The dysregulated autophagy signaling is partially responsible for defective podocyte development in wt1a mutant zebra fish
2018-06-14XuemeiZhangQiaohongLinFanRenJinZhangFarmanUllahDawarJieMei
Xuemei Zhang ,Qiaohong Lin ,Fan Ren,Jin Zhang,Farman Ullah Dawar,Jie Mei
Key Laboratory of Freshwater Animal Breeding,Ministry of Agriculture,College of Fisheries,Huazhong Agricultural University,Wuhan 430070,China
1.Introduction
Wilms'tumor suppressor gene(WT1)is a transcription factor that plays an important role in organ development(Chau&Hastie,2012).Mutations in WT1 cause Wilms’tumor,a pediatric kidney cancer(Call et al.,1990;Gessler et al.,1990).For example,WT1-deficient mice have developmental defects in many organs,such as kidney,gonad,spleen,heart as well as retina,and the fetus dies during pregnancy(Barbaux et al.,1997;Herzer,Crocoll,Barton,Howells,&Englert,1999;Moore,Mc Innes,Kreidberg,Hastie,&Schedl,1999;Wagner et al.,2002).In mice,the expression of WT1 was confined to the metanephrogenic mesenchyme in early stages and the kidney glomeruliat later stages.WT1 playsa key role in the development of the urogenital system,particularly glomerular development and podocyte function(Armstrong,Pritchard-Jones,Bickmore,Hastie,&Bard,1993).Loss of WT1 in podocytes leads to defective podocyte maturation and failure of renal filtration(Dong et al.,2015).
Being a lysosomal degradation pathway,autophagy is essential for maintaining cellular homeostasis(Mizushima,Levine,Cuervo,&Klionsky,2008),and plays crucial roles in removing protein aggregates and organelle debris,as well as providing energy(Mizushima&Komatsu,2011).Defects in autophagy have been related to carcinogenesis,accelerated aging and some other human diseases(Madeo,Zimmermann,Maiuri,&Kroemer,2015).In addition,autophagy is involved in the pathogenesis of kidney disease and its activation can protect the kidney against acute tubular injury(Takabatake,Kimura,Takahashi,&Isaka,2014).In aging mice,the podocyte-specific deletion of autophagy-related protein 5(Atg5)causes glomerulopathy(Hartleben et al.,2010).Meanwhile,autophagy plays an important role in kidney development by regulating the process of podocyte differentiation(Zhang,Li,Wen,&Yang,2017).
In contrast to mammals,reptiles and amphibians,fi sh possess two wt1 paralogs,wt1a and wt1b.In developing zebra fish,both wt1a and wt1b are expressed in the intermediate mesoderm and gradually become restricted to the pronephric field.Finally,both wt1a and wt1b are expressed in the podocytes after completion of pronephros differentiation(Bollig et al.,2006,2009;Serluca&Fishman,2001).Morpholino-based gene knockdown studies indicated that knockdown of wt1a led to the failure of glomerular differentiation and developmental defects in nephrogenesis(Hsu,Lin,&Chung,2003;Perner,Englert,&Bollig,2007).However,the role of wt1b during nephrogenesis is still not clear,since conflicting results have been reported in the studies regarding loss of function due to wt1 deficiency.Morpholino-based gene knockdown of wt1a resulted in developmental defects at later stages of nephrogenesis,such as cysts in the glomeruli region at 2 days post-fertilization(2 dpf)and subsequent pericardial edema at 4 dpf(Perner et al.,2007).Another study suggested that wt1b might play a redundant role with wt1a and be dispensable for normal podocyte development,because the phenotype of dual knockdown of wt1a and wt1b was no more severe than wt1a knockdown alone(Perner et al.,2007;O'Brien et al.,2011;Miceli,Kroeger,&Wingert,2014).
In common with what occurs in mammals,Wt1 deficiency causes podocyte injury and nephrogenesis defects in fish species(Dong et al.,2015;Perner et al.,2007).Due to non-specific effects in previous morpholino-based knockdown studies(Schulte-Merker&Stainier,2014),the exact functions of wt1a and wt1b in fish species still need to be discovered.In the present study,the functions of wt1a and wt1b were investigated using CRISPR/Cas9 system to generate zebra fish mutant lines.Our findings revealed that wt1a is required for normal podocyte development,whereas loss of wt1b function has no effects on podocyte development.We also discovered that autophagy signal cooperates with the wt1a mutant signal to regulate podocyte development.
2.Materials and m ethods
2.1.Zebra fish husbandry
AB strain zebra fish were used in this study and were reared in the fish facility of Huazhong Agricultural University.The maintenance and breeding of zebra fish was performed following standard methods(Kimmel,Ballard,Kimmel,Ullmann,&Schilling,1995;Wester field,2000).All animal experiments were conducted in accordance with the guidelines and approval of the Animal Research and Ethics Committees of Huazhong Agricultural University.
2.2.Establishment of wt1a and wt1b mutant zebra fish
Wt1a and wt1b mutant zebra fish were generated by CRISPR/Cas9 technology.The DNA template preparation,gRNA and Cas9 m RNA in vitro transcription and purification were performed as previously described(Lin et al.,2017).In brief,gRNA target sites were designed with ZIFIT Targeter.A BLAST search was performed in the zebra fish genome to avoid off-target effects,and using the principles reported previously(Cong et al.,2013).In vitro transcription of gRNA was done with a Transcript Aid T7 High Yield Transcription Kit(Thermo)using 1μg purified DNA as the template for 4 h at 37°C.The Cas9 plasmids were linearized with Xba I and purified by ethanol precipitation.Cas9 m RNA was produced by in vitro transcription of 1μg linearized template DNA with a m MESSAGE m MACHINE T7 ULTRA Kit(Ambion)according to the manufacturer's instructions.The Cas9 m RNA(300 ng/μL)and gRNA(20 ng/μL)mixture was microinjected directly into one-cell-stage zebra fish embryos,and the mutants were analyzed by genomic PCRand sequencing as described previously(Yang,Wang,Li,Zhou,&Gui,2017).Primers used in this study are shown in Table 1.Finally,the wt1a-/-homozygous embryos/larvae were identified by genotyping and the number of defective embryos/larvae by genotype recorded.
2.3.Whole mount RNA in situ hybridization and western blot analysis
Embryos at different developmental stages were collected,fixed and pretreated as described(Mei,Zhang,Li,Lin,&Gui,2008).DIG-labeled(Roche,Germany)antisense and sense probes for podocin,nephrin and cdh17 were generated from PCR products,which included a T7 RNA polymerase-binding sequence at the 3′end.In situ hybridization was performed as described previously(Lu et al.,2017;Zhong,Zhou,Li,Wang,&Gui,2014).Western blot analysis was performed as previously reported(Jing et al.,2015).In brief,protein was extracted from 6 dpf wild-type and homozygous zebra fish using the One Step Animal Tissue Active Protein Extraction Kit(Sangon,China).Equal amounts of protein per sample were separated by 10% SDS-PAGE gel electrophoresis and thentransferred to nitrocellulose membranes(NCP,Merck Millipore)using standard protocols(Xiong et al.,2017).After blocking NCP with 5%defatted milk diluted in TBST at room temperature,the membranes were incubated with the primary antibodies,anti-SQSTM1/P62(MBL,PM 045),anti-LC3A/B(Cell Singnaling Technology,4108s)and anti-β-actin(Cell Singnaling Technology,4967s)overnight at 4°C.After washing,the membranes were incubated with HRP-conjugated secondary antibodies,and detection performed using ECL Western Blotting Detection Reagents(GE Healthcare).
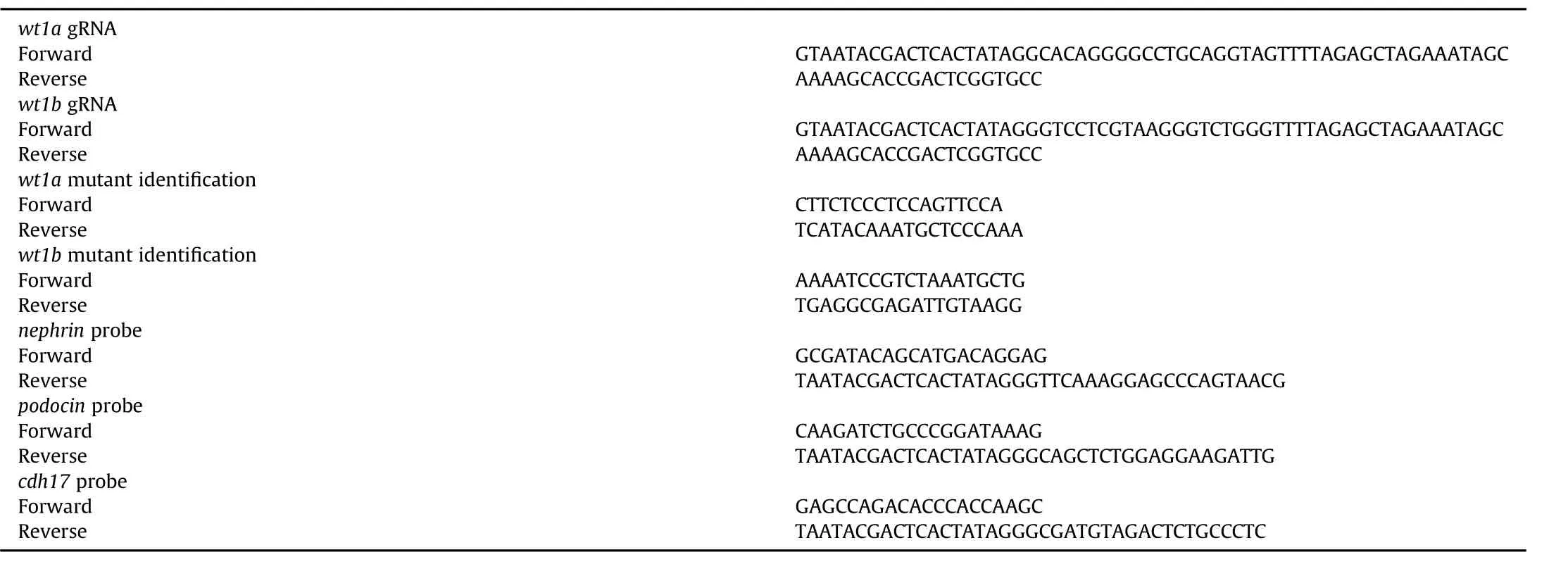
Table 1 Primers used in this study(5′-3′).
2.4.Rapamycin treatment
Rapamycin treatment of zebra fish embryos was performed according to a previous description with minor modifications(Sucularli et al.,2016).Rapamycin(Med Chem Express)was dissolved in an appropriate volume of dimethyl sufloxide(DMSO)solution to prepare a 10 m mol/L stock solution.In brief,the F2-generation of wt1a-mutated embryos at 4 h post-fertilization(hpf)were collected and seeded(30 embryos per well)in 6-well plates containing 4 m L embryo medium with different concentrations of rapamycin,and 0.1%DMSOsolution(the treatment vehicle)was used as the control.The embryos were incubated with rapamycin at 28°Cfor 44 h and fixed with 4%paraformaldehyde in PBS at 4°Covernight for further examination.
2.5.Statistical analysis
Data is presented as mean±SD(standard deviation).Statistical analysis was performed with SPSS software(SPSS Inc.).The data was assessed using a Student's t-test.A probability(P)of<0.05 was considered statistically significant.
3.Results
3.1.Generation of wt1a and wt1b mutants by CRISPR/Cas9
Zebra fish wt1a encodes a protein of 419 amino acids and the Wt1b protein comprises 404 amino acids.Both of these proteins share a high level of sequence identity(Bollig et al.,2006).To investigate the role of wt1a and wt1b in zebra fish development,CRISPR/Cas9 system was used to produce wt1a and wt1b mutant zebra fish.Both wt1a and wt1b contain 9 exons and the targeting sites were chosen in the first exon to disrupt the functional domains(Fig.1A and C).Finally,13-bp deletion(named wt1aΔ13)and 28-bp deletion(named wt1aΔ28)were identified and chosen to establish the wt1a mutant line(Fig.1A).A 7-bp deletion(named wt1bΔ7)and 10-bp insertion(21-bp insertions plus 11-bp deletions,named wt1b+10)were chosen to establish the wt1b mutant line(Fig.1C).The genotypes of WT and all homozygotes were confirmed by genomic sequencing(Fig.1Band D).All mutations in wt1a and wt1b resulted in an open reading frame(ORF)shift that caused the formation of a premature termination codon on the third and first exon,respectively.
3.2.Wt1a,not wt1b is required for zebra fish podocyte development
The wt1a-mutated zebra fish did not have an obvious abnormal phenotype before 3 dpf,when compared with the wild-type.At 3.5 dpf,the homozygous wt1a mutants displayed pericardial edema,and subsequently developed yolk sac edema at 4.5 dpf and general body edema at 5.5 dpf.In the wt1a F2 generation,all the homozygous larvae died before 13 dpf presumably due to the inability to eat,and only heterozygotes and wild-type zebra fish survived to adulthood(Fig.2A).All the homozygous wt1b mutants were normal and as viable as the heterozygotes and wild-type zebra fish during development(Fig.2B).
Embryonic kidney development was examined by in situ hybridization using the podocyte markers podocin and nephrin,which are both specifically expressed in podocytes and essential for pronephros development(Kramer-Zucker,Wiessner,Jensen,&Drummond,2005).Wild-type embryos at 36 and 48 hpf displayed normal podocin and nephrin expression in the pronephric glomeruli(Fig.3A and B),as previously described(O'Brien et al.,2011).Wt1a mutation resulted in complete loss of expression of both marker genes at 36 and 48 hpf(Fig.3A and B).These data indicate the absence of podocytes in wt1a-mutated embryos.However,the expression of cdh17,a marker for the pronephric duct was similar in wt1a-mutated embryos and the wild-type embryos at 36 and 48 hpf(Fig.3C).In wt1b-mutated embryos,both the podocyte markers and pronephric duct marker had a similar expression pattern to the wild-type embryos(Fig.3D),suggesting that wt1b is not required for podocyte differentiation and pronephric duct development.
3.3.Defects of podocyte development in zebra fish wt1a mutants can be partially rescued by activation of autophagy signals
The autophagy signal was evaluated by assessing the protein expression of LC3 and P62(Pankiv et al.,2007).Western blot experiments showed a significant down-regulation of the LC3 protein and up-regulation of the P62 protein in wt1a-mutated embryos relative to the wild-type embryos and indicated that autophagy signaling is reduced in wt1a mutants(Fig.4A).To investigate whether rapamycin,a stimulator of autophagy signals,could rescue the developmental defects in wt1a mutants,different concentrations of rapamycin were used to treat wild-type embryos to get an optimal dose.Results indicated that rapamycin treated wild-type embryos had a larger yolk,less pigmentation and a shorter body size and the effect was concentration-dependent(Fig.4B),as has previously been reported(Sucularli et al.,2016).In situ hybridization of wt1a F2 generation embryos(produced by crossing wt1a+/-males and females)treated with 0.5,1.0,5.0 and 10.0μmol/L rapamycin was performed with the podocyte marker podocin.Statistical analysis revealed that the percentages of rescued embryos were about 5%,29%,28%and 21%in 0.5,1.0,5.0 and 10.0μmol/L rapamycin treated wt1a homozygous mutants,respectively.The number of embryos in each group was greater than 80(Fig.4Cand D).Thus,defective podocyte development in wt1a-mutated embryos was partially rescued by rapamycin treatment and the optimal dose was 1.0μmol/L.This suggests that the induced autophagy signal repaired damaged podocyte development in wt1a mutants.
4.Discussion
The Wilms tumor suppressor gene WT1 is essential for kidney development.In teleosts fish,there are two wt1 co-orthologs(wt1a and wt1b)due to the teleost-specific genome duplication.Morpholino-based knockdown studies in zebra fish showed that wt1a and wt1b control different steps of kidney development and the knockdown of each of them induces defective kidney development(Perner et al.,2007).In this study,the CRISPR/Cas9 system was used to knockout wt1a and wt1b genes in zebra fish and the phenotypes were checked.Interestingly,we found that wt1a is required for kidney development,whereas wt1b might play a redundant role with wt1a and be dispensable for normal podocyte development.

Fig.1.Targeted disruption of zebra fish wt1a and wt1b genes by CRISPR/Cas9.(A and C)Schematic diagram of the genomic structure of zebra fish wt1a and wt1b.Nucleotide sequences of the CRISPR/Cas9 target sites are shown in blue and mutations are shown by dotted red lines.(B and D)Sequencing results of the targeted region from WT and homozygotes.
As described before,WT1 plays a key role in the development of the urogenital system,since the WT1 knockout mouse lack a kidney and adrenal gland(Herzer et al.,1999;Moore et al.,1999).In medaka,either wt1a,wt1b or wt1a/b morphants were embryonic lethal for defective pronephros development,suggesting that both wt1a and wt1b are essential for embryonic pronephros development(Kluver,Herpin,Braasch,Driessle,&Schartl,2009).The wt1a homozygous mutation resulted in kidney defects in zebra fish embryos,similar to the phenotypes in the WT1 mutation mouse and wt1a morphant zebra fish embryos(Moore et al.,1999;Perner et al.,2007).The wt1b morphant zebra fish embryos develop cysts in the glomeruli,tubules and pericardial edema(Perner et al.,2007).However,the wt1b-/-embryos of both zebra fish and tilapia were phenotypically normal(Jiang et al.,2017),suggesting that wt1b may have become redundant relative to wt1a in kidney development during evolution.

Fig.2.Effects of homozygous mutations of wt1a and wt1b on zebra fish larval development.(A and B)Morphology comparisons between wildtype(WT),wt1a and wt1b mutant larvae,respectively.Pericardial edema is marked by red arrows and yolk sac edema by black arrows.
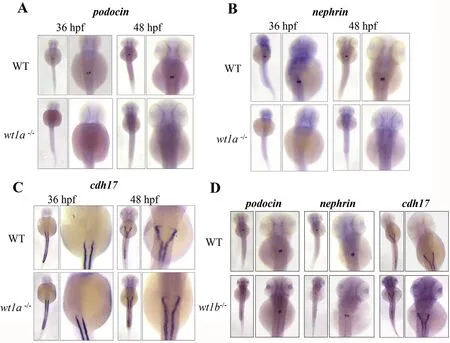
Fig.3.Wt1a deletion impairs podocyte development in zebra fish.RNA in situ hybridization was performed with podocin(A),nephrin(B)and cdh17(C)probes in WT and wt1a mutants at 36 and 48 hpf.(D)In situ hybridization with these markers in WT and wt1b mutants at 48 hpf.
Autophagy is a highly conserved pathway that plays important roles in organ development and maintenance of cellular function.Abnormal autophagy may lead to cell death and human diseases,such as defective renal development including the specification of podocytes and proximal tubular cells(Mizushima&Komatsu,2011;Mizushima et al.,2008).Dysfunction of podocyte autophagy is a key event that is responsible for the progression of diabetic nephropathy(Liu,Zhang,Wang,&Zeng,2017).Autophagy has been revealed as a novel therapeutic target for diabetic nephropathy.For example,rapamycin,a stimulator of the autophagy signal,rescued podocyte injury in the nephritis model rats,in which autophagy was inhibited(Kume&Koya,2015;Wu et al.,2013).In the current study,the autophagy signal was inhibited in the wt1a mutant zebra fish and podocyte differentiation was impaired as shown by the loss of expression of the podocyte differentiation maker podocin.Interestingly,rapamycin treatment could partially rescue the defects in podocyte development.
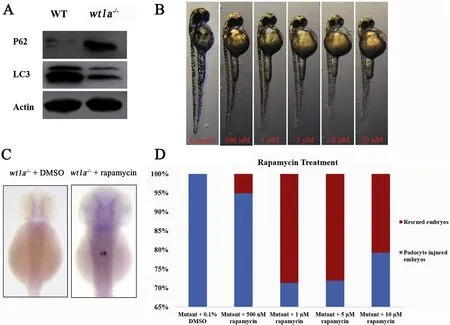
Fig.4.Podocyte injury can be partially rescued by rapamycin treatment in wt1a mutants.(A)Western blot analysis with anti-SQSTM1/P62 and anti-LC3A/B to examine the autophagy signaling.(B)Effects of rapamycin on wild-type embryo development.(C)In situ hybridization with podocin in the wt1a-/-embryos(right)and rapamycin treated wt1a-/-embryos(left)at 48 hpf.(D)Statistical analysis of the percentage of rescued embryos in wt1a homozygous mutants treated with different doses of rapamycin.
In conclusion,wt1a plays an essential role in kidney development depending on the precise regulation of autophagy pathway,while wt1b is not essential for the kidney development.Besides the effects on the autophagy pathway in wt1a mutant zebra fish,other molecules or signaling pathways involved in the defective phenotypes need to be further investigated.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities(2662017PY013)and the National Natural Science Foundation of China(31672635).The funders had no role in study design,data collection and analysis,decision to publish,or preparation of the manuscript.
Armstrong,J.F.,Pritchard-Jones,K.,Bickmore,W.A.,Hastie,N.D.,&Bard,J.B.(1993).The expression of the Wilms'tumour gene,WT1,in the developing mammalian embryo.Mechanisms of Development,40,85-97.
Barbaux,S.,Niaudet,P.,Gubler,M.C.,Grunfeld,J.P.,Jaubert,F.,Kuttenn,F.,et al.(1997).Donor splice-site mutations in WT1 are responsible for Frasier syndrome.Nature Genetics,17,467-470.
Bollig,F.,Mehringer,R.,Perner,B.,Hartung,C.,Schafer,M.,Schartl,M.,et al.(2006).Identification and comparative expression analysis of a second wt1 gene in zebra fish.Developmental Dynamics,235,554-561.
Bollig,F.,Perner,B.,Besenbeck,B.,Kothe,S.,Ebert,C.,Taudien,S.,et al.(2009).A highly conserved retinoic acid responsive element controls wt1a expression in the zebra fish pronephros.Development,136,2883-2892.
Call,K.M.,Glaser,T.,Ito,C.Y.,Buckler,A.J.,Pelletier,J.,Haber,D.A.,et al.(1990).Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms'tumor locus.Cell,60,509-520.
Chau,Y.Y.,&Hastie,N.D.(2012).The role of Wt1 in regulating mesenchyme in cancer,development,and tissue homeostasis.Trends in Genetics,28,515-524.
Cong,L.,Ran,F.A.,Cox,D.,Lin,S.,Barretto,R.,Habib,N.,et al.(2013).Multiplex genome engineering using CRISPR/Cas systems.Science,339,819-823.
Dong,L.,Pietsch,S.,Tan,Z.,Perner,B.,Sierig,R.,Kruspe,D.,et al.(2015).Integration of cistromic and transcriptomic analyses identifies Nphs2,mafb,and Magi2 as Wilms'tumor 1 target genes in podocyte differentiation and maintenance.Journal of the American Society of Nephrology,26,2118-2128.
Gessler,M.,Poustka,A.,Cavenee,W.,Neve,R.L.,Orkin,S.H.,&Bruns,G.A.(1990).Homozygous deletion in Wilms tumours of a zinc-fi nger gene identified by chromosome jumping.Nature,343,774-778.
Hartleben,B.,Godel,M.,Meyer-Schwesinger,C.,Liu,S.,Ulrich,T.,Kobler,S.,et al.(2010).Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice.Journal of Clinical Investigation,120,1084-1096.
Herzer,U.,Crocoll,A.,Barton,D.,Howells,N.,&Englert,C.(1999).The Wilms tumor suppressor gene wt1 is required for development of the spleen.Current Biology,9,837-840.
Hsu,H.J.,Lin,G.,&Chung,B.C.(2003).Parallel early development of zebra fish interrenal glands and pronephros:Differential control by wt1 and ff1b.Development,130,2107-2116.
Jiang,D.,Chen,J.,Fan,Z.,Tan,D.,Zhao,J.,Shi,H.,et al.(2017).CRISPR/Cas9-induced disruption of wt1a and wt1b reveals their different roles in kidney and gonad development in Nile tilapia.Developmental Biology,428,63-73.
Jing,J.,Xiong,S.,Li,Z.,Wu,J.,Zhou,L.,Gui,J.F.,et al.(2015).A feedback regulatory loop involving p53/miR-200 and growth hormone endocrine axis controls embryo size of zebra fish.Scientific Reports,5,15906.
Kimmel,C.B.,Ballard,W.W.,Kimmel,S.R.,Ullmann,B.,&Schilling,T.F.(1995).Stages of embryonic development of the zebra fish.Developmental Dynamics,203,253-310.
Kluver,N.,Herpin,A.,Braasch,I.,Driessle,J.,&Schartl,M.(2009).Regulatory backup circuit of medaka Wt1 co-orthologs ensures PGC maintenance.Developmental Biology,325,179-188.
Kramer-Zucker,A.G.,Wiessner,S.,Jensen,A.M.,&Drummond,I.A.(2005).Organization of the pronephric filtration apparatus in zebra fish requires Nephrin,Podocin and the FERM domain protein Mosaic eyes.Developmental Biology,285,316-329.
Kume,S.,&Koya,D.(2015).Autophagy:A novel therapeutic target for diabetic nephropathy.Diabetes&Metabolism J,39,451-460.
Lin,Q.,Mei,J.,Li,Z.,Zhang,X.,Zhou,L.,&Gui,J.F.(2017).Distinct and cooperative roles of amh and dmrt1 in self-renewal and differentiation of male germ cells in zebra fish.Genetics,207,1007-1022.
Liu,Y.,Zhang,J.,Wang,Y.,&Zeng,X.(2017).Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes.Cell Death&Disease,8,e3006.
Lu,C.,Wu,J.,Xiong,S.,Zhang,X.,Zhang,J.,&Mei,J.(2017).MicroRNA-203a regulates fast muscle differentiation by targeting dmrt2a in zebra fish embryos.Gene,625,49-54.
Madeo,F.,Zimmermann,A.,Maiuri,M.C.,&Kroemer,G.(2015).Essential role for autophagy in life span extension.Journal of Clinical Investigation,125,85-93.
Mei,J.,Zhang,Q.Y.,Li,Z.,Lin,S.,&Gui,J.F.(2008).C1q-like inhibits p53-mediated apoptosis and controls normal hematopoiesis during zebra fish embryogenesis.Developmental Biology,319,273-284.
Miceli,R.,Kroeger,P.,&Wingert,R.(2014).Molecular mechanisms of podocyte development revealed by zebra fish kidney Research.Cell&Developmental Biology,3,1000138.
Mizushima,N.,&Komatsu,M.(2011).Autophagy:Renovation of cells and tissues.Cell,147,728-741.
Mizushima,N.,Levine,B.,Cuervo,A.M.,&Klionsky,D.J.(2008).Autophagy fights disease through cellular self-digestion.Nature,451,1069-1075.
Moore,A.W.,McInnes,L.,Kreidberg,J.,Hastie,N.D.,&Schedl,A.(1999).YAC complementation shows a requirement for Wt1 in the development of epicardium,adrenal gland and throughout nephrogenesis.Development,126,1845-1857.
O'Brien,L.L.,Grimaldi,M.,Kostun,Z.,Wingert,R.A.,Selleck,R.,&Davidson,A.J.(2011).Wt1a,Foxc1a,and the Notch mediator Rbpj physically interact and regulate the formation of podocytes in zebra fish.Developmental Biology,358,318-330.
Pankiv,S.,Clausen,T.H.,Lamark,T.,Brech,A.,Bruun,J.A.,Outzen,H.,et al.(2007).p62/SQSTM 1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy.Journal of Biological Chemistry,282,24131-24145.
Perner,B.,Englert,C.,&Bollig,F.(2007).The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebra fish pronephros.Developmental Biology,309,87-96.
Schulte-Merker,S.,&Stainier,D.Y.(2014).Out with the old,in with the new:Reassessing morpholino knockdowns in light of genome editing technology.Development,141,3103-3104.
Serluca,F.C.,&Fishman,M.C.(2001).Pre-pattern in the pronephric kidney field of zebra fish.Development,128,2233-2241.
Sucularli,C.,Shehwana,H.,Kuscu,C.,Dungul,D.C.,Ozdag,H.,&Konu,O.(2016).Functionally conserved effects of rapamycin exposure on zebra fish.Molecular Medicine Reports,13,4421-4430.
Takabatake,Y.,Kimura,T.,Takahashi,A.,&Isaka,Y.(2014).Autophagy and the kidney:Health and disease.Nephrology Dialysis Transplantation,29,1639-1647.
Wagner,K.D.,Wagner,N.,Vidal,V.P.I.,Schley,G.,Wilhelm,D.,Schedl,A.,et al.(2002).The Wilms'tumor gene Wt1 is required for normal development of the retina.The EMBO Journal,21,1398-1405.
Wester field,M.(2000).The zebra fish book.A guide for the laboratory use of zebra fish(Danio rerio).Zebra fish Book A Guide for the Laboratory Use of Zebra fish.
Wu,L.,Feng,Z.,Cui,S.,Hou,K.,Tang,L.,Zhou,J.,et al.(2013).Rapamycin upregulates autophagy by inhibiting the mTOR-ULK1 pathway,resulting in reduced podocyte injury.PLoSOne,8,e63799.
Xiong,S.,Mei,J.,Huang,P.,Jing,J.,Li,Z.,Kang,J.,et al.(2017).Essential roles of stat5.1/stat5b in controlling fish somatic grow th.Journal of Genetics and Genomics,44,577-585.
Yang,Y.J.,Wang,Y.,Li,Z.,Zhou,L.,&Gui,J.F.(2017).Sequential,divergent,and cooperative requirements of Foxl2a and Foxl2b in ovary development and maintenance of zebra fish.Genetics,205,1551-1572.
Zhang,C.Y.,Li,W.,Wen,J.K.,&Yang,Z.(2017).Autophagy is involved in mouse kidney development and podocyte differentiation regulated by Notch signalling.Journal of Cellular and Molecular Medicine,21,1315-1328.
Zhong,J.X.,Zhou,L.,Li,Z.,Wang,Y.,&Gui,J.F.(2014).Zebra fish Noxa promotes mitosis in early embryonic development and regulates apoptosis in subsequent embryogenesis.Cell Death&Differentiation,21,1013-1024.
杂志排行
Aquaculture and Fisheries的其它文章
- Temperature stress response of heat shock protein 90(Hsp90)in the clam Paphia undulata☆
- Variation of lipids and fatty acids composition in the tissues of wild devil stinger(Inimicus japonicas)during sexual maturation
- Evaluating the bioavailability of heavy metals in natural-zeoliteamended aquatic sediments using thin-fi lm diffusive gradients
- Abundance and biomass of assorted small indigenous fish species:Observations from rural fish markets of West Bengal,India
- Fish kidney cells show higher tolerance to hyperosmolality than amphibian
