsule. When an avulsion fracture at the tibial insertion of the posterior cruciate ligament occurs, this articular capsule and other soft tissues often cause the fracture reduction to be difficult andfixation to be notfirm, which inturn is unsuitable for performing early functional exercise.21
Features of this study
In this study, we designed and proposed a novel method tofix the avulsion fracture at the tibial insertion of the knee posterior cruciate ligament. Compared to arthroscopic reconstruction, this novel method provides morefirmfixation,reduces operative time, enables reconstruction of knee posterior cruciate ligament, provides better ligament tension, and maintains a more stable knee function, which offers patients with early opportunities of postoperative functional exercise.The anatomical design of thefixation device was approved by the national patent organization for its simple light weight feature and fewer adverse reactions.
Limitations of this study
In this study, random grouping will not be used, and the proposed follow up time is short. These factors will influence the accuracy of experimental results.
Significance of this study
Findings from this study will provide evidence to identify whetherfixation of crushed bones using bone plates inserted through an inverted “L”-shaped incision in the popliteal fossa and reconstruction of ligament tension provides superior repair and safety in the treatment of avulsion fracture at the tibial insertion of the knee posterior cruciate ligament compared to arthroscopic reconstruction.
Additional file
Additionalfile 1: SPIRIT checklist.
Author contributions
This study was designed by GDC. YJN and HMD will be responsible for participant recruitment. YZ will be responsible for data collection and analysis. TJC will assist with surgery. ZLS will be responsible for project coordination. All authors approved thefinal version of this manuscript for publication.
Conflicts of interest
All authors declare that no competing interests exist.
Financial support
None.
Research ethics
This study was approved by Medical Ethics Committee of Cangzhou Central Hospital of China (approval No. 2017-120-01). The study will be performed in accordance with the Declaration of Helsinki.
Declaration of patient consent
The authors certify that they will obtain patient consent forms. In the form, patients will give their consent for their images and other clinical information to be reported in the journal. The patients will understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables,figures, and appendices) will be in particular shared. Study protocol and informed consent form will be available. The data will be available immediately following publication without end date. Results will be disseminated through presentations at scientific meetings and/or by publication in a peerreviewed journal. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License,which allows others to remix, tweak, and build upon the work noncommercially, as long as the author is credited and the new creations are licensed under identical terms.
REFERENCES
1. Ambra LF, Franciozi CE, Werneck LG, et al. Posteromedial vesus direct posterior approach for posterior cruciate ligament reinsertion. Orthopedics. 2016;39:e1024-1027.
2. Zehir S, Elmalı N, Şahin E, et al. Posterior cruciate ligament reconstruction via tibial inlay technique in multiligament knee injuries. Acta Orthop Traumatol Turc. 2015;49:579-585.
3. Chen LB, Wang H, Tie K, et al. Arthroscopicfixation of an avulsion fracture of the tibia involving the posterior cruciate ligament: a modified technique in a series of 22 cases. Bone Joint J. 2015;97-B:1220-1225.
4. Muhm M, Winkler H. The posterocentral approach to the posterior tibial plateau. Oper Orthop Traumatol. 2015;27:80-93.
5. Jia KJ, Guan JJ, Yang CL, et al. Cannulated screwfixation through posteromedial approach screw for the treatment of tibial avulsion fracture of the tibial attachment of the posterior cruciate ligament. Zhongguo Gushang. 2013;26:727-729.
6 Li Q, Song K, Sun Y, Zhang H, Chen D, Jiang Q. Severe cartilage damage from a broken absorbable screw head afterfixation of an avulsion fracture of the tibial attachment of the posterior cruciate ligament: a case report. Medicine (Baltimore). 2016;95:e5180.
7. Mamatkerimulla T, Xu G, Wang X, et al. Clinical observation of one-stage arthroscopic reconstruction and strict immobilization for treatment of knee dislocation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016;30:412-415.
8 Espejo-Baena A, López-Arévalo R, Urbano V, et al. Arthroscopic repair of the posterior cruciate ligament: two techniques. Arthroscopy. 2000;16:656-660.
9. Choi NH, Kim SJ. Arthroscopic reduction andfixation of bony avulsion of the posterior cruciate ligament of the tibia. Arthroscopy. 1997;13:759-762.
10. Buckley SL, Sturm PF, Tosi LL, et al. Ligamentous instability of the knee in children sustaining fractures of the femur: a prospective study with knee examination under anesthesia. J Pediatr Orthop. 1996;16:206-209.
11. Barton TM, Torg JS, Das M. Posterior cruciate ligament insuffi ciency. A review of the literature. Sports Med. 1984;1:419-430.
12. Wu Y, Li Y, Chen B. Effect of posterior cruciate ligament retaining or not on knee-joint proprioception. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013;27:851-854.
13. Bischoff MJ, van Raaij TM, Reininga IH, van Raay JJ. Patellar resurfacing in posterior cruciate ligament retaining total knee arthroplasty (PATRES): design of a randomized controlled clinical trial. BMC Musculoskelet Disord. 2014;15:358.
14. Song EK, Park HW, Ahn YS, Seon JK. Transtibial versus tibial inlay techniques for posterior cruciate ligament reconstruction:long-term follow-up study. Am J Sports Med. 2014;42:2964-2971.
15. Risberg MA, Holm I, Steen H, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7:152-159.
16. Knop C, Oeser M, Bastian L, et al. Development and validation of the Visual Analogue Scale (VAS) Spine Score. Unfallchirurg.2011;104:488-497.
17. Zuo JZ, Shi FD, Liu SJ, et al. Clinical study of arthroscopic anterior cruciate ligament rupture combined with ramp injury.
18. Hu LY, Jia QY, Cao Y, Yu Y, Zheng SQ. Study on treatment of tibial avulsion fractures of posterior cruciateligament combined with anterior cruciate ligament. Chongqing Yixue. 2017;46:2802-2805.
19 Chen SY, Chen CY, chang SS, et al. Arthroscopic suturefixation fou avulsion fractures in the tibialattachment of the posterior cruciate ligament. Arthroscopy. 2012;28:1454-1463.
20. Frosch KH, Balcarek P, Walde T, et al. A new posterolateral approach withoutfibula osteotomy for the treatment of tibial plateau fractures. J Orthop Trauma. 2010;24:515-520.
21. Nicandri GT, Klineberg EO, Wahl CJ, et al. Treatment of posterior cruciate ligament tibial avulsion fractures through a modified open posterior approach: operative technique and 12-to48-month outcomes. J Orthop Trauma. 2008;22:317-324.
杂志排行
Clinical Trials in Orthopedic Disorder的其它文章
- Functional outcomes and health-related quality of life after open repair of rotator cuff tears: a prospective cohort study
- Evaluation of a computer-assisted orthopedic training system for learning knee replacement surgery:a prospective randomized trial
- Effects of balance training prior total knee replacement surgery: study protocol for a randomized controlled trial
- Total hip arthroplasty via the direct anterior approach versus lateral approach: study protocol for a randomized controlled trial
- Information for Authors - Clinical Trials in Orthopedic Disorders (CTOD)
