Characterization of m od if ied m esop orous silica nanop articles as vectors for siRNA d elivery
2018-05-15AnnSlitAnnEgorovEudldCslsAntonKiselevJessicRosenholm
Ann Slit ,Ann Egorov ,Eudld Csls,Anton Kiselev ,Jessic M.Rosenholm ,∗
a Pharmaceutical Sciences Laboratory,Faculty of Science and Engineering,°Abo Akademi University,BioCity,Artillerigatan 6A,FI 20520 Turku,Finland
b D.O.Ott Research Institute of Obstetrics,Gynaecology and Reproductology,Mendeleevskaya line,3,Saint Petersburg 199034,Russia
Keywords:Gene therapy Nanocarriers siRNA delivery Mesoporous silica nanoparticles
A B S T R A C T
1. Introd uction
Now adays new approaches for gene and drug delivery into cells are actively being developed.The m ost w idespread vectors for gene delivery are viral vectors,w hich can provide effective cell transfection.An ideal virus-based vector m ust be able to infect cells like viruses do,but at the sam e tim e such infection m ust not lead to expression of viral genes,w hich are responsible for replication and toxicity.How ever,viral vectors have som e draw backs,w hich include insertional mutagenesis,toxicity and immunogenicity[1].
The m ost popular and prom ising alternate approach is design of non-viral vectors that are non-toxic,biodegradable,have high loading capacity,can protect the cargo from degradation during the w hole route of the cargo/vector complex transit to the f inal destination,provide effective targeted delivery and intracellular or nuclear release of the cargo.They should not cause im m une response,w hich allow s repeated administration of the genetic constructs into the organism,and m oreover,they are generally cheaper.Besides these,nonviral vectors m ight be easily m odif ied w ith various m olecular signals for overcom ing barriers of gene delivery into the cells[2].
One novel and prom ising type of non-viral and non-organic vector is m esoporous silica nanoparticles(MSNs),w hich have an ordered porous structure.Porous silica particles have been known in their bulk form by materials scientists for over 40 years[3,4],but started to be used outside of m aterials engineering and petrochem icals f ields about 25 years ago[5]and have been proposed for drug delivery approxim ately 15 years ago[6,7].One of the m ajor advantages of MSNs is that they are especially suitable for the delivery of hydrophobic substances,due to the fact that drugs to be delivered are packed into the pores after the particle synthesis and thus shielded by the hydrophilic silica m atrix[8].Moreover,the size of the particles and their surface can be f lexibly and easily m odif ied[9]in different m anners for better interaction and protection of the cargo molecules.To provide stronger interaction of negatively charged cargo w ith the pore surface of MSN,it could be m odif ied w ith cationic surface groups such as am ines.For reaching a high abundance of am ine groups on the surface,the hyperbranched polymer polyethylenimine(hbPEI)is particularly advantageous[10,11].In addition,PEIcovering of loaded MSNs can provide capping of the pore entrances for better retention of the cargo by so-called“m olecular gate-keeping”[12,13]and internalization of loaded particles into cells by endocytosis[12,14].
Therapy based on RNA interference(RNAi)provides treatm ent options in those cases w hen current drug technology fails[15].RNAiis a process of gene expression silencing during different stages of genes processing.The advantage of sm all interfering RNAs(siRNA)is that siRNA is fully com plem entary to target sequences,w hich leads to silencing based on Argonaute 2–m ediated degradation[16].How ever,controlled siRNA delivery rem ains challenging for tw o m ain reasons:(1)existing delivery system s cannot be endow ed w ith high loading eff iciency,stability and slow release,hence long-term therapeutic effects w ould require repeated drug adm inistration;(2)biological barriers are still rem aining a challenging obstacle[17,18].Moreover,for potential in vivo use,the nanocarrier must retain the oligonucleotide molecules very tightly during circulation and cellular internalization,but slow ly and sustainably dissociate them in the intracellular environm ent according to individual requirem ents.MSNs have the potential for meeting both challenges,due to established low toxicity and high uptake.
PEI is know n to be an effective vector for gene delivery since it exhibits a unique“proton-sponge”effect to exit endosomal compartm ents.The interest now adays is focused on applying of this unique property of PEI to nanocarriers,e.g.by conjugation of PEIm olecules w ith silica nanoparticles[12].This m odif ication also provides positive charge on the surface of MSNs for loading of negatively charged nucleic acids.Several studies have been published on utilizing PEIof different molecular sizes and degrees of branching.The only disadvantage of PEIis cytotoxicity,though it is dependent on its m olecular w eight.Xia and coauthors dem onstrated toxicity of MSN-PEI(25 k Da)com plexes on m acrophage(RAW 264.7)and bronchial epithelial(BEAS-2B)cell lines[19].PEIof different m olecular w eights w as tested,w hereby the results conf irm ed the absence of toxicity of PEIs w ith low er m olecular w eight,such as 0.6,1.2 and 1.8 k Da.In addition,this study also show ed high nucleic acid binding capacity that w as m ostly independent of the PEI MW[19].At the sam e tim e,Li w ith coauthors w ere the f irst ones in 2011 w ho succeeded to load siRNA not only on the surface of m agnetic MSNs,but inside the pores[20].To prepare a reliable vector for siRNA delivery,they treated the loaded particles w ith PEIto block the detachm ent of siRNA.Low toxicity of such constructions w as proven although branched PEIw ith the w eight 25 k Da w as chosen for further experim ents.
In one of our previous publications,MSN-PEIhybrid carrier coupled to redox-sensitive surface linkers that break under intracellular conditions w as developed,thereby converting the surface charge to negative w hich drives the nucleic acids to release from the pores[21].These MSNs w ere capped w ith PEG chains,w hich did not prom ote effective endosom al escape after cellular uptake.In the subsequent study,PEGwas replaced w ith pre-polym erized PEI(in contrast to the surface-grafted PEI w hich w as grow n via surface-initiated polym erization),w hich lead to enhanced cellular uptake and endosom al escape[22].Hence,the w ork described in the current manuscript is to be considered a follow-up of this study by Prabhakar et al.2016[22]devoted to transfection of MDA-MB 231 cell line w ith m odif ied MSN particles loaded w ith cell-killing siRNA.The used AllStars Hs Cell Death Control siRNA mechanism of action involves f ive different target genes,w hich m akes it relatively unspecif ic.Thus,in the current study,w e decided to use GFP-expressing MDA-MB 231 cell line to transfect w ith MSN/anti-GFP siRNA com plexes to conf irm the silencing origin of the observed effect.Further,w e com pare the siRNA delivery eff icacy w ith non-PEI-m odif ied MSNs,to conf irm the advantage of PEIas construct.MDA-MBcell lines,MDA-MB 231 in particular,are very im portant for breast cancer research[23],w hile GFP-MDA-MB cell lines are useful for optim ization of siRNA transfection w ith non-viral vectors.
2. Materials and m ethods
2.1. Materials
Unloaded MSNs(in 96%ethanol,3 m g/m l),w ith cystam ine-PEI coated pores,w ere synthesized as described previously[21].All the in vitro experim ents w ere perform ed w ith the use of MDA-MB-231 cell culture constitutively expressing GFP gene.PEI 1300 Da(Lupasol G 20 WF)w as used for MSN pores covering w ith hyperbranched PEI after siRNA loading.For transfection experim ents,anti-GFPsiRNA w as used:sense 5′-CAA GCU GAC CCU GAA GUU Ctt-3′,antisense 5′-GAA CUU CAG GGU CAG CUU Gtt-3′.siRNA w as purchased from Syntol JSC,Russia.Tw o non-viral vectors w ere used as controls:peptide-based vector L1,modif ied w ith CXCR4 receptor ligand developed in Laboratory of Prenatal Diagnostics of Inherited and Inborn Disorders in The Research Institute of Obstetrics,Gynaecology and Reproductology nam ed after D.O.Ott,Saint Petersburg[24,25]and polyethyleneim ine(branched PEI 25 k Da;Sigm a-Aldrich).Weight ratios used in transfection experim ents w ere the follow ing:siRNA/L1-1/8.42 and siRNA/PEI-1/1.1
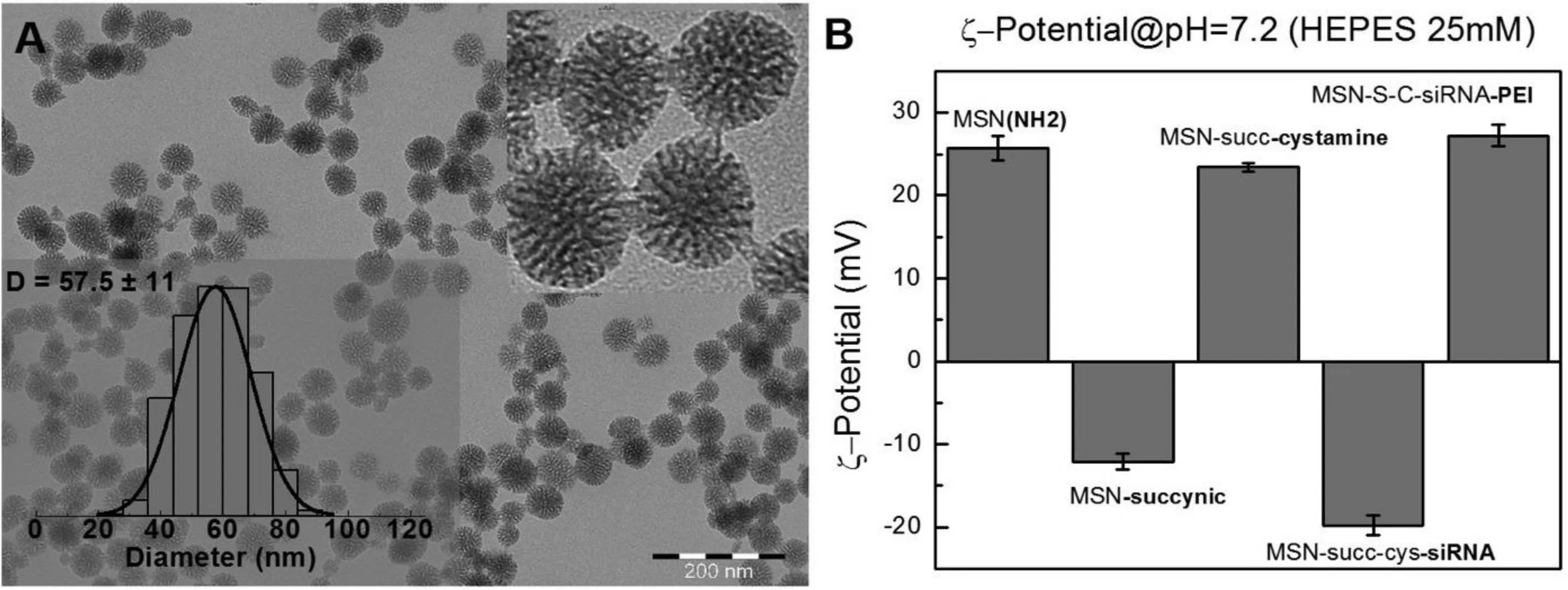
Fig.1–Characterization of the MSN system used in this w ork.(A)Rep resentative TEM im ages and size distribution of the starting MSN-NH2 particles.(B)Zeta potential m easurem ents of the MSN-NH2 particles after synthesis and after the different sequential functionalization step s w ith succinic anhyd rid e,cystam ine,siRNA and PEI.Particles w ere d ispersed in HEPES buffer(10 m M;p H 7.2)at a 0.1 m g/m l m ass concentration for com parability of the m easurem ents.It can be observed the exp ected m od if ication of the particle surface charge after each step(negatively charged after succinylation and siRNA load ing and positively charged for the MSN after synthesis,after cystam ine conjugation and f inally after PEI ad sorp tion onto MSN-siRNA loaded particles.
2.2. Methods
2.2.1. Particles preparation
siRNA loading and PEIcovering of loaded MSN w as perform ed according to Prabhakar et al.[22].Brief ly,MSN-PEIhybrid carrier nanoparticles of roughly 60 nm in diam eter(Fig.1A)coupled to redox-sensitive surface linkers(cystam ine molecules w ith an intracellularly cleavable S–S bond)w ere prepared through sequential steps involving f irst the succinylation of the MSN surface am ines,follow ed by the linking of cystam ine to the succinic acid residues by EDCcoupling.After this,complexes of siRNA/MSN w ere form ed in follow ing w eight ratios:1/15,1/10,1/7(200 n M of siRNA corresponding to 41.2μg/m l,27.4μg/m l and 9.59μg/m l MSNs).Specif ically,Anti-GFP-siRNA was added to the prepared suspension of MSN-cystamine in MES buffer at these w eight ratios.It is necessary to state that GFP is not a natural protein in MDA-MB-231 cells and is know n to not to affect cellular physiology.Final solution w as m ixed for 1 h and centrifuged(5000×g/10 m in).For PEI covering the com plexes w ere resuspended in ethanol.Then PEI(1300 Da)and N,N′-disuccinim idyl carbonate(DSC)w ere added under stirring to provide PEIsurface polymerization.The solution w as incubated at room tem perature for 30 m in,then centrifuged(5000×g/10 min)for purif ication.The f inal solution w as resuspended in HEPESbuffer.The success of the different functionalization steps w as assessed by zeta potential m easurem ents,w here the expected m odif ication of the particle net surface charge can be observed after each step(Fig.1B).MSN binding to siRNA w as m onitored using Sybr Green f luorescence quenching m ethod.The particles w ere analyzed at follow ing w eight ratios(1/15,1/10,1/7).
2.2.2. Particles characterization
MSN sam ples w ere characterized by m eans of different techniques such as transm ission electron m icroscopy(TEM),zeta potential(ζ-potential)and dynam ic light scattering(DLS).Transmission electron microscopy(TEM)im ages were obtained using a JEM 1400-Plus(JEOL Ltd.,Tokyo,Japan)operating at 80 k V w ith a tungsten f ilam ent and an 11-Mpx CCD cam era.Preparation of the sam ples w as perform ed onto a carbon coated copper TEM grids by drop casting.Different parts of the grid w ere observed and im ages w ere taken at different m agnif ications to ensure obtaining statistically signif icant results for MSNs size distribution.More than 1000 particles were counted using Image Jsoftware(Fig.1A).Zeta potential(ζ-potential)m easurem ents w ere perform ed using a Malvern Zeta Sizer Nano ZS Instrum ent operating at a light source w avelength of 532 nm and a f ixed scattering angle of 173°for detection.After synthesis and after the different functionalization steps,a volum e of 0.8 m l of the MSNs(0.1 m g/m l)dispersed in HEPES(10 m M;p H 7.2)w ere placed into the specif ic cuvette,and the softw are w as arranged w ith the specif ic param eters of refractive index and absorption coeff icient of the MSN and viscosity of the solvent,data required to obtain the correctζ-potential value for the sam ples(Fig.1B).Finally,a slight increase in dynamic light scattering(DLS)values along w ith the m aintenance of the polydispersity index indicates that MSN m aintain their colloidal stability after the different functionalization steps(data not show n).
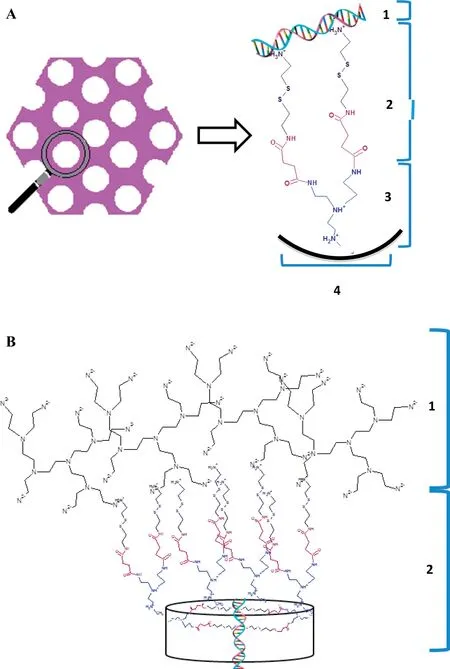
Fig.2–Schem atic rep resentation of(A)the construction of cleavable organic linkers on MSN pores surface through hyp erbranched PEI w ith load ed NA:1.NA,2.Cystam ine w ith am ino-group and disulf id e bond,3.PEI,4.MSN p ore surface;(B)the NA load ing,particle surface grafting of ex terior PEI:1.Hyperbranched PEI,2.MSN-PEI-Linker.
2.2.3. Loading eff iciency
Sybr Green displacem ent w as assayed follow ing the decrease in em ission f luorescence at 590 nm(485 nm excitation).After loading of MSN-based vectors w ith prelabeled siRNA solution w ith 1×Sybr Green(AMRESCO),one part of the loaded particles solution w as covered w ith hyperbranched PEI,w hile the rest were left uncovered.The concentration of siRNA was 0.2μg per w ell.Tw o w eight ratios of siRNA and MSN w ere tested:1/7 and 1/10.Fluorescence m easurem ents w ere perform ed by m eans of Wallac 1420Dscanning m ultilabel counter(Therm o Fisher Scientif ic Oy,Vantaa,Finland).Displacem ent w as calculated as(F-Ff)/(Fb-Ff),w here Ff and Fb are the f luorescence intensities of Sybr Green in the absence and presence of siRNA,respectively.
2.2.4. Transfection experiments and estimation of transfection eff iciency
Transfection experiments were performed in triplicate.Before transfection,cell culture m edium w as replaced w ith m edium w ithout FBS.siRNA-MSN com plexes at three w eight ratios established before(1/15,1/10,1/7)w ere added and incubated with MDA-MB-231 cells for 4 h.The f inal concentration of siRNA w as 200 n M in each w ell,or 1.37μg.The concentration of siRNA for transfection experim ents w as set based on existing protocols previously show n to provide a good readout[25],according to w hich the amounts of MSN were calculated(41.2,27.4 and 9.59μg/m l).After incubation in norm al culture m edium for the next 48 h,the m edia w as rem oved from w ells and 80μl of lysis-buffer w ere added to each w ell w ith transfected cells.To provide eff icient lysis of cells they were put into freezer(-70°)for at least 1 h.To m easure the GFP f luorescence intensity the cells w ere centrifuged(2500×g/5 m in)and 50μl of supernatant w as taken to Wallac 1420 Multilabel Counter(em ission 535 nm/excitation 485 nm).The GFP gene expression w as norm alized by the total protein concentration of the cell extracts,m easured w ith Bradford reagent(Helicon,Russia).Fluorescence of MDA-MB-231 cells transfected w ith naked siRNA w as used as a control w ith m axim al f luorescence taken as 100%.
2.2.5. Hemolysis assay
Hem olysis assay w as perform ed according to m ethodology described before by Evans et al.[26].5 m l of blood w as obtained into tube w ith Na2EDTA from an anonymous human donor according to ethical requirem ents.Red blood cells(RBCs)w ere w ashed three tim es w ith NaCl and phosphate-buffered saline(PBS)(p H 7.4)and diluted up to 50 tim es w ith PBSp H 7.4.Stock solutions of MSN-PEI-Linker-PEI of different concentrations w ere prepared.For each assay w e took 200μl of RBCand 800μl of particles stock solutions to reach the f inal concentration of MSN-PEI-Linker-PEI 41.2,27.4 and 9.59μg/m l.For positive control tubes w e added 800μl of 1,25%Triton X-100 to 200μl.For negative control tubes,w e added 800μl of PBS p H 7.4.Tubes w ere incubate at 37°Cfor 1 h and 24 h.After incubation tubes w ere centrifuged for 5 m in at 500×g to pellet intact erythrocytes.Hem olysis w as estim ated in UV/Vis spectrum,from the 540 nm absorbance of hem oglobin released into the supernatant using NanoDropTM2000,the results w ere expressed as%hemolysis.Negative control w hich corresponds to 0%hem olysis w as taken as the absorbance of the supernatant of erythrocyte suspensions in PBS p H 7.4.Absorbance of the supernatant of erythrocyte suspensions in presence of Triton X-100 w as taken as 100%hemolysis.
2.2.6. Statistics
Statistically signif icant differences were analyzed by Student’s t-test,using Instat(Graph Pad Softw are Inc.,USA).P<0.05 w as considered statistically signif icant.
3. Results and discussion
The present study is focused on evaluation of hyperbranched PEI covered MSN as vehicles for siRNA delivery into MDAMB-231 breast cancer cells for GFPexpression inhibition.The structure of MSN particles and surface functions m ay be variable and f lexibly tuned for increasing their loading capacity[27].Considering this,the interaction of oligonucleotides and surface-m odif ied MSNs and their behavior inside pores w ere studied previously[21,22].As a result,it has been show n that short oligonucleotides(up to 20 bp)can be m ore effectively bound by functionalized pores in com parison w ith longer DNA m olecules[28],w hich led to further studies of siRNA delivery by MSNs.Recently it has been show n that modif ication of MSN pores w ith am ino groups is a prom ising approach to increase the am ount of siRNA w hich could be loaded into MSNs[29].
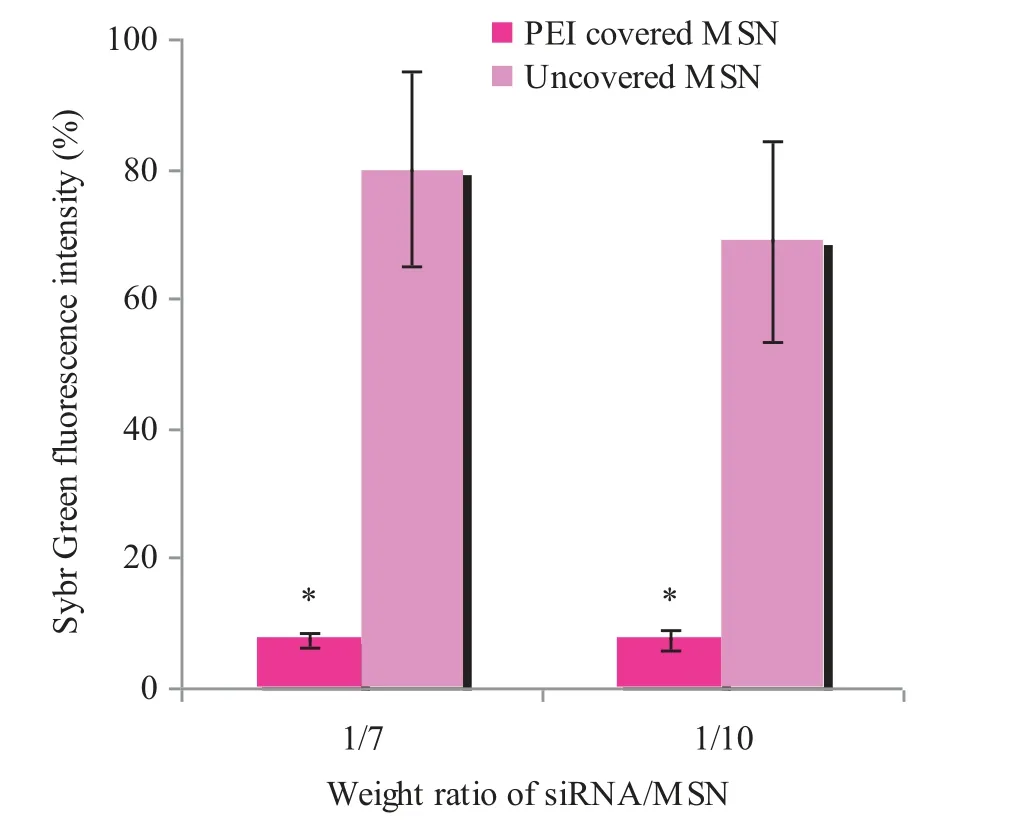
Fig.3–Sybr Green exclusion from com plexes of siRNA and MSNs w ith and w ithout PEI covering.∗P<0.05 w hen com p ared w ith uncovered MSN p articles.
The m ost effective m eans to increase siRNA loading of MSN is to achieve low N/P ratio inside the functionalized pores,ranging from 0.7 to 1.0,by providing high density of am ine-term inated organic linkers(one linker per 3.3–3.7 nm2)[21].Here,cystamine w as chosen as organic linker because it contains am ino-groups and a disulf ide bond,w hich could be easily disrupted inside cells(Fig.2).
To provide a better protection of siRNA inside pores,increased cellular uptake and endosomal escape,the MSN surface w as covered w ith a coating of pre-polym erized PEI(1300 Da)after siRNA loading.It has been show n experim entally that low-m olecular w eight PEI polym ers are not toxic for cells[30]and especially when used as a construct in silica hybrid m aterials,the cytotoxicity can be suppressed even for higher MW PEI[29].Besides,the outside layer of PEI provides the“proton sponge”effect for endosom al escape of siRNA/MSN complexes[19].
To estim ate the eff iciency of MSN/anti-GFP siRNA complexes form ation,exclusion tests w ere perform ed.Intercalating dye Sybr Green releases out of DNA during com plex form ation,w hich causes f luorescence intensity decrease.Free DNA w as used as a control w ith m axim al f luorescence taken as 100%.Data obtained prove that the PEIcoating prevents Sybr-Green access to siRNA inside pores,w hich results in decreased f luorescence in com parison w ith uncovered particles(Fig.3).

Fig.4–Relative GFP f luorescence intensity(%)after transfection of MDA-MB-231 cells w ith anti-GFP siRNA/MSN com p lexes at w eight ratios 1/15,1/10 and 1/7.Com plexes of anti-GFP siRNA and vectors L1 and PEI w ere used as controls.The result of transfection w ith naked anti-GFP siRNA is taken as 100%.∗P<0.05 w hen com pared w ith uncovered MSN particles.
Tw o of the m ost im portant characteristics of vectors for intracellular delivery are toxicity and transfection eff iciency.Previously,Prabhakar and co-authors show ed MSNPEI-Linker-PEIvectors to be not toxic for MDA-MB 231 cell culture[22]by WST-1 assay,w hich is based on the conversion of the tetrazolium salt WST-1 into a colored dye by m itochondrial dehydrogenase enzym es.
Transfection experiments were subsequently perform ed for evaluation of siRNA delivery eff iciency by MSNs into cells.For these experim ents,MDA-MB-231 cell culture w as used because previously low toxicity of MSN/NA com plexes w as proved for this cell line[22].Uncovered particles(w ithout an outer coating of pre-polym erized PEI)w ith the w eight rate of siRNA/MSN 1/15 w ere taken for com parison(MSN-PEI in Fig.4).As control,w e used branched PEI 25 k Da and previously described peptide vector L1,m odif ied w ith ligand to CXCR4 receptor[24,31].As show n earlier,MDA-MB-231 cells overexpress CXCR4 receptors on their surface[32].Transfection eff iciency w as estimated by the level of GFPexpression silencing in the transfected cells.No differences in transfection eff icacy in MDA-MB-231 cells betw een PEI-m odif ied siRNA/MSN form ed at low w eight ratios and unm odif ied siRNA/MSN w ere found,whereas eff icacy of siRNA/MSN form ed at w eight ratio 1/15 w as signif icantly decreased after PEIm odif ication(Fig.4).
Results obtained show that PEI-covered com plexes w ith higher siRNA/MSN w eight ratios provide m ore effective delivery of siRNAs(Table 1).Thus,our results conf irm the assum ption about the advantages of PEI-covered siRNA/MSN.
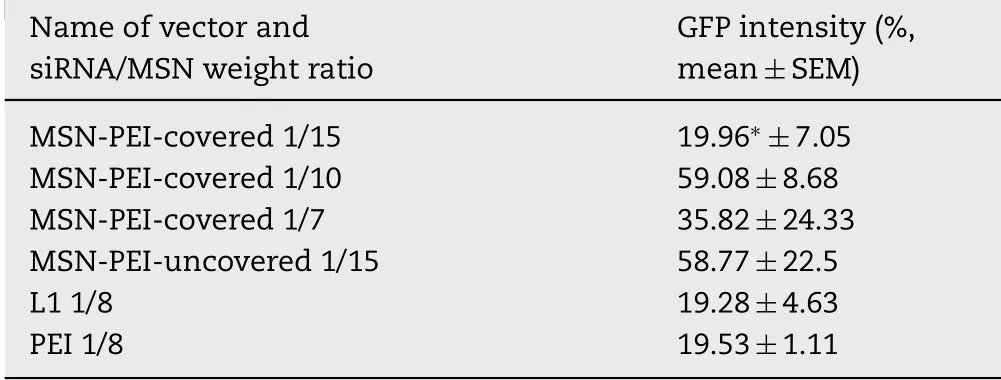
Table 1–Relative GFP f luorescence intensity(%)in MDAMB-231 cell culture after transfection w ith com p lexes of anti-GFPsiRNA w ith MSNs of w eight ratios 1/15,1/10,1/7,and com plexes of anti-GFPsiRNA w ith L1 and PEIas controls.The level of GFPintensity is ex pressed in p ercentage term s,w here result of transfection w ith naked anti-GFP siRNA is taken as 100%.∗P<0.05 w hen com paring w ith uncovered p articles.
Fig.5–Exam ple of visualization of RBC after incubation w ith MSN-PEI-Linker-PEIvectors:(A)41.2μg/m l,(B)negative control(PBS p H 7.4),(C)p ositive control(Triton X-100).
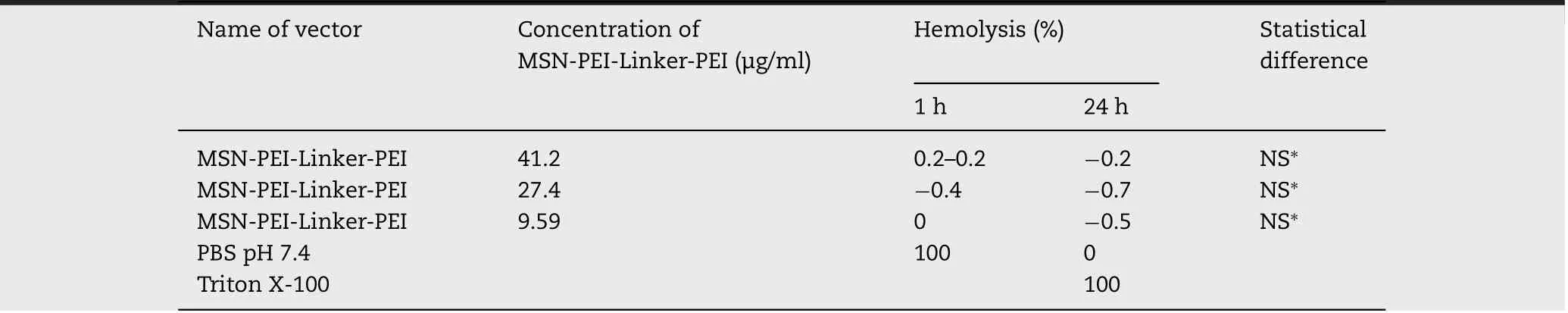
Table 2–Hem olytic activity of MSN-PEI-Linker-PEI p articles of different concentrations after 1 h and 24 h of incubation.The hem olytic activity is ex pressed in p ercentage term s.∗no signif icant difference.
The hem olysis assay is required for all devices or m aterials which are introduced into the body via the blood stream to def ine hem olytic properties.This test m easures the dam age to red blood cells w hich can occur due to nanocarrier exposure,and com pares it to positive and negative controls.
Three different concentrations of MSN-PEI-Linker-PEIwere chosen for the assay in accordance w ith concentrations utilized for transfection eff iciency test:41.2μg/m l,27.4μg/m l and 9.59μg/m l.Before analyzing the absorbance level,the effect of delivery com plexes can be detected visually(Fig.5).
For m easuring the absorbance level of supernatants of each assay m ixture,a w avelength of 540 nm w as chosen,and the percentage of hem olysis w as counted according to a previously reported formula[26].It can be seen that our particles do not exhibit any hem olytic activity in com parison w ith the positive control.Results obtained for both tim e points,1 h and 24 h,show no signif icant difference betw een the negative control(0%of hem olysis)and the studied sam ples(Table 2).
Although the investigated siRNA delivery system m ust still be further optim ized for clinical studies,w e can already envision that these types of delivery system s w ill have a signif icant im pact.There is a great dem and for the developing of effective siRNA carriers that w ould exhibit high specif icity,signif icant therapeutic effect,m inor side effects and ease of synthesis.Moreover,the f ield of use for siRNA therapy is quite broad;the silencing m echanism is effective against m any genetic and infectious disorders,such as cancer or hepatitis C.With the f irst gene therapy for a genetic disease approved by the FDA in late 2017[33],based on a viral vector,w e envision the development of gene delivery system s w ill continue to prom ote m ore effective gene therapies in the future.
4. Conclusion
During the past decades,vectors for gene therapy are being rapidly developed,but almost all of them have disadvantages,such as toxicity,short-term effect,low transfection eff iciency,high price etc.Use of non-viral vectors m ay solve m ost of these problem s,w hile non-organic non-viral vectors,like MSNs,are also much cheaper and robust.In the current study,w e dem onstrated that MSNs conjugated w ith cationic polym ers can provide safety of the fragile NA cargo and higher transfection eff iciency in comparison w ith pure MSNs.Thus,w e can conclude that m odif ied m esoporous silica nanoparticles can be considered as suitable vectors for delivery of siRNA into cells.
Conf licts of interest
The authors declare that there is no conf licts of interest.The authors alone are responsible for the content and w riting of this article.
Acknow led gm ents
This w ork w as supported in part by Russian Science Foundation grant 17-15-01230(biological characterization),Academ y of Finland project nos.284542,384542(JMR)and Jane and Aatos Erkko Foundation(EC).Anna Egorova is supported by President of Russian Federation scholarship(SP-2162.2015.4).Anna Slita w as supported by the scholarship w ithin Saint Petersburg State University bilateral exchange program for study abroad.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Sp ecial issue on“Form ulation strategies and m anufacturing technologies to enhance non-invasive drug delivery”
- Inf luence of solvent m ix tures on HPMCAS-celecox ib m icrop articles prepared by electrospraying✩
- Ad d itive m anufacturing of p rototyp e elem entsw ith p rocess interfaces for continuously op erating m anufacturing lines
- Tunable and sustained-release characteristics of venlafax ine hyd rochlorid e from chitosan–carbom er m atrix tablets based on in situ form ed p olyelectrolyte com p lex f ilm coating
- PEPT1-m ed iated p rod rug strategy for oral d elivery of p eram ivir
- Prep aration,characterization,and in vitro/vivo evaluation of p olym er-assisting form ulation of atorvastatin calcium based on solid d ispersion technique
