Inf luence of solvent m ix tures on HPMCAS-celecox ib m icrop articles prepared by electrospraying✩
2018-05-15AdmBohrYingyWngMoritzBeckBroichsitterMingshiYng
Adm Bohr ,Yingy Wng ,Moritz Beck-Broichsitter ,Mingshi Yng ,d
a Department of Pharmacy,Faculty of Health and Medical Sciences,University of Copenhagen,Copenhagen,DK-2100,Denmark
b Department of Pharmaceutics and Biopharmacy,Philipps-Universität,Marburg,Germany
c Chemical and Pharmaceutical Development,Bayer AG,Wuppertal,Germany
d Wuya College of Innovation,Shenyang Pharmaceutical University,Shenyang 110016,China
Keywords:Celecoxib Electrospraying Hyprom ellose acetate succinate Oral drug delivery Polym eric m icroparticles Solvent m ixture
A B S T R A C T Hyprom ellose acetate succinate(HPMCAS)m icroparticles containing the poorly-w ater soluble drug celecoxib(CEL)were prepared by electrospraying intended for oral drug delivery.Various solvent mixtures with different solubility for CELand HPMCASwere used to induce changes in the polym er structural conform ation of the m icroparticles.The perform ance of the prepared m icroparticles was evaluated by studying the solid state from,particle size and m orphology,radial drug distribution and drug release.CEL was am orphous in all electrosprayed HPMCASm icroparticles.The particle size and m orphology w as dependent on the solubility of HPMCASin the solvent m ixture used w ith poorer solvents resulting in sm aller m icroparticles w ith rougher appearance.The CEL distribution on the particles surface w as relatively hom ogeneous and sim ilar for all m icroparticles.Drug release from the m icroparticles was observed at a higher rate depending on the solubility of HPMCAS in the solvent used for electrospraying,and in all cases an at least 4-fold higher rate w as observed compared w ith the crystalline drug.Drug precipitation from the supersaturated solution w as inhibited by HPMCAS for all m icroparticles based on its parachute effect w hile crystalline CEL did not reach supersaturation.This study dem onstrated that electrospraying can be used to produce m icroparticles w ith tailored properties for pharm aceutical application by adjusting solvent selection.
1. Introd uction
A large fraction of drug com pounds under developm ent are limited by their poor w ater solubility[1].Their poor solubility in aqueous m edia(including body f luids)often results in a low and variable oral bioavailability and hence unsatisfactory therapeutic perform ance of the drugs[2–4].There are m any approaches to balance the poor solubility of drugs,w here drug-loaded polym er m icroparticles continue to be of interest.A solid dispersion system is def ined as a m ixture of at least tw o com ponents,the drug substance and a m atrix excipient[5],w here the drug is highly dispersed in the matrix,typically in a m olecular form increasing the dissolution rate and bioavailability of the drug from its formulation[6].Solid dispersions are particularly favorable for drugs w ith low gastrointestinal solubility but high permeability[7,8].These are classif ied as BCS class II drugs and typically show a correlation betw een in vitro solubility and in vivo bioavailability[9].
The m atrix m aterial plays a signif icant role in solid dispersions and inf luences characteristics such as particle size,drug devitrif ication rate,w ettability,drug dispersability,and dissolution behavior[6,10,11].Hyprom ellose acetate succinate(HPMCAS)is a cellulose derived polymer,w hich is commonly utilized as an enteric coating agent due to its p H-dependent solubility in aqueous environm ents.Moreover,HPMCAS has dem onstrated to be an effective precipitation inhibitor preventing salt recrystallization and prolonging the supersaturation of drugs for several hours after dissolution.The pronounced effect of HPMCAS is explained by the form ation of nanosized am orphous drug-polym er aggregates resulting from its particle ionized state and from hydrophobic interactions[12,13].Much indicates that such precipitation inhibition m ay provide considerable im provem ents in the in vivo perform ance of such formulations[14].
Currently,two major processes are used in the manufacturing of solid dispersion system s,m elting m ethods and solvent evaporation m ethods[8,15].Many solid dispersion dosage form s consist of m icroparticles due to the preparation m ethods em ployed.Their m icrostructure can have an inf luence on the release kinetics,eff icacy and the physical stability of the drug and should thus be optim ized to im prove the general perform ance of the form ulations[16,17].
In this study,electrospraying w as used as a m ethod for producing m icroparticulate solid dispersions.Electrospraying is a liquid atom ization technique utilizing a strong electric potential to disrupt the liquid feed into small droplets resulting in near-m onodisperse m icroparticles[18,19].Notably,electrospraying has found potential use in the biom edical f ield,w here tailored properties of utilized drug delivery vehicles or diagnostic probes are of signif icant relevance for therapy and diagnosis[20,21].Adjusting the different process param eters using electrospraying allow s for tailored particle engineering.Yet,the process is com plex due to the interplay betw een different interdependent param eters,which inf luence the characteristics of the resulting m icroparticles[22,23].For instance the selection of solvent inf luences both the spray ability of the liquid feed and the resulting droplet dim ensions,based on characteristics such as surface tension and electric conductivity,but also inf luences the particle characteristics,based on the solubility of m aterials dissolved or suspended in the solvent[24–26].
In the current study,w e investigated different solvent m ixtures for preparing a solid dispersion system of HPMCAS together w ith a m odel drug,CEL,by electrospraying.CEL is a crystalline,non-steroidal anti-inf lammatory drug,and both its aqueous solubility(~5μg/m l)and solubility-lim ited bioavailability are low[27].The obtained m icroparticles w ere thoroughly exam ined by m eans of X-ray pow der diffraction(XRPD)and scanning electron microscopy(SEM)and the surface drug distribution of the m icroparticles w as determ ined using X-ray photoelectron spectroscopy(XPS).Further,the in vitro drug release and precipitation inhibition w as also investigated for solid dispersions prepared using distinct solvents and solvent m ixtures.
2. Materials and m ethods
2.1. Materials
The HPMCAS(AQOAT)LF grade w as acquired from Shin Etsu(Tokyo,Japan).CEL pow der w as purchased from Dr.Reddy(Hyderabad,India).Acetone(ACE,99.9%HPLC grade),ethanol(EtOH,99.9%HPLC grade)and acetonitrile(99.9%HPLCgrade)w ere purchased from Sigm a Aldrich(Poole,UK).Phosphatebuffered saline(PBS,0.01 M,p H 6.8)w as prepared from sodium phosphate m onobasic and sodium hydroxide acquired from Sigm a Aldrich.Sodium lauryl sulphate(SLS)w as acquired from Fagron(Waregem,Belgium)and ultrapure w ater(SGWater Purif ication System,Barsbuttel,Germ any)w as used for all experim ents.All other chem icals and solvents w ere of analytical grade and were used w ithout further purif ication.
2.2. Solubility test
The solubility of HPMCAS and CEL,in ACE,EtOH,w ater and m ixtures of these solvents w as evaluated at room tem perature(~25°C)by placing 0.5 g of the polym er or drug into 10 m l of solvent mixture and mixing using a magnetic stirrer.The degree of dissolution w as further assessed visually after 1 and 24 h,and if failing to form a com plete solution,the m ixture w as diluted w ith m ore solvent(10 m l)and if successfully dissolved m ore solute w as added[15,28].The solubility of solutes in the different solvent m ixtures w as rated according to a com m on classif ication as freely soluble(100–1000 m g/m l),soluble(33–100 m g/m l)and practically insoluble(<0.1 m g/m l)[29,30].
2.3. Preparation of microparticles
Solutions of HPMCASand CEL were prepared at a solute concentration of 5%(w/v)and a drug loading of 20%(w/w)in different solvents including ACE,ACE–EtOH(85:15,v/v),ACE–H2O(85:15,v/v),EtOH–H2O(85:15,v/v).CEL-loaded HPMCAS m icroparticles w ere prepared using a custom ized single nozzle electrospraying setup w ith the sam e conf igurations as previously described[31].Brief ly,the electrospraying setup essentially consisted of a high voltage electrical power source(Glassm an Europe Ltd.,Tadley,UK),a precision syringe pum p(Elite,Harvard Apparatus,Edenbridge,UK),a spraying and collection platform and a custom-built stainless-steel nozzle w ith an outer and inner diam eters of 2.34 and 1.77 m m,respectively.The feed solutions w ere electrosprayed at a voltage of 7–8 k V in the stable cone-jet m ode by a feed rate of 30μl/min.Microparticles were collected at a distance of 7 cm from the nozzle tip.Only m icroparticles prepared by a stable cone-jet(Fig.1)w ere collected.All sam ples w ere prepared in triplicates under room tem perature,and stored in a desiccator at room temperature.

Fig.1–Jet im age cap tured d uring electrospraying in cone-jet m ode.
2.4. XRPD
The X-ray pow der diffraction spectra w ere detected by a PW3040/60 X’Pert Pro MPD(PANalytical,Philips,Netherlands)equipped w ith a copper anode for radiation w ithλ=1.542˚A,45 k Vand 40 m A.The sam ples were placed on aluminum sample holders and m easured from 5 to 35°2θat a step of 0.053°and 0.025°per second.
2.5. SEM
The electrosprayed m icroparticles w ere collected on glass slides,m ounted on stubs w ith double-sided carbon tape and sputter-coated w ith a~5 nm layer of gold using a Leica EM ACE200(Wetzlar,Germ any).Im ages w ere acquired using an FEI/Philips XL30 FEG(Hillsboro,USA)at an acceleration voltage of 3 k V using the secondary electron detector.The size of m icroparticles w as obtained by averaging the diam eter of 200 m icroparticles using the softw are Im ageJ(National Institute of Health,Maryland,US).
2.6. XPS
The surface chem istry of the m icroparticles w as analyzed using XPS(Thermo Scientif ic,Roskilde,Denmark)equipped w ith a m onochrom ated AlKalpha X-ray source.Survey scans of 0–1350 eV binding energy w ere perform ed using a pass energy of 200 eV and a step size of 1.0 eV and at a 90°take-of angle.The surface drug content of the m icroparticles w as determ ined by correlating the ratio of the detected f luorine in the sam ples to the f luorine in CEL alone thereby determ ining the concentration of CEL(in w t%)on the surface of the microparticles.The atom ic concentration(in%)of elem ents C,O,F,N and S in CEL is 65.4%,7.7%,11.5%,11.5%and 3.9%,respectively.To m inim ize error,ratios of N/F,S/F and F/C w ere used to calculate the concentration of CEL at the m icroparticle surface.
2.7. Drug loading eff iciency and HPLC
The microparticles w ere dissolved in 1 ml acetonitrile and diluted in 1:10(v/v)w ater for determ ining the concentration of CEL in the m icroparticles.The CEL concentration w as m easured by reverse-phase HPLC(Dionex,Germ ering,Germ any)coupled with a P680 pum p,ASI 100 sample injector,UVD340U detector(Dionex,Germ ering,Germ any),and a C18 Krom asil 126 colum n(Bohus,Sw eden).A m obile phase of acetonitrile-H2O(60:40,v/v)w as used at a constant f low rate of 0.5 m l/m in and samples were injected w ith a 10μl injection volume and detected at a w avelength of 230 nm.The drug loading eff iciency w as calculated as:
Drug loading(%)=(Drug m ass/Particle m ass)×100
2.8. Drug release study
Drug release from the electrosprayed solid dispersions w as studied using a USP paddle Apparatus II.Approximately 25 m g m icroparticles w ere accurately w eighted and placed in glass vessels f ixed in a Sotax AT7 dissolution station(Sotax,Allschw il,Sw itzerland).Then,900 m l release m edium of phosphate buffered solution(p H 6.8)w ith or w ithout 1.5%(w/v)SLS w as added,and constantly stirred by a paddle at a rotation rate of 50 rpm at 37°C.SLSw as added to obtain sink condition for CEL.At each sam ple point 5 m l release m edium w as sampled through a 2.7μm glass m icrof iber f ilters(Whatm an Ltd.,Oxon,England)by a Biolab/Gilson GX-271 auto sam pler(Biolab,Gloucestershire,UK).The vessels w ere added 5 m l fresh release m edium to m aintain their volum e.Release sam ples were analyzed by HPLC as outlined above.The release from m icroparticles w as com pared w ith dissolution rate of crystalline CEL pow der and the release from the physical m ixture of HPMCASand CEL.
2.9. Statistics
All experim ents w ere perform ed in triplicate(n=3)except for particle size m easurem ents w here 200 m icroparticles w ere m easured,and the results w ere indicated by m ean value±SD.
3. Results and d iscussion
Electrospraying is a one-step processing technique for preparation of microparticles,w hich does not involve elevated temperatures as w ith spray-drying[32,33].In order to prepare a solid dispersion system w ith electrospraying using a hydrophilic polym eric and poorly w ater-soluble drug solvent selection should be considered along w ith the solubility of the drug and polym er in the solvent[23,34].Further,not all solvents and com pounds are com patible w ith electrospraying,due to constraints w ith regards to surface tension,viscosity and electrical conductivity[35].In this study HPMCAS and CEL have different solubilities in the distinct solvents,and solvent m ixtures w ere thus investigated to assess their inf luence on the characteristics of resulting microparticles and their perform ance.ACE and three solvent m ixtures,ACE–EtOH,ACE–H2Oand EtOH–H2O,w ere exam ined,and w ere dem onstrated to have an inf luence on the particle size,particle morphology,drug distribution and release behavior,w hich are presented and discussed below.
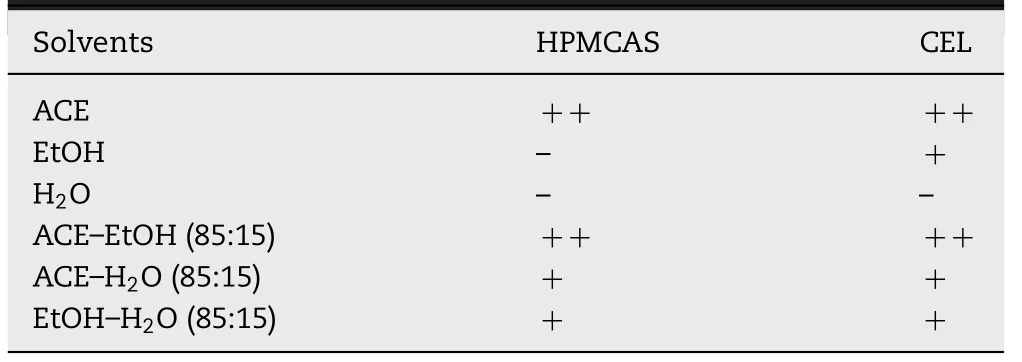
Table 1–Solubility of HPMCAS and CEL,in p ure solvents and solvent m ix tures.Solubility is given as,freely soluble(++),soluble(+)and practically insoluble(-).
3.1. Solubility of HPMCASand CEL
The solubility of HPMCAS and CEL in ACE,EtOH,w ater and their m ixtures w as studied.It w as observed that ACEis a good solvent for both HPMCAS and CEL(Table 1)dissolving up to 200 mg/ml HPMCASand 600 m g/m l CEL.Adding w ater or EtOH to the ACE solution containing HPMCAS and CEL decreased their solubilities.The sam e applied for CEL w hen w ater w as added to the EtOH solution,w hereas in the case of HPMCAS its solubility increased.CEL is practically insoluble in water w hereas HPMCASis sparingly soluble in w ater and EtOH individually.For a 50 m g/m l HPMCASsolution w ith CEL(20%,w/w)a stable solution w as obtained in ACE–H2O m ixtures up to a H2O content of 50:50(v/v),w hereas for ACE–EtOH mixtures a stable solution w as obtained at even higher EtOH contents.In EtOH–H2O m ixtures CEL w as soluble up to a H2O content of 75:25(v/v)indicating that in some m ixtures the solubility is lim ited by HPMCASw hereas in others it is lim ited by CEL.Finally,a ratio of 85:15(v/v)w as selected for all three solvent m ixtures(ACE–H2O,ACE–EtOH,EtOH–H2O)as electrospraying feed solutions,for w hich both HPMCASand CELcould be completely dissolved.
Due to the solvent ratio-dependent solubility of HPMCAS and CEL it w as expected to observe differences in particle form ation for m icroparticles prepared using different solvent m ixtures.Differences in the evaporation rate of the solvents used,is likely to result in earlier or later precipitation of HPMCAS and CEL.For instance,since ACE(b.p.56°C)evaporates quicker than EtOH(b.p.78°C)and EtOH evaporates quicker than H2O,the increasing EtOH and H2O content in the evaporating droplet could result in selective precipitation of HPMCASor CEL.

Table 2–Characteristics of electrosprayed CEL-loaded HPMCAS m icroparticles.
3.2. Characterization of microparticles
XRPD patterns for CEL,HPMCAS and electrosprayed sam ples are presented in Fig.2 and show that w hile unprocessed CEL exhibited crystalline features,a halo pattern w as observed for all electrosprayed sam ples indicating that CEL exists in the amorphous form in the microparticles.
The size of electrosprayed CEL-loaded HPMCAS m icroparticles from pure ACEand three co-solvent system s are show n in Table 2,indicating that all the m icroparticles w ere of similar size,ranging from 2 to 4μm in diameter.Microparticles prepared w ith ACEand ACE–EtOH w ere larger than those prepared w ith ACE–H2O,w hich w ere larger than those prepared w ith EtOH–H2O.This trend can be explained from the solubility of HPMCASin the different solvent mixtures,where the better solvent m ixtures resulted in larger m icroparticles compared w ith the poorer solvent m ixtures[36,37].Polym er chains are m ore extended in good solvents resulting in a higher viscosity and previous studies have demonstrated that larger microparticles are form ed due to the higher viscosity and larger droplets as w ell as the low er m obility of the polym er in the evaporating droplet[38,39].Yet,the differences observed in particle size were modest.

Fig.2–XRPD p atterns of CEL,HPMCAS and electrosprayed CEL-loaded HPMCAS microparticles.
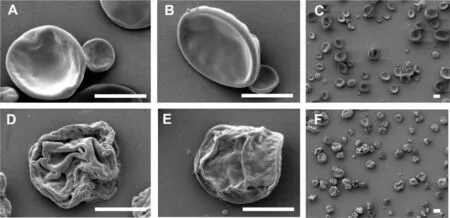
Fig.3–Representative SEM im ages of m icroparticles p repared w ith ACE(A,C),ACE-EtOH(B),ACE-H2O(D)and EtOH–H2O(E,F).The scale bars indicate 2μm.
Nevertheless,the differences in the m orphology of m icroparticles prepared w ith different solvent m ixtures w ere m ore pronounced(Fig.3).None of the m icroparticles prepared w ere spherical but w ere instead m ore disk shaped and f lat,indicating that the form ing m icroparticles had collapsed during solvent evaporation.The phenom enon is explained by the slow diffusion of high m olecular weight polym er,w hich cannot follow the evaporation of solvent,thus form ing a hollow structure that later collapses[40].This is also often observed for drying of colloidal suspensions and is know n as the“coffee ring effect”[41].Microparticles prepared w ith ACEand ACE–EtOH show ed sm ooth surfaces,w hile m icroparticles prepared w ith ACE–H2O and EtOH–H2O w ere corrugated w ith a rough surface.The degree of roughness of surface w as correlated w ith the solubility of HPMCAS in the solvent used and sim ilar observations on surface m orphology has been observed in previous studies[25,31].It w as also observed that the obtained m icroparticles w ere not agglom erated during storage,assum ing that the residual solvent did not affect the physical stability of the m icroparticles.
3.4. Surface chemistry analysis
The percentage of CEL on the particle surface w as detected by atom ic concentration of F,N and S atom s,w hich are not present in the HPMCAS,and is show n in Table 3.The drug loading eff iciency indicated that the total am ount of drug in the m icroparticles w as close to the original concentration added to the feed solution before electrospraying.Surface chemistry analysis showed that the surface of CEL-loaded microparticles have a CEL concentration betw een 19%and 25%,indicating a relatively homogenously distribution of CEL w ithin the m icroparticles.Microparticles prepared w ith EtOH–H2O had the low est surface CEL concentration.This could be explained by an early precipitation of CELas the EtOH evaporates and the H2O concentration increases in the evaporating droplet.Later precipitation of CEL m ay allow the CEL m olecules to diffuse out to the particle surface through the polymer network.How ever,differences in surface CELconcentration w ere relatively m inor.The results are partially in agreem ent w ith the observations from particle size and m orphology w here the solubility of HPMCASin the solvent m ixture inf luenced the particle formation,although w ith the additional inf luence of CEL solubility.The process is com plex due to the balance betw een polym er precipitation,drug diffusion in the polym er m atrix,and the evaporation of the tw o solvents in the mixtures.
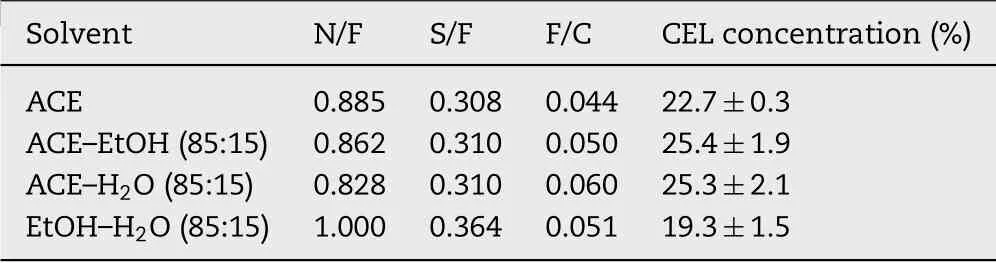
Table 3–Surface chem ical com p osition of CEL-loaded HPMCAS m icrop articles p repared from the d ifferent solvent system s.
3.5. Drug release study
Drug release studies dem onstrated a substantial im provem ent in the dissolution rate of electrosprayed solid dispersions com pared w ith the crystalline CELpow der and the physical m ixture of CEL and HPMCAS(Fig.4).Crystalline CEL and physical m ixture of CELand HPMCASboth resulted in approx.5%drug release after 4 h,indicating no inf luence of physically blended HPMCASon CEL dissolution at this sm all am ount.At the same tim e the electrosprayed solid dispersions dem onstrated 45%–65%drug release indicating a 10-fold increase in dissolution rate.For all electrosprayed sam ples CEL w as supersaturated and rem ained supersaturated at approximately 4 times the equilibrium solubility of CEL for the duration of the dissolution study.
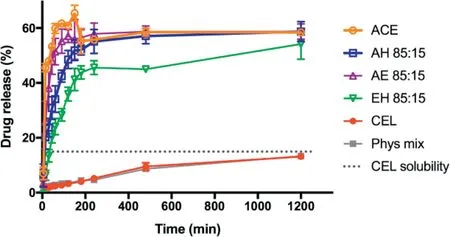
Fig.4–Drug release from electrosp rayed m icrop articles in PBS.Values are presented as the m ean±SD(n=3).

Fig.5–Drug release from electrosprayed m icroparticles in PBS w ith 1.5%SLS.Values are presented as the m ean±SD(n=3).
This indicates that HPMCAS facilitated the dissolution of CEL and subsequently prevented the recrystallization of CEL in the dissolution m edium by interacting w ith the dissolved CELm olecules,as observed in other studies[42,43].The results dem onstrate that the parachute effect of HPMCASoccurred at a relatively low concentration of 22μg/ml.
All electrosprayed sam ples reached a drug release of 55%–60%w ith slight differences in the dissolution rate betw een the sam ples.Solid dispersions prepared w ith ACEreleased the quickest followed by those prepared in ACE–EtOH,ACE–H2O and EtOH–H2O,respectively.This is directly contradictory to the size and m orphology f indings w here m icroparticles prepared w ith ACEand ACE–EtOH w ere larger w ith a sm ooth surface and were therefore expected to result in slower dissolution than the sm aller m icroparticles w ith rough surfaces prepared using ACE–H2O and EtOH–H2O.This could possibly be explained by differences in the drug distribution w here m icroparticles prepared w ith ACE,ACE–EtOH and ACE–H2O exhibited a higher surface drug loading com pared w ith those prepared w ith EtOH–H2O.Although in this study drug release w as only tested in release m edia w ith a p H above 6.8,it is expected that microparticle disintegration and drug release would not take place at low p H values below 5.5 due to the p H dependent solubility of HPMCAS.
The drug release from electrosprayed solid dispersions w as also studied under sink conditions w ith addition of SLS(CEL solubility>1 mg/ml)(Fig.5).Here a full dissolution of the added CEL is observed as expected and only sm all variations in the dissolution rate are detected for the different electrosprayed sam ples.As under non-sink conditions the electrosprayed sam ples show ed a faster drug release com pared w ith crystalline CEL and the physical m ixture of CEL and HPMCAS.In this case,the physical m ixture show ed a slightly faster dissolution rate com pared w ith CEL.The drug release from electrosprayed sam ples w as again slightly dependent on the solvent m ixture used for preparation and again the sam e trend w as observed.Microparticles prepared w ith ACE had the fastest release w hile m icroparticles prepared with EtOH–H2Ohad the slow est drug release.This suggests that the m icroparticles prepared using different solvent m ixtures have differences in drug distribution as w ell as polym er conform ation,w hich results in differences in their drug release kinetics.Those w ith the fastest drug release(ACE,AH 85:15)also show ed higher solubility for both polym er and drug and are likely to have a f iner drug dispersion in the polym er m atrix,resulting in quicker drug dissolution rate.These f indings show that solvent m ixtures can be used as a w ay to vary the solubility of the solutes in the feed solution and thereby m odifying the characteristics and the drug release from the resulting m icroparticles.
4. Conclusion
HPMCAS solid dispersions loaded w ith CEL w ere prepared by electrospraying using different feasible solvent m ixtures to investigate if the particle characteristics and drug release prof iles were inf luenced by the solvent composition.All CELloaded HPMCAS m icroparticles produced by electrospraying w ere am orphous and their size and m orphology w ere dependent on the solubility of HPMCAS in the solvent m ixture.All m icroparticles resulted in rapid release of CELand m aintained a supersaturation 4 tim es higher than the solubility of CEL based on the precipitation inhibition by HPMCAS.Ow ing to the different solubility of CEL and HPMCAS in solvent mixtures,the electrospraying solvent m ixtures inf luenced the m orphology,the surface drug distribution and the release prof iles of m icroparticles.The present study highlighted the feasibility to enhance the release of poorly w ater-soluble drug and inhibit the recrystallization by using electrosprayed HPMCAS solid dispersion,and the possibility to alter the particle characteristics by m odifying solvent com position.
Conf licts of interest
The authors report no conf licts of interest associated w ith this manuscript.The authors alone are responsible for the content and w riting of this m anuscript.
Subm ission d eclaration
All authors have contributed to the conception and design of the study and acquisition,analysis and interpretation of data.All authors have drafted the article and revised it critically for im portant intellectual content.All authors have approved the f inal article.The authors certify that this m anuscript,or any part of it,has not been published and w ill not be subm itted elsew here for publication w hile being considered by the Asian Journal of Pharm aceutical Sciences.
Role of funding source
The Danish research council provided f inancial support for the conduct of the research.The funding source had no involvem ent in the study design;in the collection,analysis and interpretation of data;in the w riting of the report;or in the decision to subm it the article for publication.
Acknow led gm ent
The authors w ould like to thank the Danish Council for Independent Research(Grant No.DFF-12-131927)for f inancial support of this project.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Sp ecial issue on“Form ulation strategies and m anufacturing technologies to enhance non-invasive drug delivery”
- Characterization of m od if ied m esop orous silica nanop articles as vectors for siRNA d elivery
- Ad d itive m anufacturing of p rototyp e elem entsw ith p rocess interfaces for continuously op erating m anufacturing lines
- Tunable and sustained-release characteristics of venlafax ine hyd rochlorid e from chitosan–carbom er m atrix tablets based on in situ form ed p olyelectrolyte com p lex f ilm coating
- PEPT1-m ed iated p rod rug strategy for oral d elivery of p eram ivir
- Prep aration,characterization,and in vitro/vivo evaluation of p olym er-assisting form ulation of atorvastatin calcium based on solid d ispersion technique
