Variation of lipids and fatty acids composition in the tissues of wild devil stinger(Inimicus japonicas)during sexual maturation
2018-06-14SonglinLiWenWenXuGongXuxiongHungNisongChen
Songlin Li ,Wen Wen ,Xu Gong ,Xuxiong Hung ,b,c,*,Nisong Chen
a National Demonstration Center on Experiment Teaching of Fisheries Science,Shanghai Ocean University,Shanghai 201306,China
b Research Centre of the Agriculture Ministry on Environmental Ecology and Fish Nutrition,Shanghai Ocean University,Shanghai 20136,China
c Shanghai Collaborative Innovation for Aquatic Animal Genetics and Breeding,Shanghai Ocean University,Shanghai 201306,China
1.Introduction
The quantity and quality of fish larvae are important for the aquaculture of newly exploited fish species,and wild-caught adult fish are generally used as broodstocks due to the higher quality of sperm and eggs produced(Cavalli,Tamtin,Lavens,&Sorgeloos,2001;Huang et al.,2010).Therefore,biochemical and metabolomic characterization of wild-caught fish during its maturation is important to understand nutrition requirement of fish broodstocks.
During maturation,lipid deposition,especially fatty acids,is crucial for reproduction and larval development due to their key role in fish metabolism(Ghaedi,Kabir,&Hashim,2016;Huang et al.,2010;Ling,Kuah,Muhammad,Kolkovski,&Shu-Chien,2006;Ng&Wang,2011;Watanabe&Vassallo-Agius,2003).Particularly,long chain polyunsaturated fatty acids(LC-PUFAs),such as docosahexaenoic(DHA),eicosapentaenoic(EPA)and arachidonic(ARA)acids,are essential for the normal development and function of fish larvae nervous system(Benítez-Santana et al.,2012;Ishizaki et al.,2001;Turkmen et al.,2017)and sensory organs(Benítez-Santana et al.,2007).However,during early larval stages,especially the endogenous nutrition stage,rely on yolk lipid reserves for the supply of energy and essential fatty acids to maintain normal growth performance.The yolk lipid reserves re flect the maternal nutrient reserves and depend on the broodstock diet(Sargent,1995).Therefore,more attention should be paid on the lipid level and fatty acid pro files of the broodstock diet.In addition,excessive LC-PUFAs content exhibited negative effects on fish performance due to its higher susceptibility to peroxidation(Kjær,Todorˇcevi′c,Torstensen,Vegusdal,&Ruyter,2008;Saera-Vila et al.,2009)and thus,lipid and LC-PUFAs contents in the diets should be controlled within a limited range.
The devil stinger(Inimicus japonicas)is a valuable demersal scorpaenid fish,with high nutritional and medical value.The biological aspects of this fish species has been reported in few studies(Huang,Feng,Wen,Chen,&Wei,2013;Nozakiet al.,2003),and no significant differences in gonadal development exists between male and female.Gonadal development begins between January and February and spawning occurs in May(Nozaki et al.,2003).With the establishment of successful artificial fish propagation(Takushima,Nozaki1,Kadomura,Yasumoto,&Soyano,2003),the devil stinger has become a newly exploited fish species for mariculture in southern China.However,wild-caught stinger is still used as broodstocks due to the scarce information about nutrient requirements.Meanwhile,nutrient content and changes in broodstocks tissues during the reproductive season may contribute for the development of a specific enrichment diets(Huang et al.,2010).In this study we have characterized the lipid and fatty acid pro files in the gonad,liver and muscle of wild-caught devil stinger broodstocks at various stages of gonad development to better understand nutrient reserves and to collect basic information that can be used to develop artificial diets to be used in captivity.
2.Materials and methods
2.1.Sampling and processing
Stinger broodstocks were collected from the Xiamen sea zones(E118.16,N24.42)between April and May(during the reproductive season)to include animals with different gonad developmental stages.The broodstocks were transferred to the lab and fish length and weight were measured.Fish were dissected on ice,and the gonad and liver were individually weighed.The gonadosomatic index(GSI)and hepatosomatic index(HSI)were calculated according to the following formula:

The developmental phase of the broodstock gonad was determined according to the method described by(Mai et al.,2005).Muscle,liver and gonad were sampled,stored at-80°Cand dried at-46°C using a freeze-dry system and were subsequently individually ground to powder.
2.2.Total lipid content and lipid class
Total lipids(TLs)content in the muscle,liver and gonad were individually extracted using chloroform-methanol(2:1,v/v)containing 0.01%butylated hydroxytoluene(BHT)as an antioxidant according to Cejas,Almansa,and Jerez(2004)with some modification(Huang et al.,2013),and the extracted lipids were vacuumdried until constant weight was obtained.The extracted total lipids were used to assay lipid class and total lipid content was separated into polar(PL)and neutral lipids(NL)according to the method described by Berry,2004.Brie fly,total lipids were dissolved in petroleum ether and 95%methanol(1:1,v:v)by oscillation and the mixture was left to rest to promote separation into an upper layer of petroleum ether and a lower layer of methanol.The upper and the lower layers were rinsed twice with an equal volume of 95%methanol and petroleum ether,respectively.The combined 95%methanol(containing polar lipids)and petroleum ether(containing neutral lipids)layers were collected and evaporated to dryness for the measurement of PLand NL.
2.3.Fatty acid content
The PL and NL were esterified to fatty acid methyl esters(FAMEs)with 14%BF3in methanol(Morrison&Smith,1964)and the FAMEs extracted with hexane.FAMEs were detected in duplicated on an HP6890 gas chromatograph according to the method described by Huang et al.(2010).Peak identification was performed by comparing the retention times with commercial standard(Sigma Chemical Co,St.Louis,MO,USA),and the fatty acid C19:0(nonadecanoic acid,Sigma)was used as an internal standard for fatty acid quantification.The internal-standard calculation was described as:

in which Cxcorresponds to the concentration of a specific fatty acid in the dried sample(mg/g);C19the concentration of C19:0(mg/m L);V19the additive volume of C19:0(m L);M19the molecular weight of C19:0 FAME;S19the peak area of C19:0 FAME;Mxthe molecular weight of a specific fatty acid;Sxthe peak area of a specific fatty acid;and m the sample dried weight(g).
2.4.Statistical analysis
Data was expressed as the mean±standard deviation and analysed with One-Way ANOVA and correlation analysisperformed in SPSS19.0 for Windows.Duncan's multiple range test was chosen as a multiple comparison test and a significance level of 5%was used.
3.Results
3.1.Biological characteristics of the broodstocks
A total of 67 devil stinger broodstocks were caught from the sea and the biological and biochemical compositions of the wildcaught stinger were analysed(Table 1).The female broodstocks mean body weight and GSI significantly increased as the ovary developed from phase IV to V(P<0.05).However,ovary development had no significant effect on the HSI(P<0.05).The mean body weight,GSI and HIS of the male broodstocks significantly increased as the spermary developed from phase IIIto IV(P<0.05)(Table 1).
3.2.Lipid content and lipid class at different stages of gonad development
Total lipids(TL)content varied in the broodstock tissues(Table 2).Total lipid level in the liver was up to 50%significantly higher than in ovary,testis and muscle.Differences were also observed in the proportion of NL to TLacross the different tissues.PL was the dominant component of TL in the ovary,sperm and muscle while NLwere more abundant in the liver.
The TL level and lipid class changed according to gonad development(Table 2).Although no significant difference was found in the TL content of the ovary from phase IIIto V(P>0.05),a significant decrease of PLand significant increase of NLwere detected in the ovary phase V(P<0.05).The TL and NL contents in the liver significantly decreased and PL increased step-wise with ovary development from phase IIIto V(P>0.05).However,PL,but not NL,content significantly increased with the development of the testis from phase III to V(P<0.05).In male,the TL and NL in the liver slightly increased with testis development from phase III to V(P>0.05),and was distinct from females.TL content in testis significantly increased with the development of sperm from phase IIIto IV,while NL and PL contents were not significantly affected(P>0.05).
3.3.Changes in fatty acid contents at different stages of gonadal development
Fatty acids contents(mg/g DW)were tissue-and lipid class specific(Tables 3-5).The fatty acids contents of PL were remarkably higher than NL.Poly-unsaturated fatty acids(PUFAs)was the main PLfatty acids in the gonad and was much higher in the ovary than in testis.The main PL fatty acids in the ovary were:22:6n-3(DHA)>16:0>20:5n-3(EPA)>20:4n-6(ARA)>18:0 and in testis,22:6n-3 (DHA)>20:4n-6 (ARA)>16:0>18:0>20:5n-3 (EPA).During the gonad development from phase IIIto IV,no significant differences were found in the fatty acids contents of the ovary(P>0.05),while SFA,MUFA,PUFA content in the testis significantly increased(P<0.05).The content of mono-unsaturated fatty acids(MUFAs)in gonad NLwas slightly higher than PUFAs,while MUFAs content in PL was significantly lower than that of PUFAs(P<0.05).The PUFAs content of the NLfrom the ovary was lower than in testis and the main fatty acids present were 16:0,24:1,18:1n-9,DHA and 16:1 while in testis were 24:1>DHA>18:0>ARA>16:0.The main NL fatty acids content of the ovary did not change significantly during development(P>0.05)but DHA,EPA and ARA contents increased significantly with testis development(P<0.05).
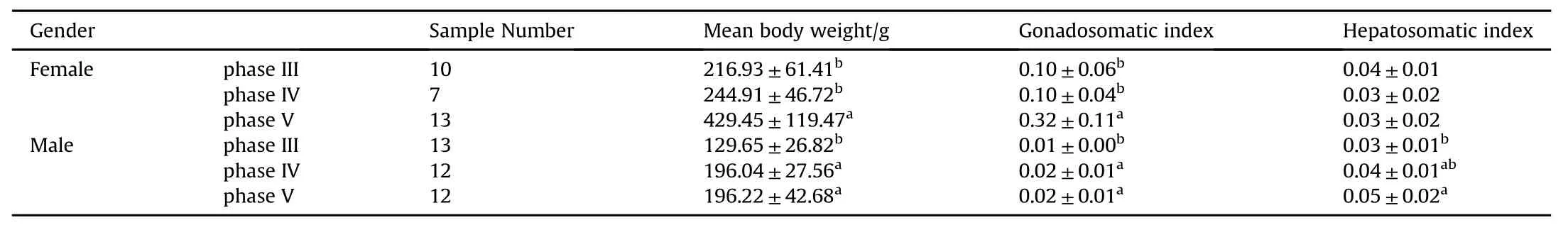
Table 1 Biological characteristics of wild-caught Inimicus japonicus broodstocks.

Table 2 Lipid class in tissues of wild-caught I.japonicus broodstocks at different gonad stages(%in dry weight).
The main fatty acids present in the liver were saturated fatty acids(SFAs)and MUFAs and NL fatty acids contents was remarkable higher than PL.In the liver of both male and female the most abundant NLfatty acids were 16:0,16:1,18:0,18:1n-9,18:1n-7,20:1,ARA,EPA and DHA and PLwere 16:0,16:1,18:0,18:1n-9 and DHA.The HUFAs,DHA,EPA and ARA contents increased significantly in PLof the female liver during gonad development from phase IV to V,while no significant differences were observed in PLof the male liver.Interesting,the content of HUFAs in NLof the male liver,but not in the female,significantly decreased with gonad development.
In the muscle the fatty acids contents of PLwere much higher than NL.The dominant PL fatty acids in both male and females were HUFAs(DHA,EPA and ARA),18:1n-9 and 16:0,and PUFA content in PLwas higher than SFA and MUFA.However,the content of MUFA in PL was higher than PUFA and SFA.In the female muscle the contents of DHA,ARA,SFA,MUFA and PUFA in PL significantly decreased with ovary development from phase IIIto IV(P<0.05).
4.Discussion
Understanding nutrient storages and metabolism in tissues during the reproductive season may contribute for the development of a specific diet for fish broodstocks(Huang et al.,2010).Nutrientscan be transported to and stored in the gonads during the maturation of fish species(Mai,Huang,Ye,Li,&Wang,2005),and in the present study this may account for the largest GSI of devil stinger broodstocks at phase V as previously reported in other fish species(Cejas et al.,2003;Huang et al.,2010;P′erez et al.,2007;Rodríguez et al.,2004).Thus,investigation of the fatty acids variation during gonad development can contribute to a better understanding of the nutrient requirement of the devil stinger broodstocks.
The devil stinger is a typical benthic carnivorous fish species,and preyed mainly on mysisshrimp,nematodes and wild fish and it has been suggested that the sampling site may affect the lipid and fatty acids composition.During sampling,no food residue was observed in the intestine tract of the stinger broodstocks and suggests that they do not feed during maturation and thus variation of lipid and fatty acidsduring maturation was poorly affected by the sampling site.
The TLcontent of the liver of the devil stinger broodstocks were significantly higher when compared with the ovary,testis and muscle.This contrasts to what has been described from other marine fishes that produce buoyant eggs such as silver pomfret(Pampus argenteus),where TL content of the ovary in the broodstocks were the highest among tissues(Huang et al.,2010).TLs functionally act as structural components(mainly PL)and energy reserves(mainly NL).Generally,the excess energy is mainly stored as neutral lipid and particularly as TAG(Sargent,Tocher,&Bell,2002)and lipid tissue content in fish is usually closely correlatedto their habit.It has been suggested that demersal marine fish,such as the red drum(Sciaenops ocellatus)and the Pacific cod(Gadus microcephalus),accumulate large amounts of fat in their liver,while swimming marine fish species tend to accumulate more fat in the muscle(Craig,MacKenzie,Jones,&Gatlin,2000).In this study,we found that the devil stinger accumulated larger amounts of lipid(approximately 50%DW)in the liver,while lipid content of the muscle was less than 3%.Therefore,lipid tissue distribution in devil stinger was consistent with its demersal habit in which fish do not require high levels of lipid deposited in the muscle to fuel swimming(Craig et al.,2000).
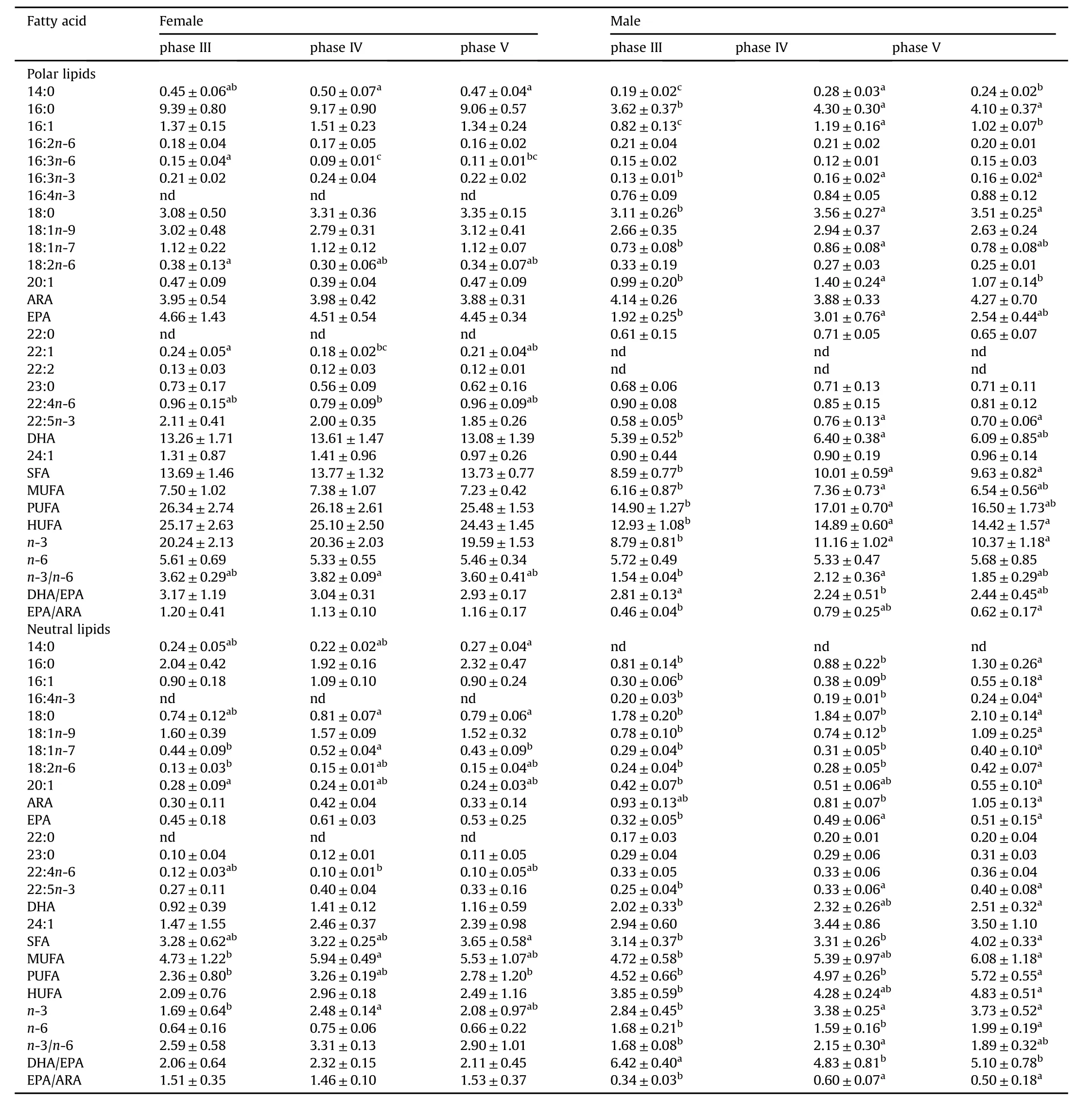
Table 3 Absolute fatty acid pro files(mg/g)in the gonads of I.japonicus broodstocks during different stages.
The dominant lipid class in the liver was NL,while PL were abundant in the ovary,testis and muscle.However,for most marine fish that produce buoyant eggs,NL is the major component of TLs inthe ovary(Cejas et al.,2003;Huang et al.,2010;P′erez et al.,2007),which may result from the accumulation of NL to provide fuel for embryo development.We also speculated that marine fish that produced buoyant eggs without visible oil droplets,such as the devil stinger,complete lipid nutrient accumulation in oocytes earlier than other marine fish that produce pelagic eggs,such as the silver pomfret.Interesting,the ovary size of the devil stinger broodstocks increased but its TL content remained constant andthis observation is distinct from what is described from other fish species.For the supply of nutrient requirements to sustain embryonic development,TLs constantly accumulate in the ovary during its development(Cejas et al.,2003;Huang et al.,2010;P′erez et al.,2007).In the devil stinger broodstocks TL content in the ovary was approximately 13%and this is lower than described for other marine fish(Pethybridge,Daley,Virtue,&Nichols,2011).Although no changes in TL content was observed,NL content significantly increased and PL decreased significantly during the development of ovary from phase III to V,which may suggest that some PL were transformed into NL during the maturation process of the ovary.For the male broodstocks,the TL and PL content increased during the development of testis while NL decreased,suggesting that NLwere converted into PL.The different lipid accumulation in the gonads reflects the differences in allocation and utilization of the energy sources between the ovary and testis.
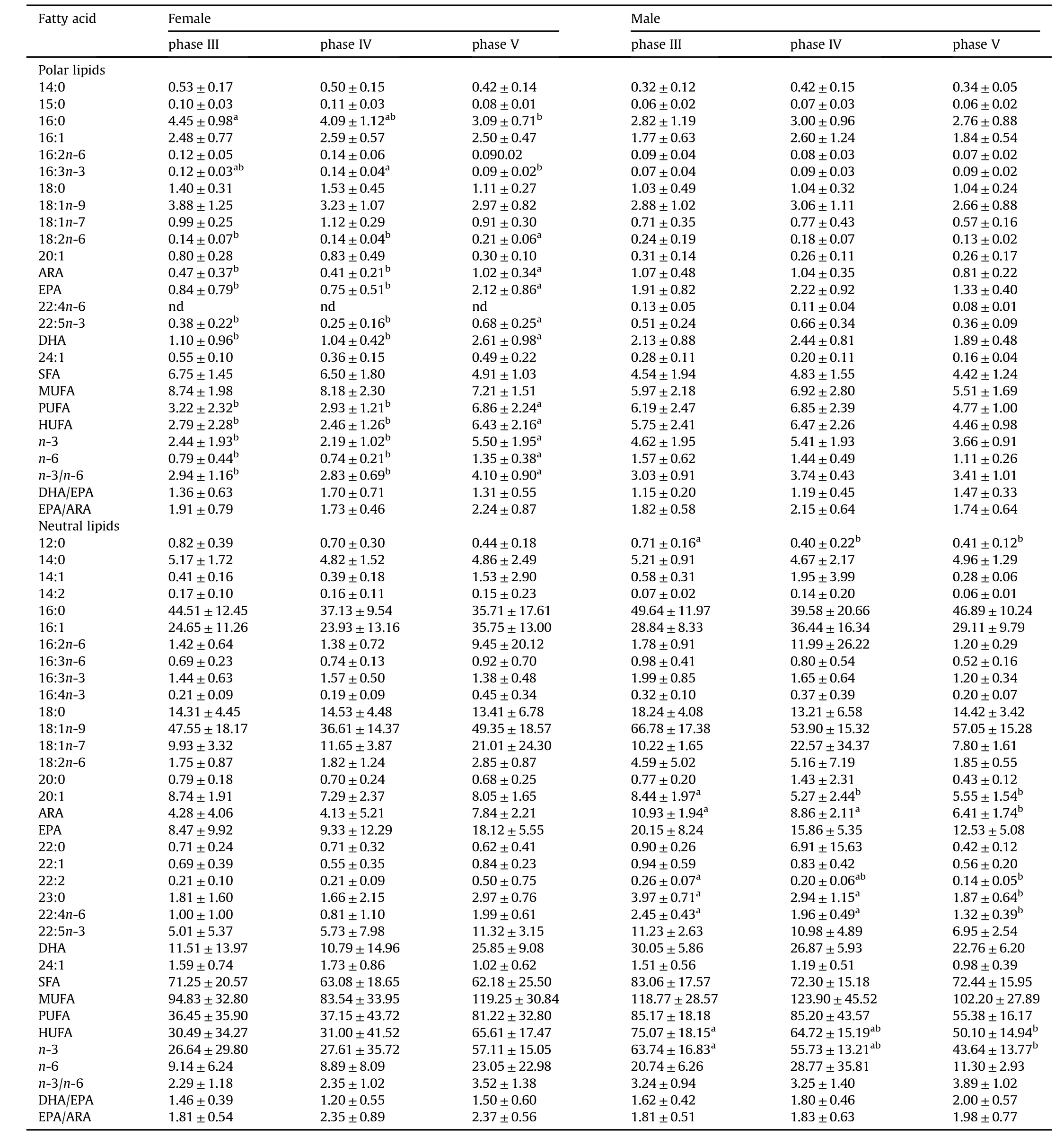
Table 4 Absolute fatty acid pro files(mg/g)in the liver of I.japonicus broodstocks during different stages.
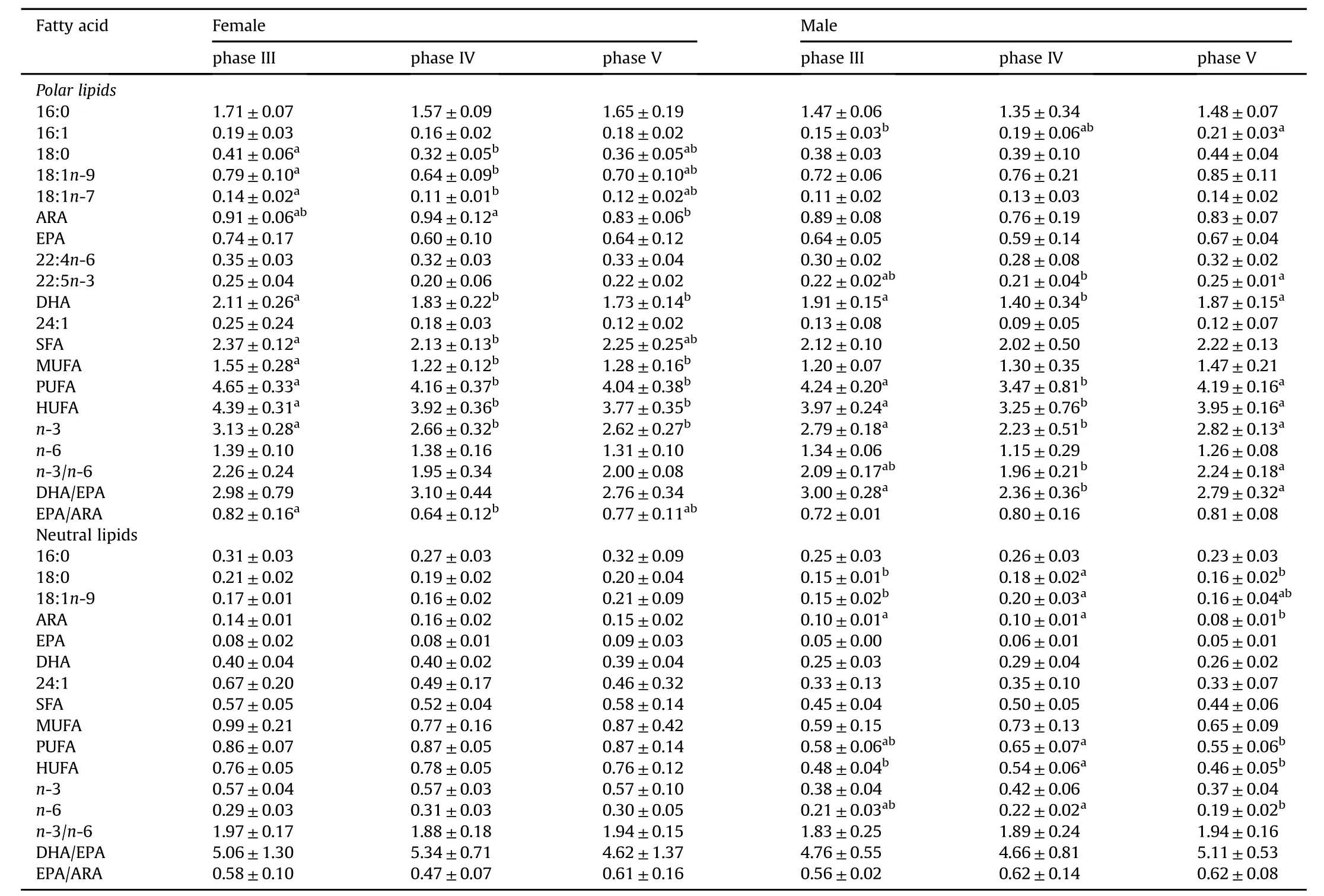
Table 5 Absolute fatty acid pro files(mg/g)in the muscle of I.japonicus broodstocks during different stages.
Dietary fatty acids and their cyclooxygenase and lipoxygenase metabolites have an extremely impact on oocyte maturation and spermatogenesis(Sorbera,Francisco Asturiano,Carrillo,&Zanuy,2001).The absolute contents(mg/g)of DHA,EPA and ARA were elevated in the gonad of devil stinger broodstocks,indicating that they have an important role during gonad maturity,fertilisation and larval development.In this study,the DHA content as well as the proportion of DHA of total PL fatty acids of the gonads were much higher than other tissues,indicating the preferentially accumulation of DHA in this tissue.Interesting,the HUFA contents,especially DHA,in the testis,but not ovary,significantly increased with gonad development indicating an important role of DHA in spermatogenesis.It has been demonstrated that HUFAs,and in particularly the DHA,were the predominant PL fatty acids in fish sperm(Pustowka,McNiven,Richardson,&Lall,2000)and the concentration of DHA in the fish testis may be positively correlated with sperm density,the number of motile sperm and sperm motility(Conquer,Martin,Tummon,Watson,&Tekpetey,1999).This may account for the increasing of DHA content during testis development.
In conclusion,lipid class and fatty acid pro files in the different tissues(gonad,liver and muscle)of the devil stinger significantly changed during gonad development from phrase III to V.HUFA,such as ARA,DHA and EPA,were selectively transferred to and stored in the ovary and testis suggesting a specific fatty acid requirement during gonadal maturation and embryogenesis.Therefore,artificial diets,may be enriched with sufficient lipids and HUFA,which should be provided,especially to the female broodstocks,prior to phase IIIof gonad development.
Acknowledgements
The authors thank Mr.Chen Qingkaifor help in collecting the broodstocks and Wu Hao for quantifying the fatty acid levels.This study was financially supported by a Innovation Project of Shanghai Education Commission,China(12ZZ166)and Shanghai Universities First-class Disciplines Project of Fisheries(2015-62-0908).
Benítez-Santana,T.,Juárez-Carrillo,E.,Betancor,M.B.,Torrecillas,S.,Caballero,M.J.,&Izquierdo,M.S.(2012).Increased Mauthner cell activity and escaping behaviour in seabream fed long-chain PUFA.British Journal of Nutrition,107,295-301.
Benítez-Santana,T.,Masuda,R.,Carrillo,E.J.,Ganuza,E.,Valencia,A.,Hernández-Cruz,C.M.,et al.(2007).Dietary n-3 HUFA de ficiency induces a reduced visual response in gilthead seabream Sparus aurata larvae.Aquaculture,264,408-417.
Berry,S.(2004).Lipid Analysis:Isolation,separation,identification and structural analysis of lipids.Nutrition Bulletin,29,72-73.
Cavalli,R.O.,Tamtin,M.,Lavens,P.,&Sorgeloos,P.(2001).Variations in lipid classes and fatty acid content in tissues of wild Macrobrachium rosenbergii(de Man)females during maturation.Aquaculture,193(3),311-324.
Cejas,J.R.,Almansa,E.,&Jerez,S.(2004).Changes in lipid class and fatty acid composition during development in white sea bream(Diplodus sargus)eggs and larvae.Comparative Biochemistry and Physiology-Part B,139,209-216.
Cejas,J.R.,Almansa,E.,Villamandos,J.E.,Badı a,P.,Bola~nos,A.,&Lorenzo,A.(2003).Lipid and fatty acid composition of ovaries from wild fish and ovaries and eggs from captive fish of white sea bream(Diplodus sargus).Aquaculture,216,299-313.
Conquer,J.A.,Martin,J.B.,Tummon,I.,Watson,L.,&Tekpetey,F.(1999).Fatty acid analysis of blood serum,seminal plasma and spermatozoa of normozoospermic vs.asthenozoospermic males.Lipids,34,793-799.
Craig,S.R.,MacKenzie,D.S.,Jones,G.,&Gatlin,D.M.,III(2000).Seasonal changes in the reproductive condition and body composition of free-ranging red drum(Sciaenops ocellatus).Aquaculture,190,89-102.
Ghaedi,A.,Kabir,M.A.,&Hashim,R.(2016).Effect of lipid levels on the reproductive performance of Snakehead murrel,Channa striatus.Aquaculture Research,47,983-991.
Huang,X.X.,Feng,L.F.,Wen,W.,Chen,Q.K.,&Wei,L.K.(2013).The changes on lipid and fatty acid pro files of devil stinger Inimicus japonicas during the development of embryo and yolk sac larvae.Journal of Fisheries of China,37,51-60.
Huang,X.X.,Yin,Y.Q.,Shi,Z.H.,Li,W.W.,Zhou,H.Q.,&Lv,W.Q.(2010).Lipid content and fatty acid composition in wild-caught silver pomfret(Pampus argenteus)broodstocks:Effects on gonad development.Aquaculture,310,192-199.
Ishizaki,Y.,Masuda,R.,Uematsu,K.,Shimizu,K.,Arimoto,M.,&Takeuchi,T.(2001).The effect of dietary docosahexaenoic acid on schooling behaviour and brain development in larval yellow tail.Journal of Fish Biology,58,1691-1703.
Kjær,M.A.,Todorˇcevi′c,M.,Torstensen,B.E.,Vegusdal,A.,&Ruyter,B.(2008).Dietary n-3 HUFA affects mitochondrial fatty acidβ-oxidation capacity and susceptibility to oxidative stress in Atlantic salmon.Lipids,43,813-827.
Ling,S.,Kuah,M.K.,Muhammad,T.S.T.,Kolkovski,S.,&Shu-Chien,A.C.(2006).Effect of dietary HUFA on reproductive performance,tissue fatty acid pro file and desaturase and elongase m RNAs in female swordtail Xiphophorus helleri.Aquaculture,261,204-214.
Mai,X.J.,Huang,W.J.,Ye,F.L.,Li,J.E.,&Wang,Y.X.(2005).Reproductive biology and artificial breeding on marine fish.Beijing:Ocean press.
Morrison,W.R.,&Smith,L.M.(1964).Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol.The Journal of Lipid Research,5,600-608.
Ng,W.K.,&Wang,Y.(2011).Inclusion of crude palm oil in the broodstock diets of female Nile tilapia,Oreochromis niloticus,resulted in enhanced reproductive performance compared to brood fish fed diets with added fish oil or linseed oil.Aquaculture,314,122-131.
Nozaki,R.,Takushima,M.,Mizuno1,K.,Kadomura,K.,Yasumoto,S.,&Soyano1,K.(2003).Reproductive cycle of devil stinger,Inimicus japonicus.Fish Physiology and Biochemistry,28,217-218.
P′erez,M.J.,Rodriguez,C.,Cejas,J.R.,Martín,M.V.,Jerez,S.,&Lorenzo,A.(2007).Lipid and fatty acid content in wild white sea bream(Diplodus sargus)broodstock at different stages of the reproductive cycle.Comparative Biochemistry and Physiology-Part B,146,187-196.
Pethybridge,H.,Daley,R.,Virtue,P.,&Nichols,P.D.(2011).Lipid(energy)reserves,utilisation and provisioning during oocyte maturation and early embryonic development of deepwater chondrichthyans.Marine Biology,158,2741-2754.
Pustowka,C.,McNiven,M.A.,Richardson,G.F.,&Lall,S.P.(2000).Source of dietary lipid affects sperm plasma membrane integrity and fertility in rainbow trout Oncorhynchus mykiss(Walbaum)after cryopreservation.Aquaculture Research,31,297-305.
Rodríguez,C.,Acosta,C.,Badía,P.,Cejas,J.R.,Santamaría,F.J.,&Lorenzo,A.(2004).Assessment of lipid and essential fatty acids requirements of black seabream(Spondyliosoma cantharus)by comparison of lipid composition in muscle and liver of wild and captive adult fish.Comparative Biochemistry and Physiology-Part B,139,619-629.
Saera-Vila,A.,Benedito-Palos,L.,Sitjà-Bobadilla,A.,Nácher-Mestre,J.,Serrano,R.,Kaushik,S.,et al.(2009).Assessment of the health and antioxidant trade-off in gilthead sea bream(Sparus aurata L.)fed alternative diets with low levels of contaminants.Aquaculture,296,87-95.
Sargent,J.R.(1995).Origins and functions of egg lipids:Nutritional implications.In N.R.Bromage,&R.J.Roberts(Eds.),Broodstock management and egg and larval quality(pp.353-372).Oxford,U.K:Blackwell Science Ltd.
Sargent,J.R.,Tocher,D.R.,&Bell,G.(2002).The lipids.In J.Halver,&R.Hardy(Eds.),Fish nutrition(pp.181-257).San Diego:Elsevier.
Sorbera,L.A.,Francisco Asturiano,J.,Carrillo,M.,&Zanuy,S.(2001).Effects of polyunsaturated fatty acids and prostaglandins on oocyte maturation in a marine teleost,the European sea bass(Dicentrarchus labrax).Biology of Reproduction,64,382-389.
Takushima,M.,Nozaki1,R.,Kadomura,K.,Yasumoto,S.,&Soyano,K.(2003).Induced ovulation using LHRHa and artificial fertilization in devil stinger,Inimicus japonicus.Fish Physiology and Biochemistry,28,521-522.
Turkmen,S.,Castro,P.L.,Caballero,M.J.,Hernández-Cruz,C.M.,Saleh,R.,Zamorano,M.J.,et al.(2017).Nutritional stimuli of gilthead seabream(Sparus aurata)larvae by dietary fatty acids:Effects on larval performance,gene expression and neurogenesis.Aquaculture Research,48,202-213.
Watanabe,T.,&Vassallo-Agius,R.(2003).Broodstock nutrition research on marine fin fish in Japan.Aquaculture,227,35-61.
杂志排行
Aquaculture and Fisheries的其它文章
- The dysregulated autophagy signaling is partially responsible for defective podocyte development in wt1a mutant zebra fish
- Temperature stress response of heat shock protein 90(Hsp90)in the clam Paphia undulata☆
- Evaluating the bioavailability of heavy metals in natural-zeoliteamended aquatic sediments using thin-fi lm diffusive gradients
- Abundance and biomass of assorted small indigenous fish species:Observations from rural fish markets of West Bengal,India
- Fish kidney cells show higher tolerance to hyperosmolality than amphibian
