NaClO、UV及UV/NaClO消毒过程中TCC的去除特性及遗传毒性
2018-05-26陆保松马晓雁李青松骆靖宇沈奇奇廖文超陈国元李国新
陆保松,马晓雁,李青松,骆靖宇,沈奇奇,廖 杰,廖文超,陈国元,李国新
NaClO、UV及UV/NaClO消毒过程中TCC的去除特性及遗传毒性
陆保松1,2,马晓雁1,李青松2*,骆靖宇3,沈奇奇4,廖 杰2,廖文超2,陈国元2,李国新2
(1.浙江工业大学建筑工程学院,浙江 杭州 310014;2.厦门理工学院水资源环境研究所,福建 厦门 361005;3.苏州科技大学环境科学与工程学院,江苏 苏州 215009;4.浙江海洋大学,国家海洋设施养殖工程技术与研究中心,浙江 舟山 316000)
采用NaClO、UV和UV/NaClO复合消毒等方式研究了三氯卡班(TCC)在消毒过程中的去除特性,考察了3种消毒方式中TCC溶液的遗传毒性变化,鉴定了TCC的降解产物并探讨了其降解机制,以UV/NaClO复合消毒为研究对象,考察了NaClO投加量、TCC初始浓度、溶液pH值和腐殖酸(HA)等因素对TCC去除的影响.结果表明,3种消毒技术对TCC的去除效果依次为UV/NaClO、UV、NaClO.消毒处理不同程度增加了TCC溶液的遗传毒性.LC-MS鉴定出了8种TCC的降解产物,降解途径主要为脱氯、加氯以及·OH/O·氧化.UV/NaClO复合消毒对TCC的去除率在97%以上;TCC的去除与其初始浓度呈负相关;TCC的去除率随pH值的增大先升高后降低;低浓度的腐殖酸(HA)对TCC的去除有促进作用,高浓度则相反.
三氯卡班;消毒;去除;产物;遗传毒性
三氯卡班(TCC)作为一种广谱抗菌剂,自1957年以来便被广泛应用于洗发水、肥皂和牙膏等日用品中[1-3].近年来它已成为水体中检出率较高的有机污染物之一[4-6].研究表明,TCC可以干扰哺乳动物的繁殖,引起人类高铁血红蛋白血症[7-10].由于其潜在的健康风险,目前TCC已成为人们关注的污染物之一[11].
净水工艺中消毒对于供水水质安全起着至关重要的作用[12]. UV、NaClO和UV/NaClO复合消毒在保障饮用水生物安全的同时对水体中的微量有机污染物有一定的去除[13-15],研究表明氯消毒过程往往伴随有毒有害副产物的生成[16-17].目前,TCC在UV、NaClO和UV/NaClO复合消毒中的去除尚未见报道,因此研究消毒过程中TCC的去除特性具有重要意义.
实验研究了TCC在UV、NaClO和UV/NaClO复合消毒等方式中的去除特性,对比了消毒后溶液的遗传毒性变化,鉴别了TCC降解产物并推测了降解途径,以UV/NaClO复合消毒技术为研究对象,探讨了NaClO投加量、TCC初始浓度、溶液pH值和腐殖酸(HA)等因素对TCC去除的影响,有助于为消毒工艺在实际水治理工程设计中的应用提供基础实验数据.
1 实验部分
1.1 试剂与仪器
三氯卡班(TCC)(德国Dr.Ehrenstorfer公司,纯度>99.5%);腐殖酸(HA)(Tech,美国Sigma- Aldrich);NaS2O3·5H2O、HCl、NaOH 均为分析纯;甲醇、乙腈(HPLC 级,德国Merck);三氯甲烷(AR); 4-硝基喹啉-1-氧化物(4-nitroquinoline- 1-oxide,4-NQO);十二烷基磺酸钠(Sodium laurylsulfonate,SDS,³95.0%);邻硝基苯β-D-半乳吡喃糖苷(o-nitrophenol-β-D-galactopyranoside, ONPG,东京化成);二甲基亚砜(Dimethyl sulfoxide,DMSO,ACS级,美国Alfa Aesar);2-巯基乙醇(AR,³99.0%);Tryptone(OXIOD);次氯酸钠(CP,活性氯³5.2%),ExTab试剂(ExStik,上海三信仪表厂);丙酮(LC,³99.5%);96孔酶标板(美国,Thermo Fisher Scientific);实验室用水均为Mili-Q 超纯水(£18.2MΩ ).
LC-20A 高效液相色谱仪(Shimadzu,日本),自动进样(SIL-20A),检测器(SPDM20-A);液质联用制备系统(配有2767样品管理器, 515色谱泵,2489紫外可见检测器,3100质谱检测器(Waters,美国);pH计(Eutevch,美国);CL200防水型笔式余氯计(ExStik,上海三信仪表厂);H-J6A 型磁力恒温搅拌器(江苏金坛峥嵘仪器);HLB SPE柱(500mg,6mL,CNW);紫外线光源(主波长254nm,杨紫特种紫外线光源,低压汞灯,20W);紫外线强度计(TN-2365A,台湾泰纳);酶标仪(美国,Spectra Max);恒温振荡器(德国,IKA).
1.2 实验方法
1.2.1 烧杯实验 反应装置见图1,烧杯中紫外灯功率为20W,波长为254nm,杯壁a处裹有锡箔纸以隔绝外界光照,a处的光强为125μW/cm2.紫外灯管外为石英玻璃套管.实验开启前加入一定量的TCC标准溶液(除TCC初始浓度影响因素和富集外,浓度均为350μg/L),启动磁力搅拌器.紫外灯在实验反应前预热30min左右让光强达到125μW/cm2,NaClO投加量为1mg/L,设定时间内取样,水样经硫代硫酸钠淬灭后过0.45μm的玻璃纤维滤膜后进样分析.
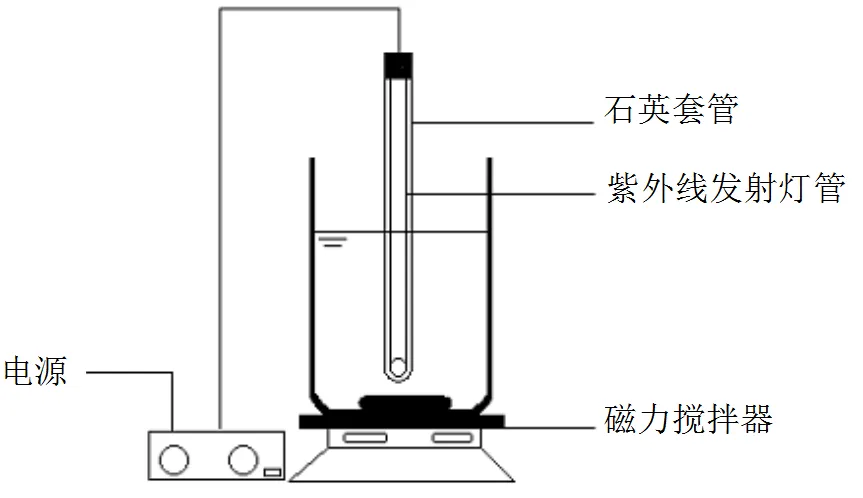
图1 实验装置示意
1.2.2 水样富集 用HLB柱固相萃取1L TCC反应溶液(SOS/umu和液质实验中的初始浓度分别为5mg/L和1mg/L,乙腈助溶),小柱依次经等体积(SOS/umu实验6mL,液质实验3mL)的甲醇与水活化后上样,采用丙酮(SOS/umu实验8mL,液质实验4mL)洗脱后经氮气吹干,然后经DMSO(SOS/umu实验200µL)和甲醇(液质实验500µL)定容后分别进行SOS/umu测试和液质分析.
1.2.3 SOS/umu实验 选择鼠伤寒沙门氏菌TA1535/1002为实验菌种,该菌种由1002质粒导入TA1535菌株而成.实验方法基本建立在Reifferscheid等[18]建立的微孔板方法基础上,具体步骤见参考文献.β-半乳糖甘酶诱导活性(β-Galactosidase Activity)计算如下:
β-Galactosidase Activity(Units/OD600)=
1000×(Abs415-1.75×Abs570)/(××Abs595) (1)
式中:为加入ONPG后的反应时间,本文中为20min;为反应菌液在显色过程中的稀释倍率,本文中为0.09;Abs595、Abs415、Abs570均为吸光度值.
本文中的β-Galactosidase Activity(unit/ OD600)值为原始计算值减去阴性空白,实验中各样品设3个平行样,实验数据的重复测定次数均为2次,取平均值.
1.3 分析方法
实验中采用HPLC和LC-MS对TCC及其降解产物进行检测分析.
HPLC色谱:色谱柱为Inertsil®ODS SP (250mm´4.6mm,5μm);流动相为乙腈:水=65:35 (:),流动相流速为1.0mL/min;检测波长为265nm;进样体积为10μL;S/N>3.
质谱条件:电喷雾离子源,负离子全扫描模式,脱溶剂温度350℃,离子源温度120℃,脱溶剂气流量500L/h,锥孔气流量50L/h,毛细管电压3000v,进样量20µL.
2 结果与分析
2.1 UV、NaClO和UV/NaClO复合消毒中 TCC的去除考察了UV、NaClO和UV/NaClO消毒中TCC的去除效果,结果见图2.
由图2可知, NaClO接触60min后TCC的去除率仅为10.20%,UV对TCC的去除率增加为80.17%;相同时间内UV/NaClO复合消毒对TCC的去除率可达到97.73%.
UV对TCC的去除主要是UV辐照产生的羟基自由基·OH参与反应[19-20].NaClO对TCC的去除主要是靠NaClO的氧化作用和其分解出的O·的强氧化性[21-22]. NaClO在UV辐照下会促使溶液中的HClO/ClO-发生光分解反应,生成·OH、Cl·、Cl2·-、ClO·等强氧化自由基,主要反应式如下[23]:
HClO + hv→·OH+ Cl·(2)
·OH + HClO→ClO·+ H2O (3)
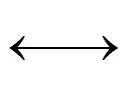
Cl2O2+ H2O→ClO2-+ OCl-+ 2H+(5)
Cl2O2+ H2O→Cl-+ O2+ OCl-+ 2H+(6)
Cl2O2→Cl2+ O2(7)
OCl-+ hv→O·-+ Cl·(8)

OCl-+ hv→Cl-+ O(3P) (10)

O(3P) + OCl→Cl-+ O2(12)
·OH+ OCl →ClO·+ OH-(13)
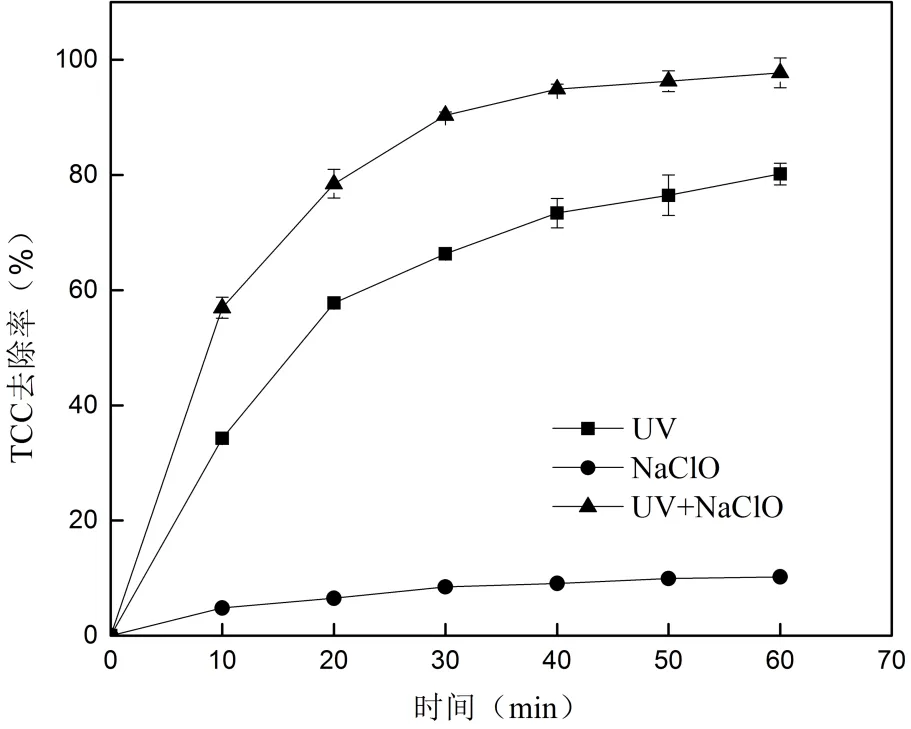
图2 UV、NaClO和UV/NaClO中TCC的去除
复合消毒溶液中的Cl·、Cl2·-和·OH等有较高的氧化还原电位[24-26],可通过单电子氧化、抽H或C—C不饱和键加成等反应有效去除芳香族、酚类和苯胺等有机化合物[26-28].
2.2 消毒过程中TCC的降解产物分析
对TCC的产物进行鉴定识别,结果如表1所示.
在经UV、NaClO、UV/NaClO消毒的溶液中分别鉴定出了Formamide,N-(4,5- dichlorophen-yl)-、Formamide,N-(4-hydrox- yphenyl)-等8种产物,据此,推测的可能反应路径见图3.
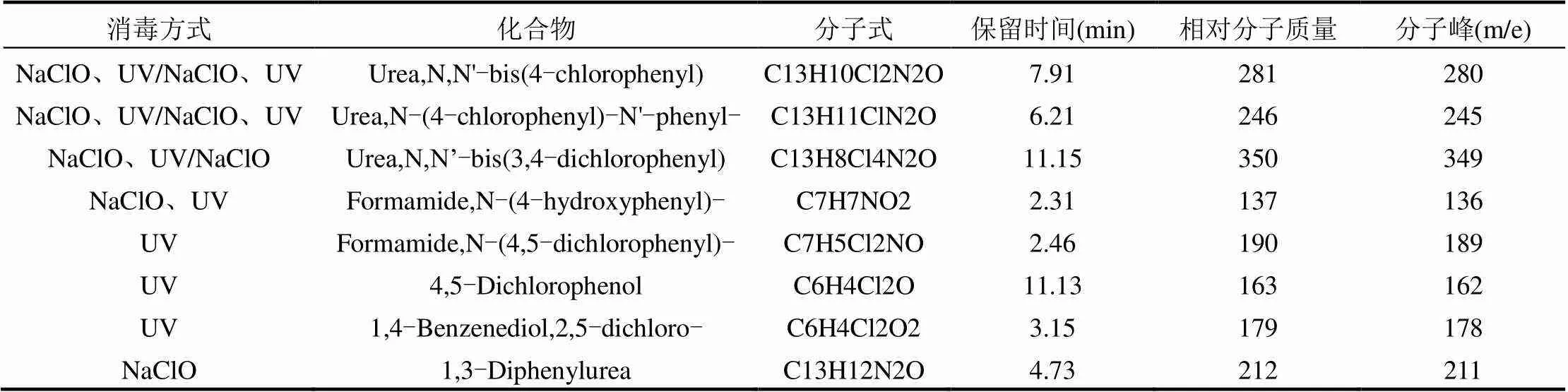
表1 TCC主要产物的质谱信息
如图3所示,推测TCC的降解途径主要有:(Ⅰ)·OH/O·氧化C—N键形成Formamide, N-(4,5-dichlorophen-yl)-,该产物脱落一个氯同时另一个氯被氧化生成Formamide,N-(4- hydrox-yphenyl)-,前者可能通过UV消毒产生,后者可能分别通过UV和NaClO消毒产生;(Ⅱ)TCC脱氯分别形成Urea,N,-N'-bis(4- chlorophenyl)、Urea,N-(4-chlorophe-nyl)-N'- phenyl-和1,3-Diphenylurea, UV、NaClO和UV/NaClO消毒均可能生成前两种产物,最后一种产物可能通过NaClO消毒生成;(Ⅲ)TCC加氯生成Urea,N, N'-bis(3,4-dichlorophenyl), UV/ NaClO和NaClO可生成该产物;(Ⅳ)·OH氧化TCC左侧C—N键形成4,5-Dichlorophenol,该产物在Cl·,·OH的继续作用下形成1,4-Benzenediol, 2,5-dichloro-,两种物质可能均由UV消毒生成.另有研究表明TCC分解的中间产物可能会苯环开环生成CO2、H2O等[29-32].
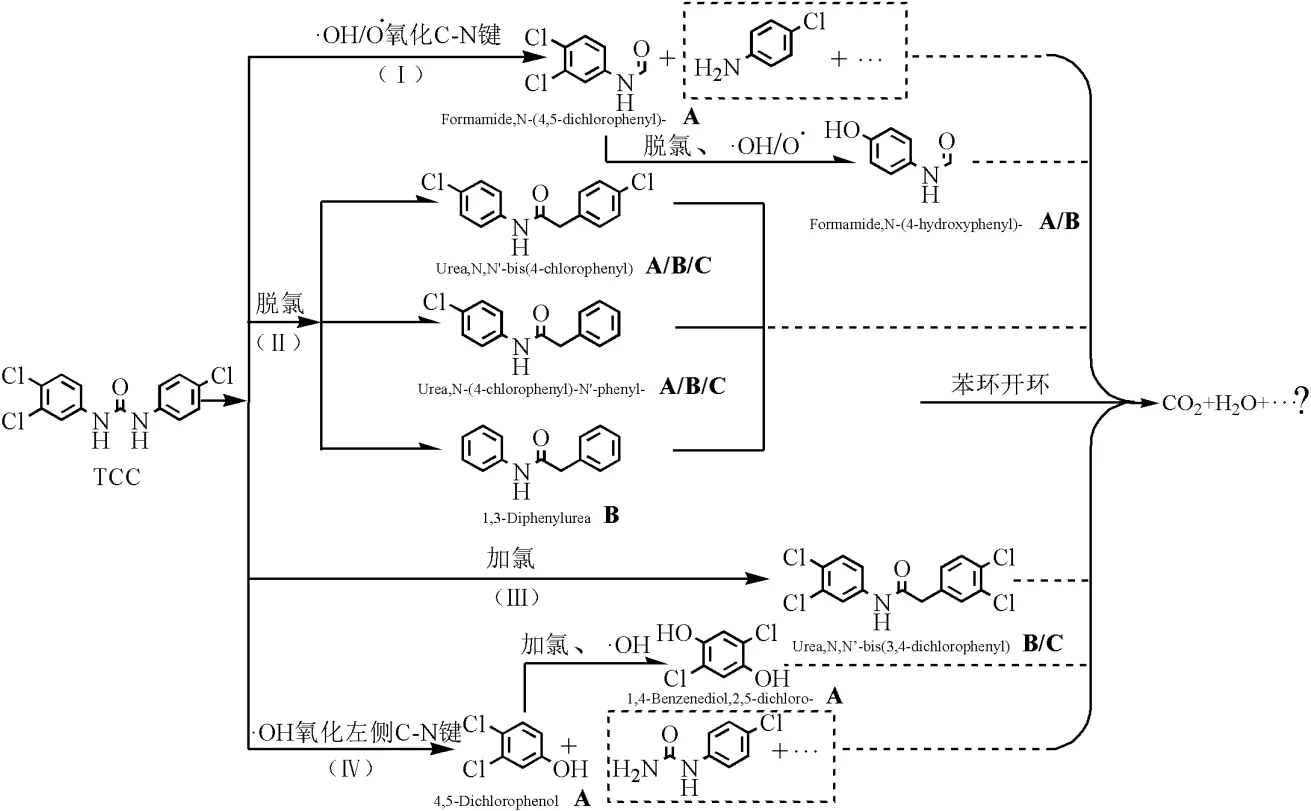
图3 TCC降解的可能途径
A:产物可能由UV消毒产生, B:产物可能由NaClO消毒产生, C:产物可能由UV/NaClO消毒产生
2.3 UV、NaClO和UV/NaClO消毒对TCC溶液遗传毒性的影响
TCC溶液经UV、NaClO和UV/NaClO消毒处理后遗传毒性诱导效果如图4所示.
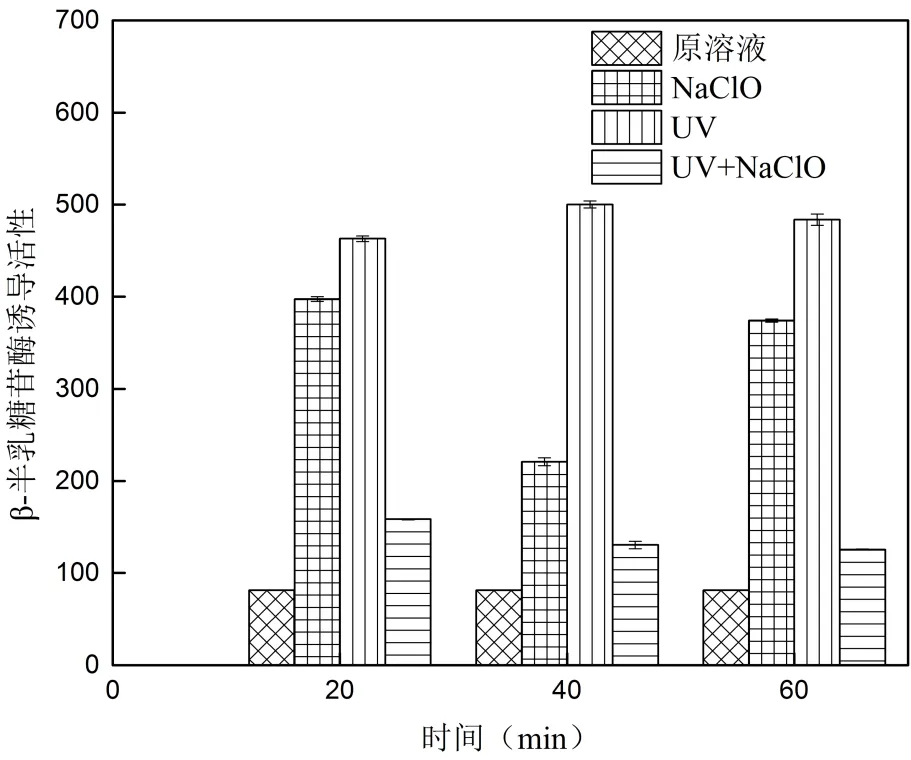
图4 消毒方式对遗传毒性诱导活性的影响
从图4中可看出,20、40和60min时NaClO消毒溶液的诱导酶含量分别为398、221和374;UV消毒溶液的诱导酶含量先是由463上升到500再降低为484;UV/NaClO复合消毒溶液的诱导酶含量呈逐步下降趋势,从158降低为131,然后继续降低为125.反应过程中消毒溶液的诱导酶含量均大于原溶液,实验表明消毒增加了溶液的遗传毒性,且:UV>NaClO>UV/ NaClO>原溶液.
不同消毒处理时TCC溶液遗传毒性变化的原因可能是: NaClO消毒时生成的中间产物增加了溶液的遗传毒性,随中间产物的继续降解,遗传毒性相应降低, 60min时溶液中可能生成了新的产物导致其遗传毒性增大;UV消毒可能会引起TCC光解生成有毒产物进而引起遗传毒性上升,60min时遗传毒性出现减弱可能是部分有毒的中间产物被紫外光进一步降解所致;UV/ NaClO溶液的遗传毒性逐步降低,这可能是因为复合工艺产生的Cl·、Cl2·-等[23]氧化自由基降解了部分有毒的中间产物生成遗传毒性更小的小分子有机物,从而遗传毒性逐渐降低.Jin等[33]用SOS/umu测定含氨基比林饮用水经氯消毒前后的遗传毒性变化呈相似的变化趋势.
2.4 UV/NaClO复合消毒对TCC去除的影响因素
在3种消毒方式中,UV/NaClO复合消毒可以有效去除TCC,且相比UV和NaClO消毒遗传毒性较低,因此选用UV/NaClO复合消毒研究了TCC去除的影响因素.
2.4.1 NaClO对TCC去除的影响 投加不同量的NaCIO,考察NaCIO投加量对TCC去除的影响.结果见图5.
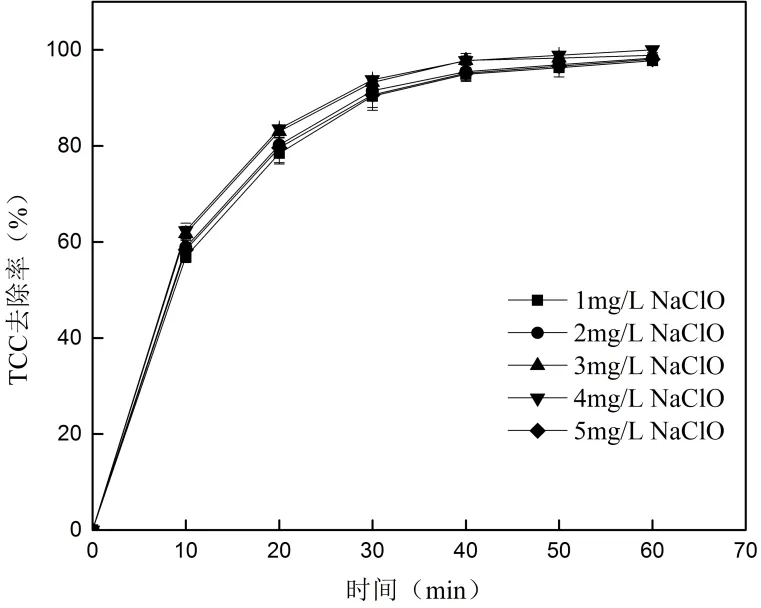
图5 NaCIO投加量对TCC去除的影响
如图5所示,NaClO投加量为1、2、3、4和5mg/L时,60min的TCC去除率分别为97.73%、98.30%、98.87%、100%和98.02%.可以看出TCC的去除随NaClO投加量的增大先上升后降低,但变化不显著.
反应溶液中Cl·、Cl2·-等活性自由基含量随NaClO浓度的增加而升高[23],因此TCC的去除随NaClO浓度(1、2、3、4mg/L)的增加而增大.相同UV辐照条件下,NaClO 溶液接收的光子数量一定[24],当NaClO投加量大于4mg/L时,根据式(12)和(13)溶液中的HClO与强氧化性活性物质的副反应可能逐渐加强,导致·OH和ClO·活性自由基含量下降从而降低了TCC的去除.
2.4.2 腐殖酸对TCC去除的影响 腐殖酸( HA)是天然水体中有机物的主要组成之一[34-35],因此实验中投加不同量的HA,考察HA对TCC去除的影响,结果见图6.
由图6可知,当HA在反应液中的浓度分别为1、3、5、7和9mg/L时,60min的TCC去除率分别为100%、98.56%、92.51%、89.91%和86.74%.TCC的去除随HA投加量的增加持续下降且低浓度的HA对TCC的去除产生促进作用.去除持续下降的结果和Westerhopp等[36]的研究相同,因为HA分子中含有酚羟基、胺基、羧基等活性基团,会消耗溶液中的活性自由基,所以对TCC的去除产生抑制作用,而且HA会增加溶液的色度从而抑制了UV的辐照效果[37].实验中低浓度的HA对去除有促进作用的原因可能是因为紫外辐照下HA会增加·OH的产生[38],产生·OH的量大于自身活性基团消耗·OH的量时将促进去除的进行.
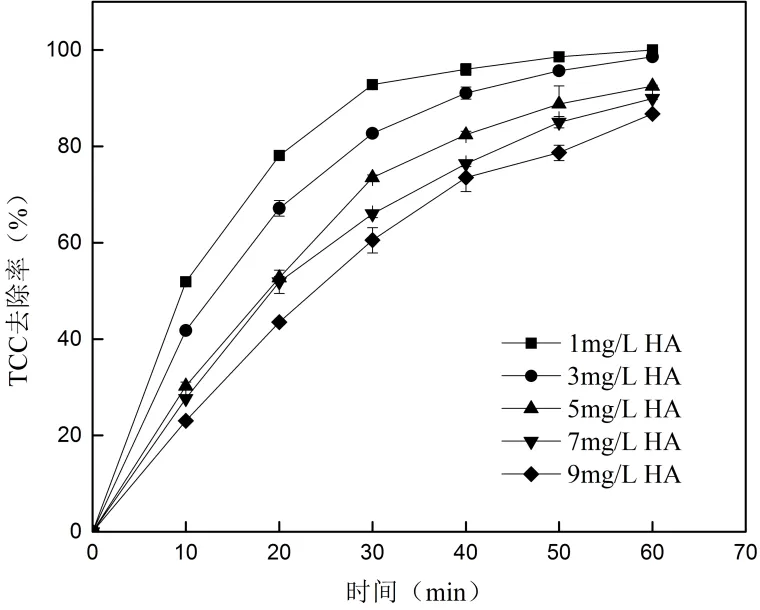
图6 HA对TCC去除的影响
2.4.3 pH值对TCC去除的影响 用盐酸和氢氧化钠调节溶液的pH值,考察pH值对TCC去除的影响,结果见图7.
从图7中可以看出,溶液的pH值为3、5、7、9和11时,60min时TCC的去除率分别为91.71%、94.86%、97.71%、100%和98.86%.pH值在3~9范围内,TCC的去除率逐渐升高;pH值在9~11阶段,TCC的去除率有所降低,pH值对TCC去除的影响不大.
TCC是一种碱性物质(pKa=12.7)[39],酸性条件下的质子化作用降低了TCC分子上氨基基团的活性[40].同时,根据Wang等[41]用紫外/氯降解卡马西平的研究,氯溶液中紫外光的吸收率随pH值(5.5~9.5)的上升逐渐增大导致溶液中氧化自由基含量增多.所以TCC的去除率随pH值(3~9)的增加逐渐升高.然而,pH值升到9以后溶液中的OH-会相应增多,根据方程(11)可以得出溶液中的·OH会对应减少,导致TCC的去除会有所降低.
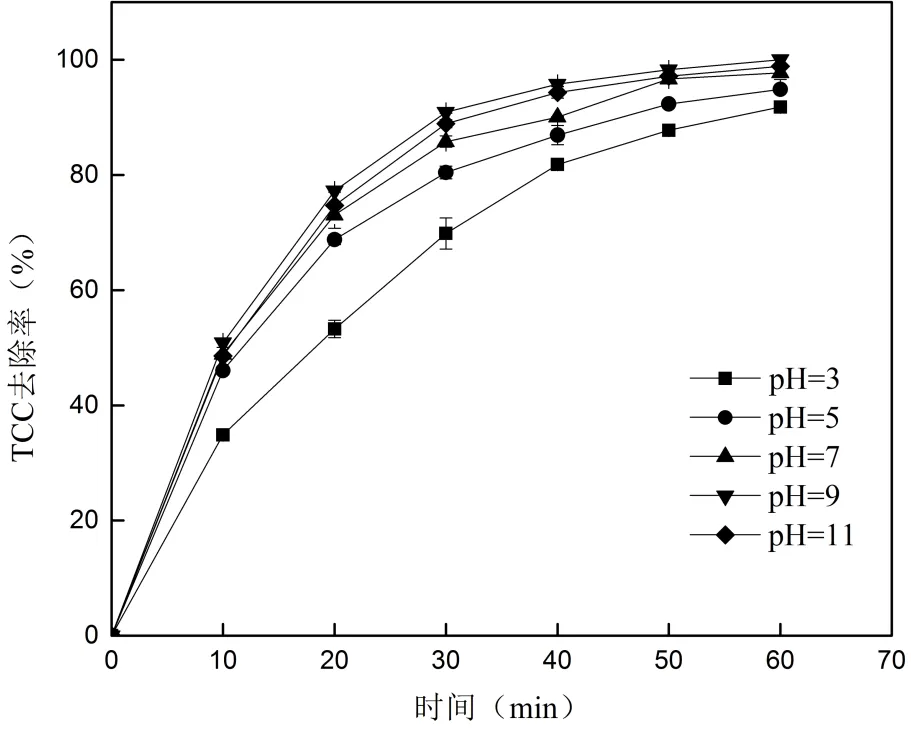
图7 pH值对TCC去除的影响
2.4.4 初始浓度对TCC去除的影响 改变溶液的初始浓度,考察TCC初始浓度对其去除的影响,结果见图8.
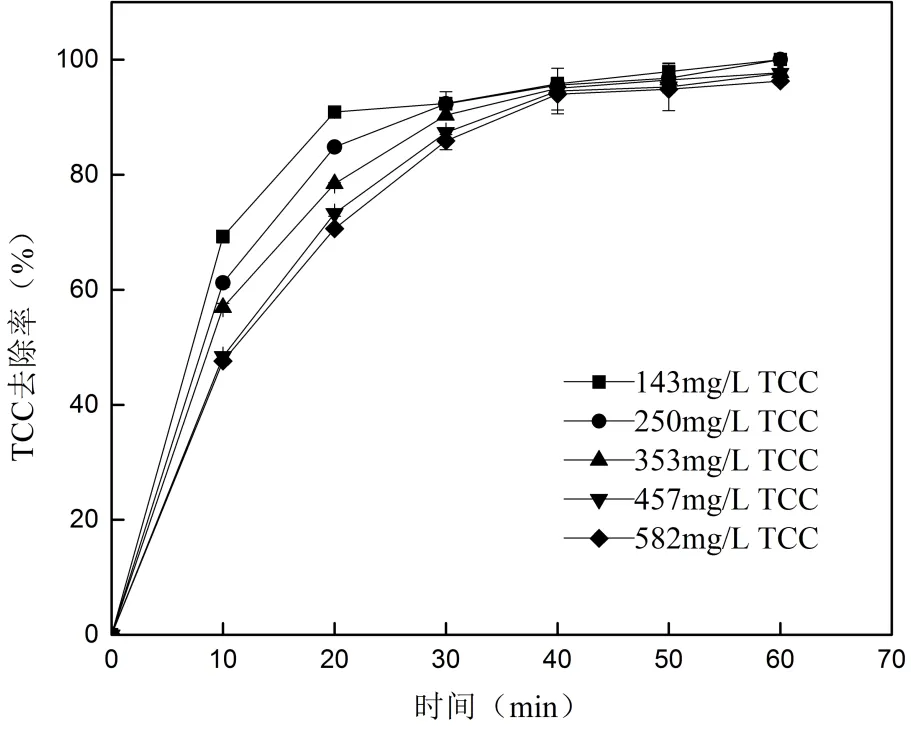
图8 初始浓度对TCC去除的影响
由图8可知,60min时TCC浓度在143、250、353、457与582mg/L时的去除率分别为100%、100%、97.73%、97.62%和96.31%.TCC的去除随初始浓度的增大而降低,较高浓度的TCC需要较长时间才能达到相同的去除效果.
这可能是因为UV辐照时间和NaCIO投加量不变时,单位TCC与氧化物质反应的几率随TCC初始浓度的增加而降低,从而TCC浓度越高去除率越低,这与李玉瑛等[42]研究UV/ClO2去除三氯生的规律相似.另一个原因可能是较高浓度的TCC会生成较多的中间产物,这些中间产物会消耗一定量的氧化自由基,进而降低了TCC的去除.
3 结论
3.1 TCC在NaClO、UV和UV/NaClO 3种消毒方式中均可被不同程度地去除,60min时TCC的去除率分别为10.20%、80.17%和97.73%.
3.2 不同方式消毒后溶液的遗传毒性有所增加.TCC溶液经3种消毒处理后的遗传毒性大小为:UV>NaClO>UV/NaClO>原溶液.
3.3 TCC的主要降解途径为:(Ⅰ) TCC的C-N键被氧化形成Formamide,N-(4,5-dic- hlorophenyl)-,产物脱落一个氯的同时另一个氯被氧化生成Formamide,N-(4-hydroxyphenyl)-; (Ⅱ)TCC脱氯分别形成Urea,N,-N'-bis(4- chlorophenyl)、Urea,N-(4-chlorophenyl)-N'- phenyl-和1,3-Diphenylurea;(Ⅲ)TCC加氯生成Urea,N,N'-bis(3,4-dichlorophenyl);(Ⅳ)TCC左侧C-N键被氧化形成4,5-Dichlorophenol,产物继续在Cl·和·OH作用下形成1,4-Benzenediol,2, 5-dichloro-.
3.4 UV/NaClO消毒中NaClO的投加量和溶液的pH值对TCC的去除率影响不大,TCC的去除率随NaClO投加量的增加和pH值的上升先升高后下降.增加HA的投加量TCC的去除率持续下降但低浓度的HA对TCC的去除有促进作用.
[1] TCC Consortium. High production volume (HPV) chemical challenge program data availability and screening level assessment for triclocarban, CAS#: 101-20-2,2002;Report [R]. No.201-14186A; http://www.epa. gov/chemrtk/tricloca/c14186cv. pdf;pp 1-38.
[2] Ying G G, Yu X Y, Kookana R S.Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with enviromental fate modelling [J].Environ Pollut 2007,150:300-305.
[3] Ahn, K C, Kasagami T, Tsai H J, et al. Animmuno assay to evaluate human/environmental exposure to the antimicrobial triclocarban [J]. Environmental. Science. Technology, 2012.46(1): 374–381.
[4] Sapkota A, Heidler J, Halden R U. Detection of tricloarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandemmass spectrometry [J]. Environmental Research, 2007, 103(1):21-29.
[5] Wu Chenxi, Spongberg A L, Witter J D. Determination of the persistence of pharmaceuticalsin biosolidsusing liquid chromatography tandem mass spectrometry [J]. Chemosphere, 2008, 73(4):511-518.
[6] Cha J, Cupples A M. Detection of the antimicrobials triclocarban and triclosan in agricultural soils following and application of municipal biosolids [J]. Water Research, 2009,43(9):2522-2530.
[7] Nolen G A, Dierckman T A. Reproduction and teratogenic studies of a 2:1mixture of 3,4,4¢-trichlorocarban ilide and 3-trif- luoromethyl-4, 4¢-dichlorocarbanilidein rats and rabbits [J]. Toxicol. Appl.Pharmacol, 1979,51(3):417-25.
[8] Duleba A J, Ahmed M I, Sun M, et al. Effects of triclocarban on intact immature male rat: augmentation of and rogenaction [J]. Reprod. Science. 2011,18(2):119–127.
[9] Higgins C P, Paesani Z J, Chalew T E, et al. Persistence of triclocarban and triclosan insoil after land application of biosolids and bioaccumulation in[J]. Environ. Toxicol. Chemical, 2011,30(3):556–563.
[10] Chung E, Genco M C, Megrelis L, et al. Effects of bisphenol A and triclocarban on brain specific expression of aromataseinearly zebra fishembryos [C]// Proc. Natl. Acad. Science. U.S.A. 2011, 108(43):17732–17737.
[11] Hinther A, Bromba C M, Wulff J E, et al. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems [J]. Environmental Science & Technology, 2011,45(12):5395-5402.
[12] 胡洪营,吴乾元,黄晶晶,等.再生水水质安全评价与保障原理[M]. 北京:科学出版社, 2011.
[13] Mckinny C, Ma Y J, Novak J T, et al. Disinfection of microconstituent antibiotic resisitance genes by UV light andsluge digestion [J]. Water Research, 2009,20(11):577-589.
[14] Gibs J, Stackelberg P E, Furlong E T, et al. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds [J]. Science of the Total Environment, 2007,377(2):255-272.
[15] Shah A D, Dotson A D, Linden K G, et al. Impact of UV disinfection combined with chlorination/chloramination on the formation of halonitromethanes and haloacetonitriles in drinking water [J]. Environmental Science & Technology, 2011,45(8): 3657-3664.
[16] Plewa M J, Dwagner E. Chemical and Biological Characterization of Newly Discovered lodoacid Drinking Water Disinfection By products [J]. Environmental. Science.Technology, 2004,38(18):4713-4722.
[17] CCC (Chlorine Chemistry Council). White paper: a review of disinfection practices and issues [R]. 1997:6-14.
[18] Reifferscheid G, Heil J, Oda Y, et al. Amicroplate version of the SOS/UMU test for rapid detection of genotoxins and genotoxic potentials of environmental samples [J]. Mutation Res., 1991, 253:215~222.
[19] MartínezZapata M, Aristizábal C, Peuela G. Photodegradation of the endocrine disrupting chemicals 4nnonylphenol and triclosan by simulated solar UV irradiation in aqueous solutions with Fe (Ⅲ) and in the absence/presence of humic acids [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2013,251:41-49.
[20] Vallejo M, San Román M F, Ortiz I, et al. Overview of the PCDD/Fs degradation potential and formation risk in the application of advanced oxidation processes (A OPs) to wastewater treatment [J]. Chemosphere, 2014,118:44-56.
[21] 曲显恩.含氯消毒剂的性能与应用[J]. 中国氯碱, 2005,1: 19-23.
[22] 刘少友,黄雪莉.用原子矩阵法对工业级次氯酸钠水溶液宏观动力学研究[J]. 新疆大学学报(自然科学版), 2004,21(1):109- 112.
[23] Fang J, Fu Y, Shang C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environmental Science & Technology, 2014,48(3):1859-1868.
[24] 韩志涛,杨少龙,郑德康,等.紫外辐照强化NaClO溶液湿法脱硝的实验研究[J]. 科学技术与工程, 2016,28(16):135-138.
[25] Hirakawa T, Nosaka Y. Properties of O2$- and OH$ formed in TiO2aqueous suspensions by photocatalytic reaction and the influence of H2O2and some ions [J]. Langmuir, 2002,18(8): 3247e3254.
[26] Beitz T, Bechmann W, Mitzner R. Investigations of reactions of selected azaarenes with radicals in water. 2.Chlorine and bromine radicals. J. Phys. Chem. A, 1998,102(34):6766−6771.
[27] Neta P; Huie R E, Ross A B. Rate constants for reactions of inorganic radicals in aqueous solution [J]. J. Phys. Chem. Ref. Data, 1988,17(3),1027−1284.
[28] Buxton G V, Greenstock C L, Helman W P, et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (×OH/×O−) in aqueoussolution. J. Phys. Chem. Ref. Data 1988,17(2):513−886.
[29] 熊重铎,程 强,施 薇,等.微波无极紫外光催化降解茜素绿的性能研究及产物分析 [J]. 环境工程学报, 2014,8(12):5185- 5190.
[30] 徐 蕾.基于硫酸根自由基反应的2,4,6-三氯苯酚氧化降解的研究[D]. 上海:东华大学, 2012.
[31] Geeta S, Rao B, Mohan H, et al. Radiation inducedoxidation of substituted benzaldehydes: A pulse radiolysis study [J]. Journal of Physical Organic Chemistry, 2004,17(17):194-198.
[32] Singh T, Gejji S, Rao B, et al. Radiation chemical oxidation of aniline derivatives [J]. Journal of the Chemical Society Perkin Transactions, 2001,7(7):1205-1211.
[33] Jin Ai-jie, Li Feng. Changes of the toxic potential of drinking water containing aminopyrine before and after chlorine disinfection as determined by the algal toxicity assay and the SOS/umu assay [J]. International Biodeterioration & Biodegradation, 2016,113:269-275.
[34] Swietlik J, Dbrowska A, Raczyk-Stanisawiak U, et al. Reactivity of natural organic matter fractions with chlorinedioxide and ozone [J]. Water Res, 2004,38(3):547-558.
[35] Palmer F L, Eggins B R, Coleman H M. The effect of operational parameters on the photocatalytic degradation of humi cacid [J]. J Photochem PhotobiolA: Chemistry, 2002,148:137-143.
[36] Westerhoppp, Mezyk S P, Cooperw J, et al. Electron pulse radiolysis determination of hydroxyl radical rate constants withSuwannee river fulvic acid and other dissolved organic matter isolates. Enviornment Science & Technology, 2007,41(13): 4640-4646.
[37] Xu B, Gao N Y, Xue X F, et al. Photochemical degradation of diethyl phthalate with UV/H2O2[J]. Journal of Hazardous Materials, 2007,B139:132-139.
[38] Martínez-Zapata M, Aristizábal C, Peuela G. Photodegradation of the end ocrine disrupting chemicals 4nnonylphenol and triclosan by simulated solar UV irradiation in aqueous solutions with Fe (Ⅲ) and in the absence/presence of humic acids [J]. Journal of Photochemistryand Photobiology A: Chemistry, 2013,251:41-49.
[39] Mc Donnell G, Russell A D. Antiseptics and disinfectants:activity, action and resistance [J]. Clin Microbiol Rev 1999,12(1):147-179.
[40] Loftsson T, Ossurardottir I B, Thorsteinsson T, DuanM, MassonM. 2005. Cyclodextrin solubilization of theantibacterial agents triclosanand triclocarban: Effect of ionization and polymers. J. Inclusion Phenom Macrocyclic Chem, 52:109-117.
[41] Wang Wen-Long, Wu Qian-Yuan, Li Zhiming, et al. Light emitting diodes as an emerging UV source for UV/chlorineoxidation: Carbamazepinede gradationand toxicity changes [J]. Chemical Engineering Journal, 2017,310:148-156.
[42] 李玉瑛,何文龙,李青松,等.UV协同ClO2去除三氯生及其降解产物的研究[J]. 环境科学, 2015,36(2):517-522.
Study on the removal characteristics and genotoxicity of trichlorocarban during disinfections by NaClO, UV and UV/NaClO.
LU Bao-song1,2,MA Xiao-yan1,LI Qing-song2*, LUO Jing-yu3,SHEN Qi-qi4, LIAO Jie2, LIAO Wen-chao2, CHEN Guo-yuan2, LI Guo-xin2
(1.College of Civil Engineering and Architecture, Zhejiang University of Technology, Hangzhou 310014, China;2.Water Resource and Environment Institute, Xiamen University of Technology, Xiamen 361005, China;3.School of Environmental Science and Engineering, Suzhou University of Science and Technology, Suzhou 215009, China;4.National Engineering Reseach Center For Marine Aqaculture, Zhejiang Ocean University, Zhoushan 316000, China )., 2008,38(5):1752~1759
The removal characteristics of trichlorocarban (TCC) during disinfections were studied by NaClO, UV and UV/NaClO combined disinfection. The genetic toxicity of TCC solution in three disinfection technologies was also investigated. Furthermore, the degradation products were identified and the degradation mechanism of TCC was discussed. The effects of several factors such as NaClO dosage, TCC initial concentration, pH value and humic acid (HA) on TCC removal were studied during UV/NaClO combined disinfection. The results showed that the removal efficiency of TCC was in the order: UV/NaClO, UV, NaClO, and the genetic toxicity of TCC solution was increased by disinfection treatment to varying degrees. Eight kinds of TCC degradation products were identified by LC-MS. The main degradation pathways were dechlorination, chlorination, and ·OH/O·oxidating. The removal rate of TCC by UV/NaClO combined disinfection was more than 97%; The removal of TCC was negatively correlated with its initial concentration; The removal rate of TCC increased firstly and then decreased with the increase of pH;Low concentration of humic acid (HA) contributed to the removal of TCC, whereas high.
triclocarban;disinfection;removal;products;genetic toxicity
X52
A
1000-6923(2018)05-1752-08
2017-09-29
国家自然科学基金资助项目(51378446,51678527,51408518);福建省科技计划引导性项目(2017Y0079);福建省高校新世纪优秀人才支持计划项目(JA14227);福建省自然科学基金资助项目(2017J01491);福建省中青年教师教育科研项目(JAT170412);厦门市科技局项目(3502Z20131157,3502Z20150051)
* 责任作者, 副研究员, leetsingsong@sina.com
陆保松(1989-),男,安徽亳州人,浙江工业大学硕士研究生,主要研究方向为水处理理论与技术.
