Total hip arthroplasty via the direct anterior approach versus lateral approach: study protocol for a randomized controlled trial
2018-06-20DanViorelNistorSergiuCaterevNicolaeCiprianBotaDanOsvaldGheorgheLucaciuAdrianTodor
Dan Viorel Nistor, Sergiu Caterev, Nicolae Ciprian Bota, Dan Osvald Gheorghe Lucaciu, Adrian Todor
Department of Orthopaedics, Traumatology and Paediatric Orthopaedics, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca,Romania
INTRODUCTION
Degenerative hip arthritis is affecting an increasing number of the general population aged > 45 years old, with more than 27% of them having radiographic hip osteoarthritis.1As is well known, and countless time mentioned, total hip arthroplasty(THA) is the main procedure to consider when trying to restore patient’s quality of life and functionality, as well as to relieve pain. Thus, THA has gained the attribute of being the most successful procedure in degenerative hip arthritis, with the possibility of restoring full functionality and quality of life,comparable to healthy individuals.1The continuous increase in prevalence as well as the tendency to adopt a fast tracking protocol has developed the need for a minimally invasive surgical procedure to deal with the higher expectations and current economic requirements when performing a THA. One approach that would comply with all of the above is the direct anterior approach (DAA), which claims to be a true muscle sparing technique, providing a faster recovery and better patient satisfaction2when compared to the classic lateral or posterior approaches. Despite all of the possible advantages of the DAA, the procedure is quite demanding, especially in more complex cases,3where surgeons could expect femur fractures, muscle damage (i.e., tensor fascia lata muscle),lateral femoral cutaneous nerve palsy, and other complications.4-6But the possibility of less muscle damage and pain,faster rehabilitation, better patient satisfaction and functional outcome7,8are more than enough to attempt adding the direct anterior approach to ones portfolio. Although the transition to the direct anterior approach can be somewhat frightful, it can be done without jeopardizing patient safety.9Taking into account that the lateral approach is one of the most widely used approaches when performing THA, with only the posterior approach surpassing it,10we considered it to be a valid approach to compare to. More so, both the lateral and the DAA approach can be performed with the patient in the same setup,a supine position, on a standard operating table.
Our hypothesis is that the use of a muscle sparing approach would cause less soft tissue damage that would yield lower levels of markers for muscle damage, better functional outcome, less pain and a faster recovery. Throughout this study we aim to compare the DAA to the lateral approach when performing a cementless hip replacement by means of markers for muscle damage, perioperative data, postoperative pain levels and rescue medication consumption, functional outcome and quality of life.
METHODS/DESIGN
Study design
This single center, randomized controlled clinical trial will be carried out at Orthopedic and Traumatology Department I, the Emergency County Clinical Hospital, affiliated to the“Iuliu Hatieganu” University of Medicine and Pharmacy Cluj-Napoca. Two hundred patients will be assigned randomly to two groups: THA performed via the DAA or the lateral approach. All THAs will be performed by the same surgeon.Patients will be evaluated daily while being hospitalized for 7 postoperative days and at follow-up visits at 6 weeks, 3, 6 and 12 months and also at 2 years.
Participants
Two hundred patients diagnosed with end-stage primary degenerative unilateral hip arthritis, confirmed on plain radiographs, that will undergo a cementless THA will be recruited for this trial.
Inclusion criteria
Participants of either sex who completely fulfill all the following criteria will be enrolled in the study:
• Diagnosis of unilateral hip arthritis
• Indication for surgical treatment by cementless THA
• Pelvic radiographs that confirm the diagnosis
• Asymptomatic contralateral hip
• Able to participate to all postoperative follow-up visits
• Over 35 years old but under 85 years
• Express willingness to comply with the study protocol
• Patients deemed capable of giving informed consent
Exclusion criteria
Patients who meet one of the exclusion criteria will not be enrolled in the study:
• Previous hip surgery
• Diagnosis of secondary or inflammatory arthritis (i.e.,avascular necrosis of the femoral head, post traumatic,developmental dysplasia of the hip, ankylosing spondylitis, rheumatoid, gout, lupus and psoriatic)
• Any muscle diseases, recent heart attacks or rhabdomyolysis
• Any physical disability caused by other than the degenerative hip arthritis
• Bilateral degenerative hip arthritis
• Subjects with a moderate or severe psychiatric illness
• Active or suspected infection
• Patients with Parkinson’s disease
• Pre-existing medical condition that would drastically limit the patient’s lifespan

Strengths and limitations
• The subject has a known metal allergy
• Difficulty of participating in follow-up visits
Withdrawal criteria
Subjects will be removed from the study if one of the following occurs:
• Patients who desire to discontinue participation at any time
• Subjects not complying with study protocol
• Any debilitating trauma or disease that started during the trial
If patients voluntarily withdraw from the study, all data collected prior will be included only after the informed consent is acquired.
Recruitment and randomization
Patients with hip symptoms are directed by their family doc tor to a checkup with an orthopedic physician. During these visits, a basic clinical evaluation and history are taken and a pelvic X-ray if needed. All patients that might meet all the criteria to participate will be informed of the existence of this study and what it means to be recruited. Regardless of the decision of participating or not, all patients willing to undergo a THA will be hospitalized and treated. All subjects that will be interested will be presented with written information about the trial. After the written informed consent is signed and all possible questions answered, they will be randomized in one of the groups. Randomization to the direct anterior approach group or the lateral approach group will be computer generated.
Blinding
Patients will be informed of undergoing the same procedure(i.e., a cementless THA), the replacement of the hip joint using a prosthesis that will be press-fitted, thus without using any surgical cement. It will be explained that the main difference between the two groups is the soft tissue dissection during the procedure,but without disclaiming any information that might converge the patient to realize the approach to be used. Together with the patient, the investigator (physician) who will assess all patients,the physiotherapist, nurses, and all the employees in the laboratory that will analyze all blood values will be blinded. Thus we hope to achieve a double blind trial.
Baseline data
Baseline data will represent all data collected prior to surgery. This includes: demographic data (age, gender, weight, height, calculated body mass index), clinical evaluation and general medical history, blood values (myoglobin, troponin, creatine kinase (CK),lactate dehydrogenase (LDH), aspartate aminotransferase (AST)and C-reactive protein (CRP) , hemoglobin), pain level on Visual Analog Scale (VAS), Harris Hip Score (HHS), Oxford Hip Score Survey, 36-Item Short-Form Health Survey (SF-36).
Surgical procedures
All patients will undergo a unilateral total hip arthroplasty, using a cementless prosthesis. A spinal anesthesia will be used, with an intravenous analgesia during surgery, at the anesthesiologist’s discretion. Antibiotic prophylaxis will start 30 minutes prior to skin incision and will consist of Cefuroxime 1,500 mg.
The DAA group
Patients randomized to this group will undergo a total hip arthroplasty via the DAA. A standard operating table will be used, with the patient in a supine position so that hip hyperextension could be achieved when the table isflexed. Both extremities will be prepped and draped separately to facilitate proximal femur exposure. The operative hip will be covered with a transparent surgical incision drape. The surgical incision will start 2–4 cm distal and lateral to the anterior superior iliac spine, spanning a distance of 8 cm over the tensor fascia lata muscle (TFL), and then lengthened if needed. An incision will be made through the fascia overlying the tensor fascia lata muscle. The muscle is then retracted laterally. After coagulating the ascending braches of the lateral circumflex femoral artery, an anterior capsulectomy will be performed.An osteotomy at the base of the femoral neck will then be performed, with an excision of a napkin ring fragment. After the head is removed, the acetabular cavity will then be prepared using a reamer with an offset handle. Before the acetabular cup and liner are to be press-fitted (with or without the use of self-tapping screws), trial components are used to assess the correct dimensions and stability.
To prepare the proximal femur, the leg will be placed in hyperextension, adducted and externally rotated, underneath the nonoperative leg. When the proximal femur will be rightfully exposed, the femur will then be prepared using instruments with offset handles. Allfinal stems and femoral heads will befitted after a trial reduction using test components, and after checking for possible limb length discrepancy. Afterfinal components are in place, the hip will be reduced.
The lateral approach group
The total hip replacement will be carried out via the lateral approach, on the same normal operating table, with the patient in a supine position. Only the operative hip and lower extremity will be prepped and draped. The incision will be made on the lateral part of the hip, spanning the tip of the greater trochanter, for 8 cm in length. If needed, the incision will be lengthened for a better exposure. Fascia lata will then be incised and the gluteus medius and vastus lateralis muscles divided and retracted. An antero-lateral capsulectomy will be performed. Unlike the direct anterior approach, the hip isfirst dislocated, and only then the neck osteotomy is performed and the femoral head extracted. Acetabular reaming and proximal femur preparation andfinal component positioning will be done using normal instruments, without an offset handle. The same as in the THA via the DAA, trial components are used to assess proper dimensions and possible limb length discrepancies. The hip prosthesis will then be reduced.
In both groups a drainage surgical system will be used together with resorbable multifilament sutures for all soft tissue(i.e., fascia, muscle, subcutaneous tissue), and a resorbable monofilament for skin suture, using an intradermal technique.
Fluoroscopy guidance will not be used when positioning the components.
Implants
All patients will receive the same implant, a Trilogy®uncemented acetabular system shell with acetabular self-tapping bone screws if needed, a polyethylene liner Trilogy®acetabular system, a cobalt-chrome Versys®32 mm diameter femoral head, and a Metabloc™uncemented femoral stem system(Zimmer Warsaw, IN, USA).
Postoperative rehabilitation
Patients will receive low molecular weight heparin as a thromboembolic prophylactic measure for 35 postoperative days, starting 6 hours after surgery. Antibiotic prophylaxis will continue on thefirst postoperative day with a second andfinal dose of Cefuroxime 1,500 mg.
After surgery the patient will receive a standardized analgesic treatment that consists of Paracetamol 1 g and Ketorol 15 mg i.v. every 8 hours. Morphine 2 mg, i.v. is set to be the rescue medication, and will be administered whenever the patient demands it. The consumption of rescue analgesia will be noted for thefirst 5 postoperative days.
During thefirst postoperative day, the patient will start physiotherapy assisted by the department physiotherapist by walking using a walker with weight bearing as tolerated. The patient will continue to walk with a walker after discharge as needed. Physiotherapy will be continued at home following a strict program designed by our physiotherapist.
Outcome measures
Our primary outcome measures will be to establish which of the two approaches produces the least amount of muscle damage with the use of biomarkers. Serum myoglobin and troponin levels will be our main biomarkers to assess the amount of muscle damage created during the procedures.11Beside these two main markers we will also evaluate other markers like: CK,LDH, AST and CRP, through which we want to evaluate not only muscle damage levels but also the inflammatory response.
Secondary outcomes will be clinical and functional result of the THA using the HHS,12Oxford Hip Score Survey,13SF-36.14Functional outcome will be assessed by asking the patient to walk a distance of 20 m (66 feet), and climb aflight of 10 stairs.15,16The usage of any walking aids, the time that takes to complete the tests and any adverse reactions will be noted. To assess postoperative pain levels, the VAS17will be used on a daily basis while the patient is hospitalized, during physiotherapy and bed rest. The patient will note pain levels after discharge using the same VAS for 3 postoperative months,but on a weekly basis.
Perioperative data will be gathered: surgery setup time,operation time (skin incision to suture), intraoperatieve blood loss, postoperative blood loss quantified via the surgical drainage system, hemoglobin levels before and after surgery, length of hospital stay after surgery (the endpoint will be when the patient is able to walk safely with a walker).
Radiographs will be performed during thefirst postoperative day to assess if the endoprosthesis are positioned within the safety zone accepted for each component. Anteroposterior and axial views will be made.
An overview of the time schedule is shown in Tables 1 and 2.
Theflow chart of the trial protocol is presented in Figure 1.
Statistical analysis
Statistical analysis will be carried out at the Department of Medical Informatics and Biostatistics, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, using R version 3.1.1 (R Core Team, Vienna, Austria). All continuous data will be checked if they are normally distributed or not using the Shapiro-Wilk test. Normally distributed data will be compared using Student’s t-test for independent samples, and will be expressed as mean and standard deviation. Non-normally distributed data will be analyzed using the Mann-Witney U test, and presented as median and Q1and Q3quartiles. The Chisquare test or Fisher exact test will be applied when analyzing categorical values between groups. A P-value lower than 0.05 will be considered significant.

Table 1: Study outcome measures relative to the timeline

Figure 1: Flow chart of the trial protocol.
An apriori power analysis was performed using G*Power 3.1.9.218 to establish the required number of patients that will need to be randomized in each group. Using the results published in a recent article19comparing the direct anterior approach to the miniposterior approach when performing THA, that reported a mean rise in postoperative myoglobin levels of 168 ± 114 for the DAA group and 378 ± 151 for the miniposterior group (difference in means: –210, 95% CI, –269to –151), we reached a total sample size of 20 subjects for a P < 0.05, two-sided test, and required power of 95%. For one of our secondary outcomes, postoperative pain levels, we used data from a similar study20who reported postoperative levels on the day of surgery (at arrival time at the intensive care unit)of 1.6 ± 1.9 for the DAA group vs. 2.7 ± 2.1 for the LA group.The power analysis using the same standards as before resulted in a total sample size of 146 patients, for a P < 0.05, two-sided test, and required power of 95%. Due to a relative high risk of patient loss to follow-up and withdrawal, we decided to increase the sample size to 200, with 100 patients per each group.
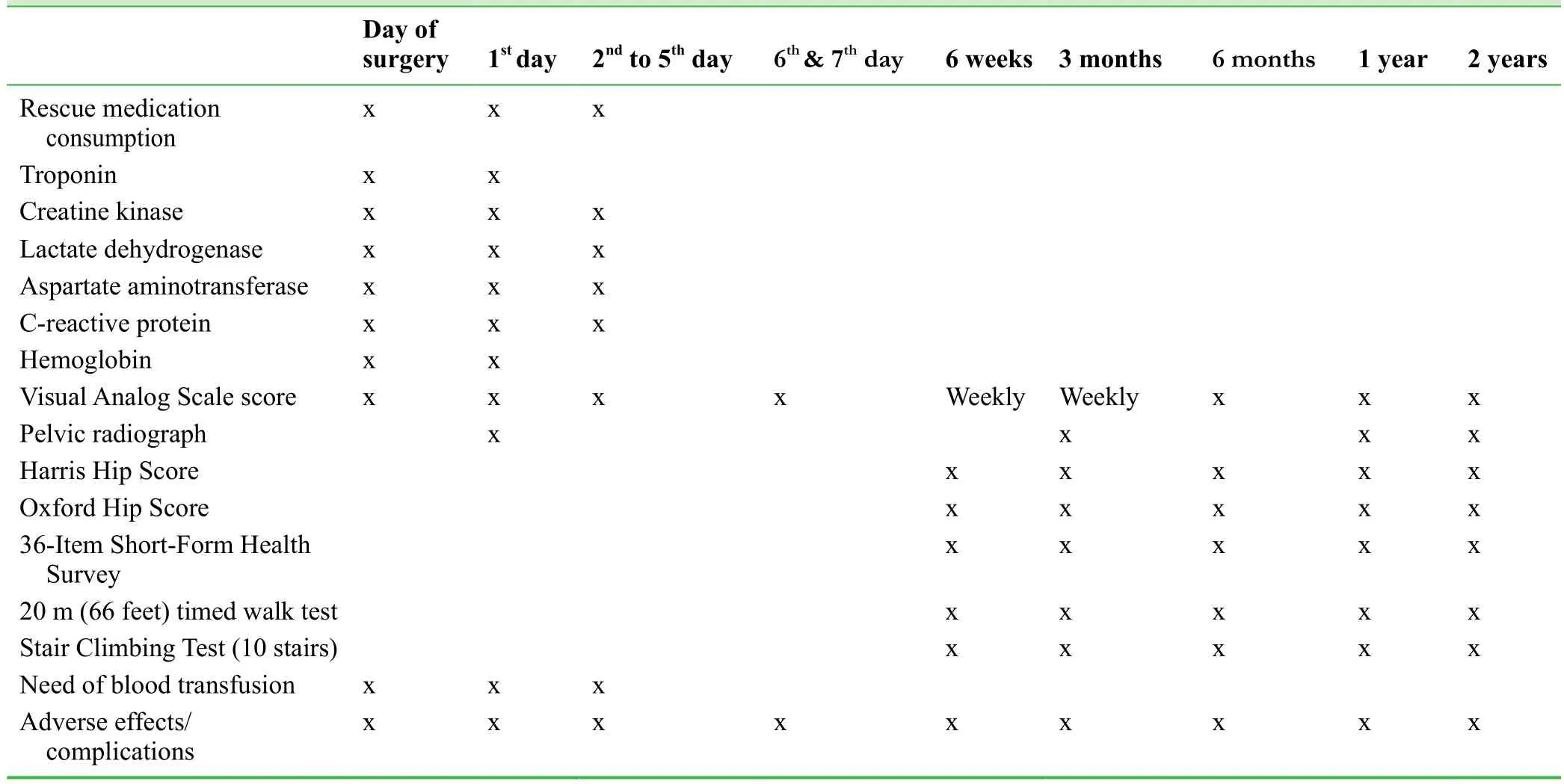
Table 2: Postoperative study outcome measures relative to the timeline
Adverse events
All adverse events will be noted, regardless of the severity. Serious adverse events will be noted and further investigated and disclosed to the review board within 24 hours. Possible adverse effects will be presented in the written information given to the patient prior to signing the informed consent. These possible adverse events are preoperative anxiety, dizziness, superficial or deep infection, superficial lateral femoral nerve palsy, hip dislocation, prosthesis loosening, deep vein thrombosis, and other possible adverse event predictable or not.
Data management
To assure that the quality of a double-blinded trial will not be lost,all personal that will know the randomization (i.e., surgical team,anesthesiologist, or any other), will sign a confidentiality agreement through which they commit not to disclose this information.Also, all personal is instructed to maintain patient confidentiality in accordance with national health laws.
All data will be collected on a case report form, and then recorded electronically using a double-data entry strategy. All electronic data collected as such (i.e., radiographs, blood values) from multiple computers in the hospital network will be than gathered and organized with the support of the University of Medicine and Pharmacy-Department of Medical informatics and biostatistics, together with their data management systems.21,22All data will not be altered in any way. The project manager will be the only one to have access to the data that will be locked in a secure location at the Emergency County Clinical Hospital – Orthopedic and Traumatology Department I, Cluj-Napoca, Romania. Original data recorded on paper will be locked in afile cabinet and electronic data will be secured via password protection, and will be backed-up on at least two secure passive storage devices, not connected to any server.
Audits
Trial progress will be reported to the Iuliu Hatieganu University ethics committee, Romania. The writing and editing of the manuscript were performed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials(SPIRIT) (Additionalfile 1).
Study protocol amendments
The study protocol will not be changed in any matter as much as possible. If changes are necessary all relevant parties will be notified.
DISCUSSION
In this randomized control clinical trial we intend to compare the direct anterior approach to the lateral approach when performing a total hip replacement. The primary outcome is differences in serum markers for muscle damage. This study protocol is designed to compare serum marker levels,considered as an objectified method of quantifying muscle damage,11with functional outcomes, postoperative pain levels,radiographic evaluations and patient satisfaction and quality of life sores. Through these comparisons we intend to provide reference information if the anterior approach is truly a muscle sparing approach relative to the lateral approach, and if this results in better patient satisfaction, lower complication rates and postoperative pain levels.
TRIAL STATUS
Recruiting at the time of submission.
Additional file
Additionalfile 1: SPIRIT checklist.
Author contributions
Trial design, data collection and writing: DVN and AT. Data collection and trial design: SC, NCB and DOGL. All authors approved thefinal version of this paper.
Conflicts of interest
The authors have no conflict of interest to declare.
Financial support
None.
Research ethics
The protocols had been approved by the University of Medicine and Pharmacy Cluj-Napoca Ethics Committee (517/2015).
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the form, the patients will give their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement
No data is reported in the article.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License,which allows others to remix, tweak, and build upon the work noncommercially, as long as the author is credited and the new creations are licensed under identical terms.
REFERENCES
1. von Rottkay E, Rackwitz L, Rudert M, Noth U, Reichert JC. Function and activity after minimally invasive total hip arthroplasty compared to a healthy population. Int Orthop. 2017;doi: 10.1007/s00264-017-3541-z.
2. Trousdale WH, Taunton MJ, Mabry TM, Abdel MP, Trousdale RT.Patient perceptions of the direct anterior hip arthroplasty. J Arthroplasty. 2017;32:1164-1170.
3. Hallert O, Li Y, Brismar H, Lindgren U. The direct anterior approach: initial experience of a minimally invasive technique for total hip arthroplasty. J Orthop Surg Res. 2012;7:17.
4. Frye BM, Berend KR, Lombardi AV Jr., Morris MJ, Adams JB. Do sex and BMI predict or does stem design prevent muscle damage in anterior supine minimally invasive THA? Clin Orthop Relat Res.2015;473:632-638.
5. Spaans AJ, van den Hout JA, Bolder SB. High complication rate in the early experience of minimally invasive total hip arthroplasty by the direct anterior approach. Acta Orthop. 2012;83:342-346.
6. Eto S, Hwang K, Huddleston JI, Amanatullah DF, Maloney WJ,Goodman SB. The direct anterior approach is associated with early revision total hip arthroplasty. J Arthroplasty. 2017;32:1001-1005.
7. Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty. 2010;25:671-679.e1.
8. Alecci V, Valente M, Crucil M, Minerva M, Pellegrino CM, Sabbadini DD. Comparison of primary total hip replacements performed with a direct anterior approach versus the standard lateral approach:perioperativefindings. J Orthop Traumatol. 2011;12:123-129.
9. Nistor DV, Caterev S, Bolboaca SD, Cosma D, Lucaciu DOG,Todor A. Transitioning to the direct anterior approach in total hip arthroplasty. Is it a true muscle sparing approach when performed by a low volume hip replacement surgeon? Int Orthop. 2017;41:2245-2252.
10. Chechik O, Khashan M, Lador R, Salai M, Amar E. Surgical approach and prosthesisfixation in hip arthroplasty world wide. Arch Orthop Trauma Surg. 2013;133:1595-1600.
11. Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757-767.
12. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737-755.
13. Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br. 1996;78:185-190.
14. Ware JE Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
15. Motyl JM, Driban JB, McAdams E, Price LL, McAlindon TE. Testretest reliability and sensitivity of the 20-meter walk test among patients with knee osteoarthritis. BMC Musculoskelet Disord.2013;14:166.
16. Bennell K, Dobson F, Hinman R. Measures of physical performance assessments: Self-Paced Walk Test (SPWT), Stair Climb Test (SCT), Six-Minute Walk Test (6MWT), Chair Stand Test(CST), Timed Up & Go (TUG), Sock Test, Lift and Carry Test(LCT), and Car Task. Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S350-370.
17. Miller MD, Ferris DG. Measurement of subjective phenomena in primary care research: the Visual Analogue Scale. Fam Pract Res J.1993;13:15-24.
18. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149-1160.
19. Poehling-Monaghan KL, Taunton MJ, Kamath AF, Trousdale RT,Sierra RJ, Pagnano MW. No correlation between serum markers and early functional outcome after contemporary THA. Clin Orthop Relat Res. 2017;475:452-462.
20. Goebel S, Steinert AF, Schillinger J, et al. Reduced postoperative pain in total hip arthroplasty after minimal-invasive anterior approach. Int Orthop. 2012;36:491-498.
21. Valeanu M, Cosma S, Cosma D, Moldovan G, Vasilescu D. Optimization for date redistributed system with applications. Int J Comput Commun. 2009;4:156-161.
22. Cosma S, Văleanu M, Cosma D, Vasilescu D, Moldovan G. Effi cient data organisation in distributed computer systems using data warehouse. Int J Comput Commun. 2013;8:367-373.
杂志排行
Clinical Trials in Orthopedic Disorder的其它文章
- Functional outcomes and health-related quality of life after open repair of rotator cuff tears: a prospective cohort study
- Evaluation of a computer-assisted orthopedic training system for learning knee replacement surgery:a prospective randomized trial
- Effects of balance training prior total knee replacement surgery: study protocol for a randomized controlled trial
- A newfixation and reconstruction method versus arthroscopic reconstruction for treating avulsion fracture at the tibial insertion of the knee posterior cruciate ligament: study protocol for a nonrandomized controlled trial and preliminary results
- Information for Authors - Clinical Trials in Orthopedic Disorders (CTOD)
