Rotifer community structure and its response to environmental factors in the Backshore Wetland of Expo Garden,Shanghai
2018-05-04LipingYinYuJiYinjiangZhangLinxuanChongLijingChen
Liping Yin,Yu Ji,Yinjiang Zhang,Linxuan Chong,Lijing Chen,*
aNational Demonstration Center for Experimental Fisheries Science Education,Shanghai Ocean University,Shanghai 201306,China
bKey Laboratory of Freshwater Aquatic Genetic Resources,Ministry of Agriculture,Shanghai Ocean University,Shanghai 201306,China
cShanghai Collaborative Innovation for Aquatic Animal Genetics and Breeding,Shanghai Ocean University,Shanghai 201306,China
dShanghai Universities Engineering Research Center of Water Ecological Environment,Shanghai Ocean University,Shanghai 201306,China
1.Introduction
The Expo Garden of Shanghai(2010)is located in downtown Shanghai,China.The Backshore Wetland lies in the southwest of the Expo Garden(31°11′N,121°28′E,6 m above sea level),covers an area of 14.2 km2,extends for 1.7 km along the coastline,and is the only natural wetland in Shanghai along the Huangpu River(Zhang,Wu,&Zhang.,2007).It provides many bene fi ts,including urban ecological security,regulation of runoff,amelioration of pollution,protection of species diversity,and beauti fi cation of the environment(Lin,Zhang,&Dai,2007).
Rotifers are important groups of zooplankton in fresh water.They are characterized by short development time,fast turnover,high production,and play vital roles in aquatic ecosystems via the circulation of matter and in energy transfer(Li,Cheng,&Chen,2005).Rotifers are indicators of nutrient levels and can be characterized by their physical and chemical attributes and as the diversity of the habitats in which they live(Zhao,Fang,&Ji,2003).Their ecological characteristics can be used as a basis for water quality evaluation because the species composition is sensitive to environmental changes.Many researchers have exploited the indicative features of rotifers for biological monitoring(Bai,Zhang,&Lu,2006;Buijse,Coops,&Staras,2002;Timothy,Sullivan Barbara,Donald,&Charles,1996)and for assessing water quality.Moreover,factors that have an influence on the biological community are often re fl ected in the population dynamics and changes in the community structure and functions of rotifers(Chovanec,Schiemer,&Waid bacher,2002;Dong,2004;Li et al.,2005).A thorough knowledge of the dynamics of rotifer community structure is essential for an understanding of the structure and function of aquatic ecosystems.
To date,there have been few reportson the community structure of rotifers in the Backshore Wetland of the Expo Garden.To explore temporal and spatial distribution of community structure,and its relationship with water quality at this location,some preliminary research was necessary to provide a scientific basis for ecological restoration,the reasonable use and protection of wetland resources,and the understanding of a benign aquatic ecological system.
2.Materials and methods
2.1.Sampling sites and time
Water enters the Backshore Wetland of Expo Garden via the Huangpu River and natural rainfall,and drainage is influenced by matrix filtration,adsorption,sedimentation,nitrogen and phosphorus removal,heavy metal adsorption,and wetland Purification.Ten sampling sites were chosen according to runoff,sediment content and water level,combined with topography,landform,water bank conditions,and river formation(Fig.1).Observations were made monthly from September 2009 to the middle of August 2010.
2.2.Methods
The collection and treatment of samples were carried out using the methods outlined by Huang(1999,pp.72—791).We collected quantitative samples at 0.5 m(the total depth of water was always less than 2 m)four times using a 2.5 L stainless steel column water collector for a total of 10 L of sampled water from each station.The collected water was put into a single bucket and mixed evenly before resampling a1 Laliquot that was fixed and dyed using Lugol's solution.Samples were preserved in a neutral formalin solution(4%)(May&O'Hare,2005).We would pour the water into the pear-shaped separatory funnel in the laboratory.After 48 h,upper-layer water was removed while we collected the bottom 50 mL into a serum bottle.The rotifer species were identified according to the classification system for freshwater rotifers proposed by Wang(1961)and Koste(1978).The approximate geometrical shape of the rotifers was decided,their volumewas estimated using the quadrature formula,biomass was determined using the volumetric method(Zhang&Huang,1991,pp.257—258).Pollution indicator species were identified according to Zhang&He,1991).
Determination of physical and chemical factors of samples(depth[Dep],water temperature[WT],pH,dissolved oxygen[DO]and water transparency[SD])were measured in situ.At the same time,1 L of water was collected for analysis of other parameters such as permanganate index(CODMn), five-day biochemical oxygen demand(BOD5),total nitrogen(TN),nitrate nitrogen(NO3-N),nitrite nitrogen(NO2-N),ammonia nitrogen(NH4-N),total phosphorus(TP),phosphate phosphorus(PO4-P),and total suspended solids(TSS).These parameters were determined in accordance with the standard methods.We used the acetone method(Zhang&Huang,1991,pp.257—258)to measure the content of chlorophyll a(Chl.a).
2.3.Biodiversity index calculations
We calculated multiple indices to describe the biological diversity within samples.These indices included:
· Shannon-Wiener diversity index(H′):the comprehensive index of species richness and a measure of the uniformity of the distribution of individuals,reflecting the complex degree and stability of community structure.Described by the formula H′=-∑(ni/N)ln(ni/N);
·Margalef richness index(D):reflects the richness of species number and individuals.Described bythe formula D=(S-1)/lnN;
·Pielou evenness index(J):reflects the evenness of the individual distribution among species.Described by the formula J=H′/lnS;
·Degree of dominance(Y)(Xie,Fu,&Liu,2004):Y=(ni/N)×fi
Where niis the density of the ith species in one site;N is the total density;S is the total number of species;fiis the appearance rate of the ith.A species is considered dominant if Y is more than 0.02.
2.4.Data processing and analysis
We used SPSS18.0 software and CANOCO4.5 package for statistical analysis of the data.The correlations between rotifers and environment parameters were investigated by Pearson analysis and canonical correspondence analysis(CCA)(Leps&Smilauer,2003,pp.168—250;Ter Braak,1986).The responses of the community structure to variable environmental factors were described by environmental variables and the main density of rotifers.SPSS18.0 was used to quantitatively analyze the influences of swap-in factors on the community structure of rotifers in the Backshore Wetland of the Expo Garden Shanghai,such as comparison of the density and biomass of different sites,by One-Wayanalysis of variance(ANOVA).
3.Results
3.1.The physical and chemical factors
The main environmental factors are shown in Table 1.There were no significant differences among sites.The preliminary Pearson correlation analysis indicated that there was a significant negative correlation between rotifer population density and pH(r=-0.891,n=120,P<0.01),whereas there were significantly positive correlations between density and TP,biomass,and DO(r=0.923,n=120 P<0.01).
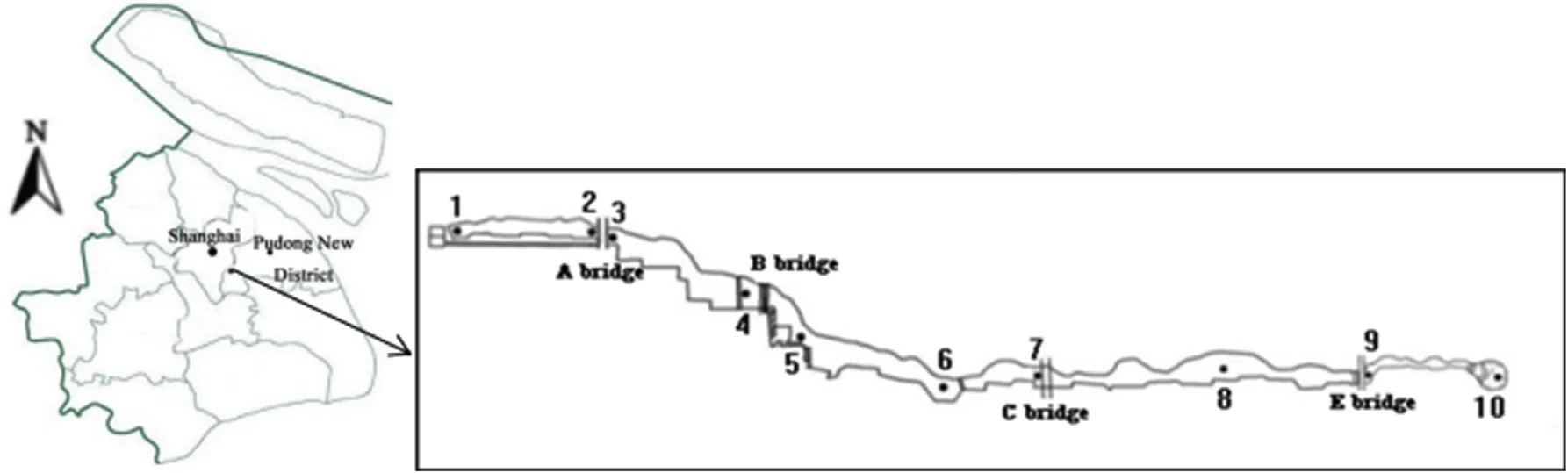
Fig.1.The distribution of sampling sites in the Backshore Wetland of the Expo Garden,Shanghai.
3.2.Rotifer species composition and pollution indicator species
We identified 116 species,belonging to 19 families and 44 genera,in the Backshore Wetland of the Expo Garden between September 2009 and August 2010(Table 2).Lecanidaewas the bestrepresented family(24 species;accounting for 20.69%),followed by Brachionidae(16 species).There was only one species from Conochilidae and Atrochidae(accounting for just 0.86%).Monostyla(14 species),Lecane(11 species),Brachionus(8 species),and Trichocerca(7 species)accounted for 12.07%,9.48%,6.90%and 6.03%,respectively.There were more rotifer species in summer(75 species)than in spring(44 species).
We found 99 pollution-indicator species in the Backshore Wetland,among which there are 34 oligomeric species,33 oligomeric-β-mesotrophic species,24 β-mesotrophic species,5 β-αmesotrophic species,and 3α-mesotrophic species,accounting for 34.34%,33.33%,24.24%,5.05%and 3.03%,respectively.
3.3.Seasonal variation of dominant species
Seven abundant species were con fi rmed according to annual density(listed here with the corresponding degrees of dominance in parentheses):Polyarthra trigla(0.2),Keratella cochlearis(0.10),Cephalodella exigua(0.04),Synchaeta oblonga(0.03),Trichocerca pusilla(0.03),Anuraeopsis fissa(0.02),and Filinia terminalis(0.02).Since the present nomenclatural status of Polyarthra trigla is species inquired(Jersabek&Leitner,2013;Segers,2007),it was replaced by Polyarthra sp..Eighteen abundant species were con fi rmed according to seasonal average density with apparently seasonal variation(Table 3).There was some seasonal variation in the abundant species and their numbers.Polyarthra sp.was the only common species that was abundant throughout the four seasons,with greatest abundance in summer and autumn;Rotaria neptunia was the abundant species of spring and summer;whereas Anuraeopsis fissa was the abundant species of summer and autumn.Keratella cochlearis and Synchaeta oblonga were the abundant species in winter and spring.Cephalodella exigua was the abundant species in autumn and winter.The others were abundant in a single season.
3.4.Monthly variation of density and biomass
The annual average(±SD)density of rotifers in the wetland was 815±1346 ind./L between September 2009 and August 2010(range:238—2723 ind./L).There were three peaks in annual density:the highest peak was in summer(June)with 2723±2926 ind./L;the second peak was in spring(April)with 1565±1556 ind./L;and the third peak was inwinter(December)with 1126±1624ind./L.The lowest density,238±271 ind./L,occurred in May.The order of density from highest to lowest was summer,spring,winter,and autumn.One-way ANOVA indicated that there were significant differences in the density of rotifers in different months(F=3.432,P<0.01)(Fig.2).
Similarly,the annual average biomass of rotifers in the wetland was 0.3649 ± 0.4940 mg/L(range:0.0476—0.7938 mg/L).Three peaks also occurred during the year:the highest peak was in summer(June)with 0.7938±0.8197 mg/L;the second peak in spring(March)with 0.6144±0.4308 mg/L;and the third peak in winter(December)with 0.4366±0.6992 mg/L.The lowest biomass,0.0476±0.0429 mg/L,occurred in May.The order of biomass from highest to lowest was winter,summer,spring,and autumn.One-Way ANOVA indicated that there were significant differences in the biomass of the rotifers in different months(F=2.128,P<0.05)(Fig.2).
3.5.Horizontal distribution of rotifers
The horizontal distribution of the rotifers ateach siteis shownin Fig.3.The highest density was at the 9thsite(1473±2623 ind./L),accounting for 18.08%of the total;the second highest density was at the 8thsite,(1409±1615 ind./L);and the lowest density was at the5thsite(327±247 ind./L),which was 4.5 times lower thanat the 9thsite(P<0.05).
The highest biomass of 0.4951±0.6454 mg/L occurred at the 1stsite,accounting for 13.62%,and the lowest of 0.1910±0.2993 mg/L occurred at the 5thsite,but the difference between them was not significant.
3.6.Spatiotemporal distribution of diversity
The Shannon-Wiener index(H′)varied from 0.79 to 1.90,with an annual average value of 1.46±0.63.The Margalef richness index(D) fluctuated from 0.51 to 1.95,with an annual average value of 1.14±0.62.The highest values of both indices occurred in June(1.87±0.87 and 1.95±0.49,respectively);the lowest values occurred in September(0.79±0.50 and 0.51±0.27,respectively).The Pielou evenness index(J)ranged from 0.64 to 0.88,with an annual average value of 0.78±0.18(Fig.4).The highest value of 0.88±0.05occurred in November and the lowest value(0.64±0.28)occurred in September.The three indices of seasonal variation followed the same pattern,i.e.,highest values in summer and lowest in spring,with significant differences among the months(PH’=0.002,PJ=0.000,PD=0.018).
Fig.5 depicts the distributions of the Shannon-Wiener index(H′),the Margalef index(D),and the Pielou index(J)among sites.The highest values of all three indices occurred at the 4thsite(1.74±0.58,1.33±0.69,and 0.87±0.08,respectively),whereas the lowest values occurred at different sites:1.21±0.57 at the 7thsite for H′;1.00± 0.59 at the 10th site for D,and 0.67± 0.22 at the 8thsitefor J.Analysis indicated that there was a significant difference in H′between the 4thand the 7thsites,no significant difference in D,and a significant difference in J(F=2.097,P=0.037).
3.7.CCA analysis between community structure and environment
Twenty- five species with a frequency of more than 40%and a relative density of more than 0.3%were used for CCA analysis,and the corresponding symbols are depicted in Table 4.The eigenvalues of the former two axes were 0.278 and 0.147,accounting for 22.5%of the total eigenvalue(Fig.6).The species-environment correlations were 0.798 and 0.770,respectively,indicating that the result were reliable and accurately reflected the relationship between rotifers and environmental factors.
Nitrites,water temperature,pH and dissolved oxygen(DO)had higher correlation with the 1staxis in the CCA biplot,whereastemperature,pH,and TP showed higher correlation with the 2ndaxis,indicating that temperature increased from left to right,and pH decreased from up to down.

Table 1 Environmental parameters of the Backshore Wetland of the Expo Garden,Shanghai,between September 2009 and August 2010.

Table 2 The rotifers observed in the Backshore Wetland of the Expo Garden,Shanghai,and the corresponding symbols of species used in canonical correspondence analysis(CCA).

Table 3 Degrees of dominance of the rotifer species in different seasons in the Backshore Wetland of the Expo Garden,Shanghai.
Fig.6 shows that temperature is an important environmental factor affecting the community structure of rotifers.In summer,there were some warm-water species,such as Anuraeopsis fissa,Conochilus hippocrepis,Keratella valga,Monostyla hamata,Rotaria neptunia,and Encentrum felis,whereas there were some cold-water species such as Keratella quadrata,Synchaeta stylata,and Synchaeta pectinata occurred in winter.Eurythermic species such as Polyarthra sp.,Monostyla closterocerca,and Diurella tenuior mostly occupied the middle of the biplot.
4.Discussion
4.1.Characteristics of species distributed in the backshore wetland
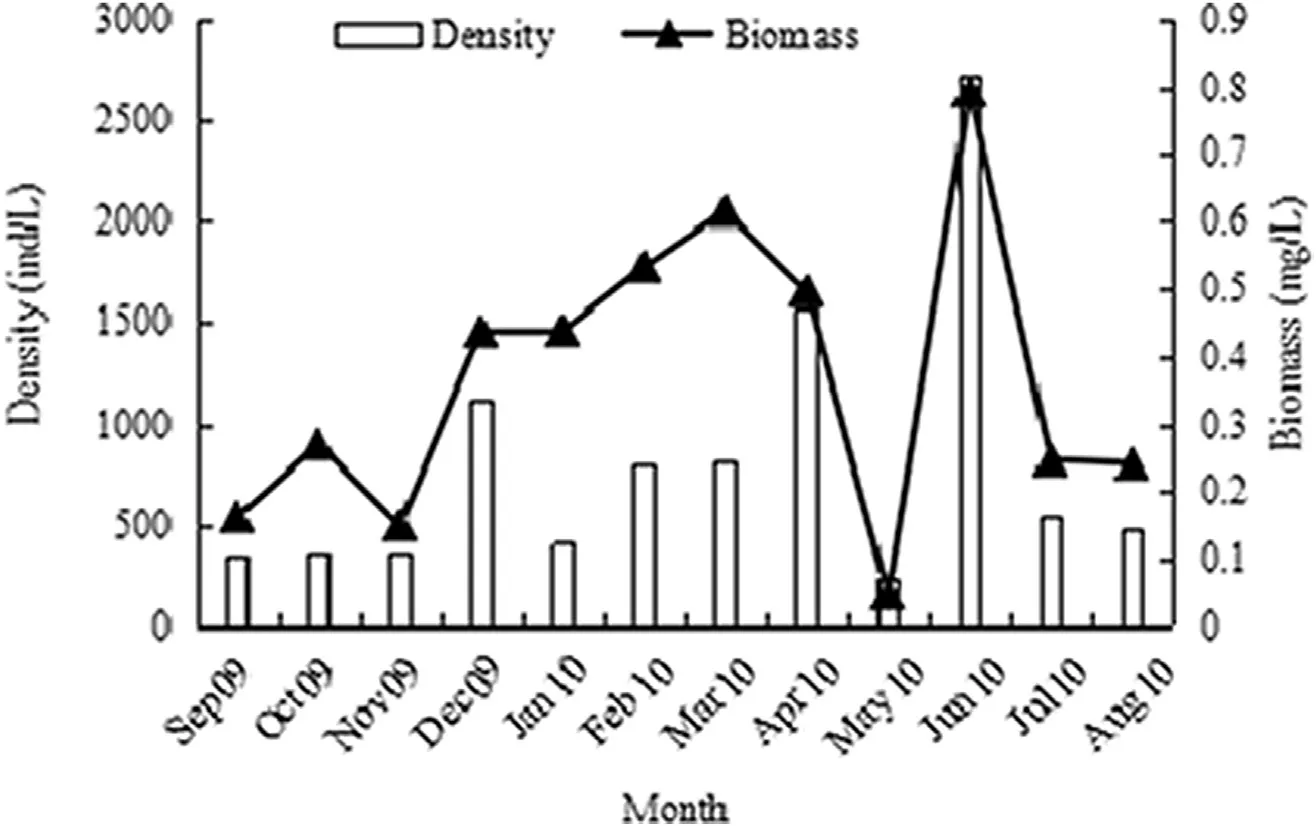
Fig.2.Annual variation in density and biomass of rotifers in the Backshore Wetland of the Expo Garden,Shanghai.
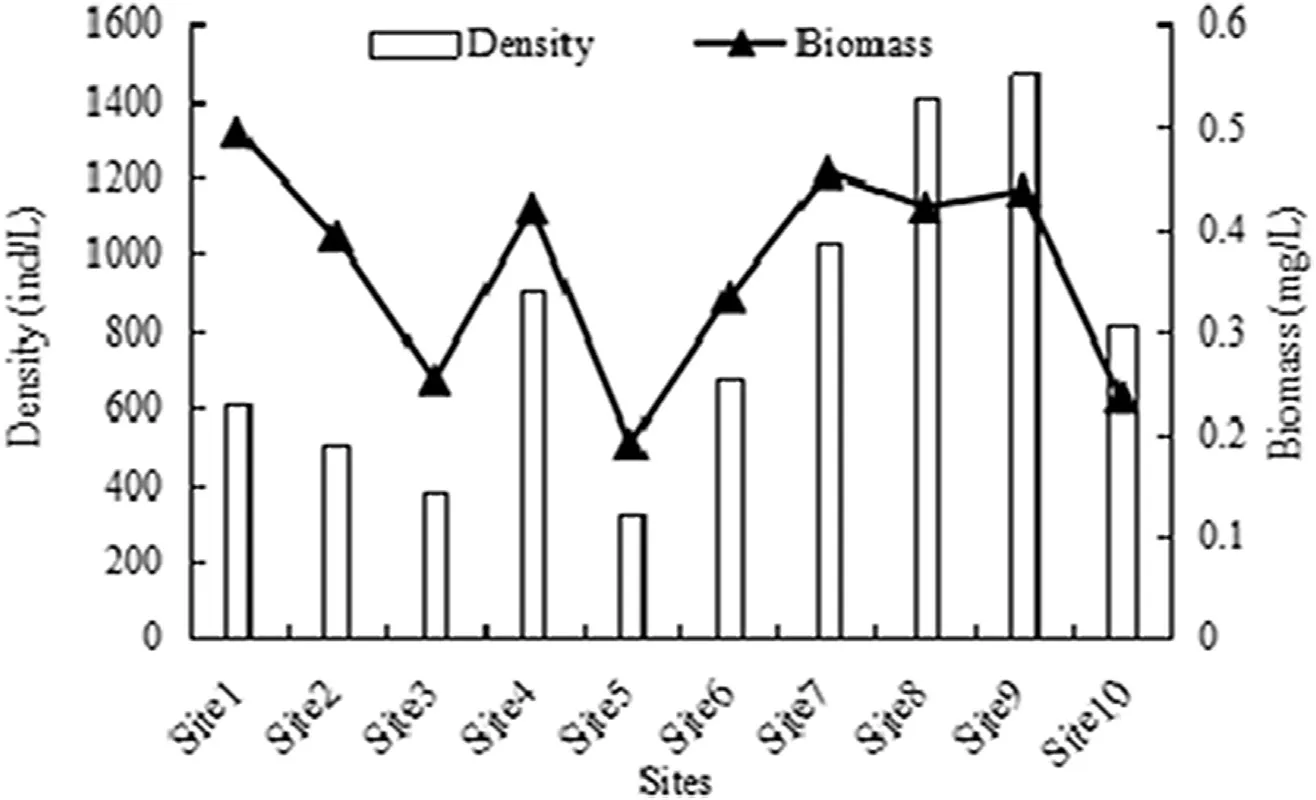
Fig.3.Horizontal variation in density and biomass of rotifers in the Backshore Wetland of the Expo Garden,Shanghai.

Fig.4.Monthly variation of diversity indexes of rotifers in the Backshore Wetland of the Expo Garden,Shanghai.
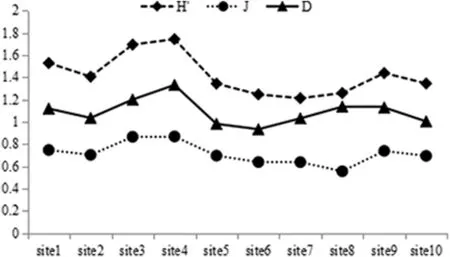
Fig.5.Horizontal variation in diversity indices of rotifers in the Backshore Wetland of the Expo Garden,Shanghai.
The genera Lecane,Brachionus,and Trichocerca account for the most common and dominant species in tropical and subtropical areas(Fernando,1980),and the majority of rotifer species.The Backshore Wetland of the Expo Garden,Shanghai,has a subtropical maritime monsoon climate,with distinct seasons,sufficient sunshine,and an average water temperature of 20.0±8.8°C.We identified 116(mostly eurythermic)rotifer species,and the generaMonostyla, Lecane, Brachionus, and Trichocerca accounted for themajority of species. With regard to habitat variation, there was anabundance of benthic and periphyton species, such as thosebelonging to the genera Monostyla, Lecane, Lepadella, Testudinella,Mytilina, Notommata, and Scaridium, which are adapted to cleanwater. The rate of water flow in the wetland may influence thespecies found there; benthic and periphyton species find favorableliving conditions, and have no competition compared with planktonicspecies living in open areas of still water, which tend to bepredated by Asplanchna and Copepoda. In the flowing water, thedensities of planktonic species were in inhibited, whereas thebenthic and periphyton species survived well feeding on bacteria andzooplankton. For instance, Monostyla bulla consumes cyanobacteriaand is widely distributed in openwater (Green, 2003). Furthermore,the number of emergent aquatic and submerged plants may beanother important factor, providing an ideal habitat for the rotifers.

Table 4 Codes of rotifera species for CCA.

Fig.6.Canonical correspondence analysis(CCA)biplot of rotifer species and environmental variables in the Backshore Wetland of the Expo Garden,Shanghai.
Rotifers are an important biological indicator of an aquaticecological system. Opinion differs among scholars on which are thebest indicator species (Arndt, 1993; Wang, Liu, & Yang, 2010). It isgenerally acknowledged that Synchaeta stylata, Ascomorpha ovalis,and Conochilus unicornis are oligotrophic species, and there arefactors, and abundance of food items (Berzins & Pejler, 1989). Keratellacochlearis, and Synchaeta oblonga consume algae, Trichocercaspecies suck the cell contents of filamentous algae (Pourriot, 1977),and Anuraeopsis fissa mainly eat detritus (Wen, Xi, & Zhang, 2006).
4.2.Effect of environmental factors on the community structure of rotifers
Dumont(1977)and Gulati(1990)found that food is the main factor that affects rotifer population abundance and seasonal succession.Generally,the abundance of rotifers depends on their food supply.Though thenumberof rotifers inawaterbody isaffectedbya series of external environmental factors,the availability of food is themostdecisivefactor(Wang,1961).Rotifersselectfood depending on their different corona and mastax.Thus,their growth,development,reproduction,and even community structures are mainly dominated by the species,shape,body volume,nutritional factors,and abundance of food items(Berzins&Pejler,1989).Keratella cochlearis,and Synchaeta oblonga consume algae,Trichocerca species suck the cell contents of fi lamentous algae(Pourriot,1977),and Anuraeopsis fi ssa mainly eat detritus(Wen,Xi,&Zhang,2006).
CCA analysis showed that water temperature,pH,total phosphorus,and nitrite nitrogen were the main factors that in fl uence distribution of rotifers.Water temperature has always been regarded as the key parameter that affects rotifer occurrence and seasonal succession(Berzins&Pejler,1989).Thermal adaptability varies among rotifers,even within the same genus,and the highest growth rate occurs as water reaches the optimum temperature(Herzig,1987).The long-term observation of Lake Dong revealed that the peak density of rotifers corresponds to water temperatures above 20°C;where,as the temperature rises,the egg development time is shortened.Simultaneously,phytoplankton and animal carcasses are quickly fragmented by the action of bacteria,thereby speeding up turnover and density(Zhang&Huang,1991,pp.257—258).Therefore,temperature,phytoplankton abundance,and rotifer numbers are inextricably linked.A CCA biplot of the main species and environmental variables also showed that water temperature was one of the most important factors that affected the abundance and distribution of rotifers in this wetland.In winter(December),the 1st distribution peak appeared as Monostyla closterocerca,Cephalodella exigua,and Synchaeta oblonga became the dominant species.Moreover,bacteria,algae,autotrophic flagellates,Balantidium minutum,and detritus are the food resources of rotifers and phytoplankton are relatively abundant in winter,and provide better quality food.The 2nd peak occurred in April as the temperature increased;Keratella cochlearis,Polyarthra sp.,and Synchaeta stylata became the dominant species,probably owing to their feeding habits.Algae are the main food of Keratella cochlearis and Synchaeta stylata;the former filter 1—15 μm suspended particles for food,whereas the latter can eat pico-level algae that dominate oligotrophic water,which conveniently avoids competition for food.Arndt(1993)considered Keratella cochlearis to be a very important consumer,and Baiao and Boavida(2000)indicated that Keratella cochlearis reaches higher density at temperatures between 11 and 21°C.Polyarthra sp.is a eurythermic species,living in areas of wide temperature range,their density increased with greater availability of food and increasing temperature(Huang,Hu,&Wu,1985).Furthermore,the mouthparts of polyarthra sp.are virgate type,tearing and absorbing the liquid of various phytoplankton cells and animal prey.Anuraeopsis fissa is a polythermal species and their intrinsic rate of population growth increases at temperatures above 25°C,which assists their rapid rise to dominance(Huang et al.,1985).Trichocerca pusilla lives in warm water,often appearing in summer at temperatures above 15°C and continuing into autumn at below 10°C,with an optimum temperature between 25 and 29°C.The CCA biplot also showed that the numberof Polyarthra sp.and Anuraeopsis fissa,located in the 4th quadrant,were significantly correlated with temperature.
Rotifer species occurrence and abundance are closely related to pH in fresh water.According to the pH preferences,rotifers can be divided into three groups:alkaliphilic,euryionic,and acidophilic.Generally,there are many species but little abundance in acidic water,whereas the reverse is true in alkaline environments(Zhang&Huang,1991,pp.257—258),as was clear in our research.The effects of nutrients on rotifers are mainly due to the indirect functions of phytoplankton.Changes in nutrient levels,especially the contents of nitrogen and phosphorus,can result in the alteration of feeding habitats of rotifers,which influences their community composition and density.Pearson analysis indicated that the species number and density of rotifers had significant negative correlation with NO2-N and NH4-N(F=-0.200,-0.256;P=0.044,0.008),but highly positive correlation with TP(F=0.218,0.253;P=0.025,0.009),which agreed with the findings of Chen(Chen,Zhong,&Zhang,2008)regarding zooplankton and its relationship with water quality.
Fund project
Project of Shanghai Municipal Committee of Fisheries Animal Genetics and Breeding Center of Shanghai Collaborative Innovation Center(ZF1206);National Science and Technology Commission of Scientific and Technological Research of Expo special(2005ba908b23);Shanghai Science and Technology Commission Expo special(05dz05823)
Acknowledgement
The authors are thankful to the anonymous referees and their valuable suggestions.This study was mainly supported by Project of Shanghai Municipal Committee of Fisheries Animal Genetics and Breeding Center of Shanghai Collaborative Innovation Center(ZF1206),Project of Science and Technology Commission of Shanghai Municipality(11dz1205000),and National Science and Technology Commission of Scientific and Technological Research Expo special.We would like to gratefully acknowledge Yan Jiang,Zhen Mei,Wei Zhang,and Hua Zhu for participating in part of the field investigation and laboratory tests.
Arndt,H.(1993).Rotifersas predators on components of the microbialweb(bacteria,heterotrophic flagellates,ciliates)-a review.Hydrobiologia,255/256,231—246.
Baiao,C.,&Boavida,M.(2000).Environmental factors determining the structure of the rotifer communities in a river-shed reservoir.Aquatic Ecology,34,369—377.Bai,S.Q.,Zhang,C.M.,&Lu,S.G.(2006).Study on urban channel ecological rehabilitation based on keeping the health of river.Yellow River,28(8),3—4(in Chinese with English abstract).
Berzins,B.,&Pejler,B.(1989).Rotifer occurrence in relation to temperature.Hydrobiologia,175,223—231.
Buijse,A.D.,Coops,H.,&Staras,M.(2002).Restoration strategies for river fl oodplains along large low land rivers in Europe.Freshwater Biology,47(4),889—907.
Chen,G.R.,Zhong,P.,&Zhang,X.F.(2008).Zooplankton and its relationship with water quality in huizhou west lake.Journal of Lake Sciences,20(3),351—356.
Chovanec,A.,Schiemer,F.,&Waidbacher,H.(2002).Rehabilitation of a heavily modi fi ed river section of the Danube in Vienna(Austria):Biological assessment of landscape linkages on different scales.International Review of Hydrobiology,87(2—3),183—195.
Dong,Z.R.(2004).The enlightenment from the Kissimmee River ecological restoration project.Water Resources and Hydropower Engineering,35(9),8—12(in Chinese with English abstract).
Duggan,C.,Green,J.D.,&Shiel,R.J.(2001).Distribution of rotifers in North Island,New Zealand,and their potential use as bioindicators of lake trophic state.Hydrobiologia,446/447,155—164.
Dumont,H.J.(1977).Biogeography of rotifers.Hydrobiologia,104,19—30.
Fernando,C.H.(1980).The freshwater zooplankton of Sri Lanka,with a discussion of tropical freshwater zooplankton composition.Hydrobiologia,65(1),85—125.
Green,J.(2003).Associations of planktonic and periphytic rotifers in a tropical swamp,the Okavango Delta.Southern Africa.Hydrobiologia,490,197—209.
Gulati,R.D.(1990).Zooplankton structure in the Loosdrecht lakes in relation to trophic status and recent restoration measures.Hydrobiologia,191,173—188.
Herzig,A.(1987).The analysis of planktonic rotifer populations:A plea for longterm investigations.Hydrobiologia,147,163—180.
Huang,X.F.(1999).Observation and analysis of lake ecological survey.Beijing:China Standard Press.
Huang,X.F.,Hu,C.Y.,&Wu,Z.T.(1985).The rotifer of wuhan east lake.Acta Hydrobiologia Sinica,2,129—143(in Chinese with English abstract).
Jersabek,C.D.,&Leitner,M.F.(2013).The rotifer world catalog.World Wide Web electronic publication.http://www.rotifera.hausdernatur.at/.
Koste,W.(1978).Rotatoria.Berlin,Stuttgart:Gebrüder Borntraeger.
Leps,J.,&Smilauer,P.(2003).Multivariate analysis of ecological data using canoco.Cambridge University Press.
Li,J.Z.,Cheng,N.N.,&Chen,Q.J.(2005).Progress in the study of bioremediation for polluted water bodies.Chinese Journal of Environmental Engineering,6(1),25—30(in Chinese with English abstract).
Lin,G.X.,Zhang,L.,&Dai,J.(2007).An image of world EXPO park Shanghai.Chinese Landscape Architecture,23(6),38—40(in Chinese with English abstract).
May,L.,&O'Hare,M.(2005).Changes in rotifer species composition and abundance along a trophic gradient in Loch Lomond,Scotland,UK.Hydrobiologia,546,397—404.
Pourriot,R.(1977).Food and feeding habits of rotifer.Arch Hydrobiol Beih Ergebn Linnol,8,243—260.
Segers,H.(2007).Annotated checklist of the rotifers(Phylum Rotifera),with notes on nomenclature,taxonomy and distribution.Zootaxa,1564,1—104.
Ter Braak,C.J.F.(1986).Canonical correspondence analysis a new eigenvector technique for multivariate direct gradient analysis.Ecology,67(5),1167—1179.
Timothy,J.,Sullivan,Barbara,McMartin,Donald,F.,&Charles.(1996).Re-examination of the role of landscape change in the acidi fi cation of lakes in the Adirondack Mountains,New York.The Science of the Total Environment,183,232—236.
Wang,J.J.(1961).Records of freshwater rotifera in China.Science Press.
Wang,Q.,Liu,L.J.,&Yang,Y.F.(2010).Response of rotifer community characteristics to environmental factors in a reservoir,Southern China.Acta Ecologica Sinica,20(13),3385—3395(in Chinese with English abstract).
Wen,X.L.,Xi,Y.L.,&Zhang,L.(2006).Analysis of community structure of rotifer and ecological assessment of water quality in Lake Jinghu,Wuhu City.Acta Hydrobiologica Sinica,30(2),152—158(in Chinese with English abstract).
Xie,G.L.,Fu,R.N.,&Liu,J.L.(2004).The community distribution of soil oribatida in Heze Peony garden.Acta Ecologica Sinica,24(4),693—699(in Chinese with English abstract).
Yoshida,T.,Urabe,J.,&Elser,J.(2003).Assessment of“top-down”and “bottom-up”forces as determinants of rotifer distribution among lakes in Ontario,Canada.Ecological Research,18,639—650.
Zhang,J.M.,&He,Z.H.(1991).Handbook of natural resources for fisheries in inland waters.Beijing:Agricultural House.
Zhang,Z.S.,&Huang,X.F.(1991).Research methods of freshwater plankton.Beijing,China:Science Press(in Chinese with English abstract).
Zhang,L.,Wu,R.W.,&Zhang,W.L.(2007).Evaluation of the internationallycollected designs for the world EXPO park Shanghai.Chinese Landscape Architecture,23(6),44—48(in Chinese with English abstract).
Zhao,S.Q.,Fang,J.Y.,&Ji,W.(2003).Lake restoration from impoldering:Impact of land conversion on riparian landscape in honghu lake area,central yangtze agriculture.Ecosystems and Environment,95,111—115.
杂志排行
Aquaculture and Fisheries的其它文章
- Immunity,feed,and husbandry in fish health management of cultured Epinephelus fuscoguttatus with reference to Epinephelus coioides
- Characterization of two splice variants of EGFR and their effects on the growth of the razor clam
- Effects of three positively buoyant dietary supplements on the buoyancy of feces,growth and intestinal health of Tilapia,Oreochromis niloticus×O.aureus
- Serum osmolality and ions,and gill Na+/K+-ATPase of spottedtail goby Synechogobius ommaturus(R.)in response to acute salinity changes
- Growth and fatty acid composition of discus fish Symphysodon haraldi given varying feed ratios of beef heart,duck heart,and shrimp meat
