Growth and fatty acid composition of discus fish Symphysodon haraldi given varying feed ratios of beef heart,duck heart,and shrimp meat
2018-05-04BinWenZaizhongChenHengchaoQuJianzhongGao
Bin Wen,Zaizhong Chen,*,Hengchao Qu,Jianzhong Gao,**
aKey Laboratory of Freshwater Aquatic Genetic Resources,Ministry of Agriculture,Shanghai Ocean University,Shanghai 201306,China
bKey Laboratory of Exploration and Utilization of Aquatic Genetic Resources,Ministry of Education,Shanghai Ocean University,Shanghai 201306,China
cShanghai Collaborative Innovation for Aquatic Animal Genetics and Breeding,Shanghai Ocean University,Shanghai 201306,China
1.Introduction
Owing to body shape,non-aggressive behavior,and appealing colors and markings,the discus fish Symphysodon spp.has been regarded as “King of Aquarium Fishes” (Livengood,Ohs,&Chapman,2009).Despite origins from the Amazon River,the breeding of Symphysodon spp.for ornamental trade has been conducted in many parts of the world,including Brazil,China,Malaysia,Korea,and Germany.Discus Symphysodon spp.aquaculture has become an important source of income for some Asian countries.Although this species is the focus of large businesses and international trade,studies on its nutritional requirements,including feed ingredient optimization,are rare(Chong,Hashim,&Ali,2000,2003).
As a carnivorous species,Symphysodon spp.cultivation generally depends on moist feed mainly formulated with beef-heart and shrimp meat,named as “beef heart hamburger”(Chong,Hashim,&Ali,2002;Song et al.,2016).Beef heart,a by-product of beef processing,is generally very expensive in Asian countries such as China(Henchion,McCarthy,&O’Callaghan,2016;Liu,Xing,Zhou,&Zhang,2017).With the rapid expansion of discus culture,the massive demand of beef heart has resulted in elevated cost for discus cultivation.Therefore,it is necessary to select relatively low cost and readily available ingredients as replacements to reduce the use of beef heart and the cost of discus cultivation.Studies on the suitability of using alternative feed ingredients as substitutes of beef heart,however,are scarce.
In aquaculture practices,easily available and low price vegetable meals,such as soybean meal and wheat meal,can be used in aqua feeds(Booth,Allan,&Anderson,2012).The plant-based ingredients,however,are generally rich in anti-nutritional factors but poor in certain essential amino acids,thus resulting in reduced feed utilization.Chong et al.(2003)revealed that dietary inclusion of soybean meal showed negative effects on the protein digestibility of discus S.aequifasciata.Compared to such plant-based ingredients,animal by-products,such as meat and bone meal(MBM),have several advantages which include a balanced amino acid pro file and lack of anti-nutritional factors(Moutinho et al.,2017).MBM also shows relatively high digestibility,but great variability among different species(e.g.,Goda,El-Haroun,&Chowdhury,2007;Li et al.,2010;Lee,Choi,Kim,Cho,&Yoo,2012).Dietary inclusion of suitable animal-source ingredients may be an effective tool to lower the cost of discus culture.Duck heart,which has relatively low cost and high availability,is a candidate for replacing beef heart within moist feeds.
Fatty acids(FA),especially long-chain polyunsaturated fatty acids(LC-PUFAs),have been regarded as essential dietary nutrients which are vital for the growth,development and reproduction of fish(Paulsen,Clemmesen,&Malzahn,2014;Tocher,2010).Dietary inclusion of alternative ingredients may provide different sources of FAs that would change the FA compositions of cultured species(García-Romero,Gines,Izquierdo,&Robaina,2014;Herath,Haga,&Satoh,2016).Therefore,the inclusion of a new feed ingredient might potentially influence the FA signatures and subsequent growth of discus fish.
We selected the duck heart as an alternative ingredient to beef heart within discus feed.The objectives of the study were to examine the effects of partial or total replacement of dietary beef heart with duck heart on the growth performance,body compositions,and FA pro files of discus S.haraldi,one of the most popular aquarium fish species,to evaluate the feasibility of using duck heart as a replacement for the traditional beef heart in moist feed for S.haraldi culture.
2.Materials and methods
2.1.Experimental diets
Ingredients and proximate compositions of the experimental diets are shown in Table 1.Duck heart was selected as a replacement of beef heart used in the moist feed.Since shrimp meat was generally added in the formulated diets for discus culture(Song et al.,2016),its suitable inclusion level was also examined.As such,nine different types of diets were formulated with three main ingredients,including beef heart,duck heart,and shrimp meat at different proportions,i.e.,10:0:0(F1),9:0:1(F2),8:0:2(F3),6:2:2(F4),4:4:2(F5),2:6:2(F6),0:8:2(F7),0:9:1(F8)and 0:10:0(F9).All feed ingredients were minced and well mixed,before adding vitamin premix,mineral premix,and dicalcium phosphate into the meat paste to form ‘beef heart hamburger’.The experimental diets were stored at-20°C prior to use.
2.2.Experiment and sample collection
The experiment was carried out at the aquarium facility of Shanghai Ocean University,Shanghai,China and lasted for 56 d.The experimental S.haraldi juveniles were collected from the Ornamental Fish Breeding Laboratory,Shanghai Ocean University.After a 14d acclimation,540 fish individuals of similar size(initial body weight 12.58±1.07g,body height 4.80±0.20cm,and body length 5.71±0.50 cm)were randomly allocated into 27 separate 80 L glass aquaria,with 20 individuals per aquarium.The aquaria werefurther divided into nine groups with three replicates for each group.During the following 56 d experiment,S.haraldi in triplicate aquaria were fed one of the nine diets with a daily total ration of 3%—4%of the body weight at 08:00,11:00,15:00,and 19:00.Water temperature was kept at 28.0±0.5°C,pH 6.2—6.8,and dissolved oxygen>6.0 mg/L.One-half of the water in each aquarium was exchanged with filtered freshwater,daily.Uneaten feed residue and feces were collected via siphoning and dried at 70°C to a constant weight.At the end of the experiment, fish individuals in each aquarium were starved for 24h,and then collected and weighed.Samples of collected body tissue were stored at-80°C for further analysis.All animal care was conducted in accordance with Administrative Measures of Experimental Animals in Shanghai City and the experimental protocols were approved by the Animal Ethics Committee of Shanghai Ocean University.

Table 1 Ingredients and biochemical compositions(%)of experimental diets.
2.3.Proximate composition and fatty acid analysis
Proximate compositions of the nine experimental diets and body tissue samples were determined according to the standard method of AOAC(1995).Moisture content was determined by drying in an oven at 110°C to a constant weight.Ash content was determined after the incineration of samples in a muf fl e furnace at 550°C for 6 h.The Kjeldhal method was employed forcrude protein content determination(N content×6.25).Crude lipids were extracted with chloroform/methanol(2:1,v/v)mixture by the method of Bligh and Dyer(1959).
Samples of experimental diets and muscle tissue were homogenized and freeze-dried for fatty acid(FA)analyses.Fatty acid methyl esters(FAMEs)were prepared by esterification using 2%sulphuric acid methanol as described in Gao,Shin,Lin,Chen,and Cheung(2006).FAMEs were separated and quantified by an Agilent 6890 gas chromatograph(Agilent Technologies,Santa Clara,CA,USA)equipped with aflame ionization detector instrument and an OMEGAWAX 320 fused silica capillary column (30m long×0.32mm internal diameter×0.25μm thickness,Supelco,Billefonte,PA,USA).The thermal gradient program was:60°C for 1.0 min,rate of 50°C/min to 170°C,2.0°C/min to 180°C and 180°C for 2min,2.0°C/min to 230°C and 230°C for 1 min and 1.0°C/min to 240°C.The 37-FAME Mix(Supelco,Bellefonte,PA,USA)was used as a standard to identify the FAMEs.FAs levels are expressed as the percentage of each FA to the total FAs.
2.4.Calculations and statistical analysis
The growth performance of S.haraldi,in terms of weight gain rates(WGR),specific growth rates(SGR),length growth rate(LGR),height growth rate(HGR),feed coefficient rate(FCR),feed intake(FI),condition factor(CF),and survival rate(SR),were evaluated as follows:

where Wfand Wiare the final and initial weights of fish individuals in each aquarium,respectively(g);Lfand Lirepresent the final and initial body length,respectively(cm);Hfand Hirepresent the final and initial body height,respectively(cm);Nfand Nirepresent the initial and final numbers,respectively;and t is the duration of the experiment(d).
Prior to analysis,raw data were diagnosed for normality of distribution and homogeneity of variance with Kolmogorov-Smirnov test and Levene's test,respectively.One-Way analysis of variance(ANOVA),followed by Tukey test for multiple comparisons at the significance level of 0.05,was used to compare the differences in growth performance,body compositions,and FA signatures among the nine diet groups.Moreover,the relative retention ratios of FAs were used to show the relationship between diets and body tissues(Ganga et al.,2005;Zhao,Wu,&Chang,2013).The statistical analysis was performed using the SPSS®20.0 software package.
3.Results
3.1.Fatty compositions of experimental diets
FA signatures of the nine experimental diets are shown in Table 2.FA contents differed significantly among the experimental diets.The highest 16:0,18:0,and saturated fatty acids(SFAs)contents were observed in the diet F8 and the lowest were found in the diet F2.The highest 16:1n-7 level was observed in the F7 diet and the lowest was detected in the diet F9.In contrast,the highest 18:1n-7 content was observed in the diet F9 and the lowest was found in the diet F7.The F4 diet showed the highest 18:1n-9 and monounsaturated fatty acids(MUFAs)contents,while the F2 diet displayed the lowest levels.The highest 18:2n-6 content was observed in the diet F9 and the lowest was found in the diet F7.In contrast,the highest 18:3n-3 level was observed in the F7 diet and the lowest was detected in the diet F9.The F3 diet showed the highest 20:4n-6 content and the F7 diet displayed the lowest level.The F1 diet showed the highest 20:5n-3 concentration while the F9 diet appeared the lowest level.The F2 diet showed the highest 22:6n-3 content while the F1 and F9 diets displayed the lowest levels.The F3 diet contained the highest polyunsaturated fatty acids(PUFAs)content while the F7 diet showed the lowest level.
3.2.Growth performance of S.haraldi
The growth of S.haraldi,fed different experimental diets,are shown in Table 3.After the 56 d feeding trial,except for the feed intake(FI),significant differences in the other parameters among the nine groups were observed(ANOVA,P<0.05).As the levels of beef heart and/or shrimp meat increased,the final body weights(FBW),weight gain rates(WGR)and specific growth rates(SGR)tended to first decrease(F1-F3)then increase(F4-F6)and thereafter decrease(F7-F9),with the highest FBW,WGR,and SGR in the group F6 and the lowest in the group F9.The FBW and WGR of the F6 group were both significantly higher than the F1 group(ANOVA,P<0.05).No significant differences in SGR between the F1 and F6 groups were observed(ANOVA,P>0.05).Similarly,as the inclusion levels of beef heart decreased,the length growth rates(LGR)and height growth rates(HGR) first decreased(F1-F3)then increased(F4-F6)and thereafter decreased(F7-F9),with the highest LGR and HGR in the group F6 and the lowest in the group F9.No significant differences in LGR and HGR between the F1 and F6 groups were observed(ANOVA,P>0.05).In contrast,the feed coefficient rates(FCR)showed the opposite trend,with the highest FCR was observed in the group F9 and the lowest was found in the group F6.No significant differences in FCR between the F1 and F6 groups were observed(ANOVA,P>0.05).Dietary inclusion of beef heart and/or shrimp meat tended to increase the condition factor(CF),particularly in the F3,F4,F5,F8,and F9 groups which were significantly higher than the F1 group(ANOVA,P<0.05).However,dietary inclusion of beef heart and/or shrimp meat tended to decrease the survival rates(SR),with the lowest rates were found in both the F7 and F9 groups.
3.3.Body composition of S.haraldi
Body compositions of S.haraldi,fed different diets,are shown in Table 4.After the 56 d feeding trial,significant differences in body compositions among the nine groups were observed(ANOVA,P<0.05).The highest moisture content was observed in the group F4 and the lowest was found in the group F5.No significant differences in moisture content between the F1 and F6 groups were observed(ANOVA,P>0.05).Dietary inclusion of beef heart and/or shrimp meat tended to decrease the crude protein and ash contents,with the lowest in the group F4.Contents of crude protein and ash in the F1 group were significantly higher than the F6 group(ANOVA,P<0.05).The highest crude lipid content was observed in the group F5 and the lowest was found in the group F8.No significant differences in crude lipid content were observed between the F1 and F6 groups(ANOVA,P>0.05).
3.4.Fatty acid composition of S.haraldi
FA compositions of S.haraldi,fed different experimental diets,are shown in Table 5.After the 56 d feeding trial,FA pro files of S.haraldi were related to those of their diets.Most of the FAs profiles in fish body reflected those of corresponding diets.For example,S.haraldi in the F3,F8,and F9 groups showed significantly lower concentrations of 16:1n-7,18:3n-3,and 20:5n-3 relative to those in the other groups(ANOVA,P<0.05).Similarly,due to the F9 and F7 diets that were high and low in 20:4n-6,respectively,the highest 20:4n-6 content was observed in the group F9 and the lowest was found in the group F7.On the other hand,although the higher content of 20:4n-6 in the diet F3 than diet F2,these two groups showed similar 20:4n-6 levels(ANOVA,P>0.05).Despite the absence of 22:6n-3 in both the F1 and F9 diets,the F1 groupshowed significantly higher content than the F9 group(ANOVA,P<0.05).The highest 16:0,18:0,18:1n-7,18:1n-9,and 18:2n-6 contents were all observed in the group F1 while the lowest levels were found in the group F3.As a result,the F1 group showed the highest SFAs and MUFAs contents while the F3 group displayed the lowest levels.The highest and lowest PUFAs contents were observed in the F4 and F7 groups,respectively.

Table 2 Fatty acid compositions of different experimental diets.Data were presented as mean±SD(n=3).Different letters within the same row indicate significant differences(ANOVA,P<0.05).

Table 3 Growth performance of S.haraldifed different diets.Data were presented as mean±SD(n=3).Different letters within the same column indicate significant differences(ANOVA,P<0.05).

Table 4 Body compositions of S.haraldifed different diets.Data were presented as mean±SD(n=3).Different letters within the same column indicate significant differences(ANOVA,P<0.05).
Table 6 shows the relationship between the body contents of FAs and their dietary levels.The relative retention ratios of 16:1n-7 and 18:3n-3 were less than 1,but showed high correlations between diets and body tissues.No obvious correlations between dietary 16:0,18:0,18:1n-7,18:1n-9,and 18:2n-6 contents and corresponding tissue levels were observed.The highest incorporationratios of 16:0,18:0,18:1n-7,18:1n-9,and 18:2n-6 were all observed in the group F1 and the lowest ratios were found in the group F3.The relative retention ratio of 20:4n-6 was less than 1 for all groups,with the highest and lowest ratios in the group F2 and F3,respectively.Similarly,the incorporation ratio of 20:5n-3 was less than 1,and exceptfor the group F2,showed nearly the similar ratios in the other groups.Except for the F3 group,the incorporation ratios of 22:6n-3 were all greater than 1.
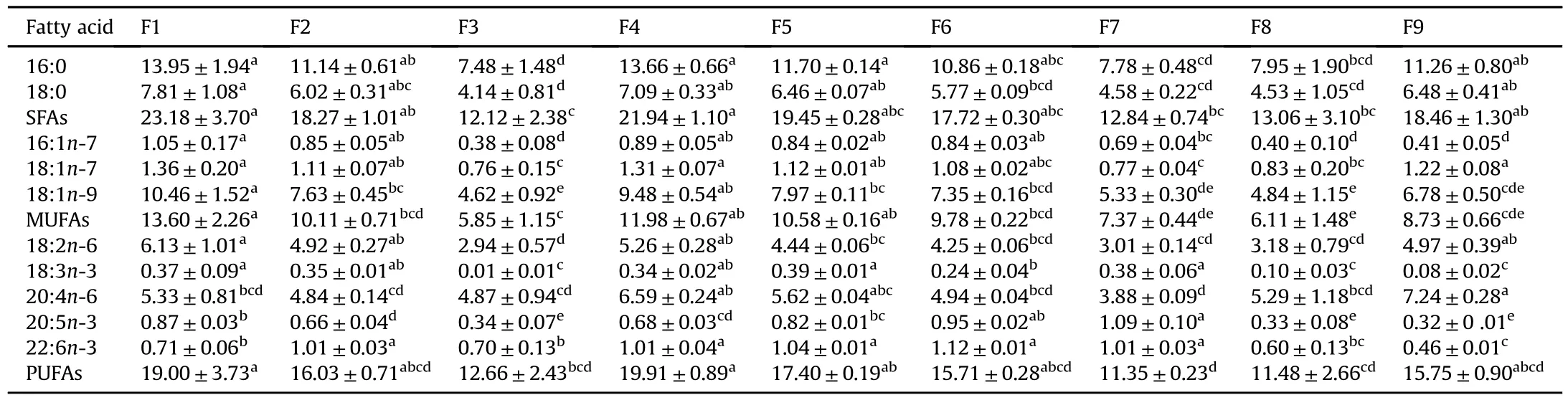
Table 5 Fattyacid compositions of S.haraldi fed different diets.Datawere presented as mean±SD(n=3).Different letters within the same row indicate significant differences(ANOVA,P<0.05).
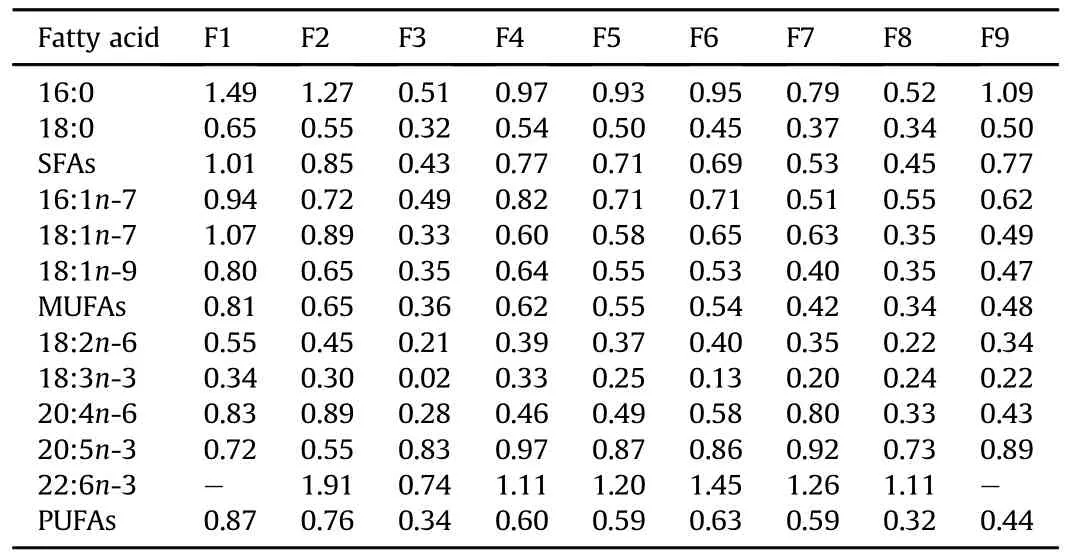
Table 6 The ratio between fatty acid(FA)percent in muscle lipid classes and dietary percent of the same FA(%FA in muscle/%FA in diet).
4.Discussion
Substituting shrimp meat for beef heart significantly reduced the speci fic growth rates(SGR),length growth rates(LGR),and height growth rates(HGR)of S.haraldi,while the substitution of shrimp meat for duck heart slightly increased the SGR,LGR,and HGR,suggesting that the shrimp meat might not necessarily need to be added into moist feed for S.haraldi culture.As for discus fed diets containing the three ingredients,i.e.,beef heart,duck heart,and shrimp meat,the dietary proportion up to 60%of duck heart(which was equivalent to 75%replacement level)showed no adverse effect on the growth of S.haraldi,but decreased their survival rates.However,the growth of S.haraldi was significantly reduced with further increasing inclusion levels(80%—100%),probably due to the low digestibility of duck heart.Similarly,the excessive mortality of S.haraldi that were fed diets containing high levels of duck heart(40%—100%)was likely due to the reduced digestibility of nutrients in diets with high proportions of duck heart.These hypotheses were supported by the relatively high feed coefficient rates(FCR)for the F7,F8,and F9 groups.Similarly,diets that replace 75%—80%of fish meal with meat,bone,and blood meal,without affecting growth performance has been observed in other fish species,such as African cat fish Clarias gariepinus(Goda et al.,2007)and grouper Epinephelus coioide(Millamena,2002).In contrast,dietary inclusion of high levels of meat and bone meal has affected the growth of species such as large yellow croaker Pseudosciaena crocea(Li et al.,2010),gibel carp Carassius auratus gibelio(Zhang et al.,2006),and olive flounder Paralichthys olivaceus(Lee et al.,2012).
The inclusion of a new feed ingredient can influence traits of quality,such as flesh quality,skin colour,and subsequent market demand(García-Romero,Gines,Izquierdo&Haroun et al.,2014;Herath et al.,2016;Hu et al.,2013).The whole-body composition could be affected by dietary ingredients(García,Kalinowski,Izquierdo,&Robaina,2010;Sørensen,Berge,Reitan,&Ruyter,2016).Similarly,in the present study,the body composition of S.haraldi was influenced by the dietary shrimp meat and/or duck heart inclusion level,except for crude lipid contents of the F5 and F8 groups,which were significant lower relative to the F1 group.Moreover,dietary inclusion of shrimp meat and/or duck heart tended toreduce the protein content.This agrees with the results of García et al.(2010),who found the whole-body protein content of red porgy Pagrus pagrus decreased with increasing dietary marine crab meal levels.The protein content of African cat fish C.gariepinus was also significantly reduced when dietary fish meal was totally replaced by poultry by-product meal(Goda et al.,2007).On the contrary,the crude protein content of Japanese sea bass Lateolabrax japonicus slightly increased with increasing dietary poultry byproduct meal(Wang,Wang,Ji,Han,&Li,2015).Other studies revealed no significant differences in crude protein content when fish were fed diets containing different dietary animal by-products levels(García-Romero,Gines,Izquierdo,&Robaina,2014;Hu et al.,2013;Moutinho et al.,2017).Body composition is regarded as an important fl esh quality characteristic for the edible fish(García-Romero,Gines,Izquierdo,&Robaina,2014,García-Romero,Gines,Izquierdo&Haroun et al.,2014).However,as ornamental fish,including discus fish,are not used for providing food,the proximate composition of discus S.haraldi might not affect their quality attributes for marketing.
The FA pro files of S.haraldi were significantly influenced by corresponding diets.Similar results were observed in several other species,such as rainbow trout Oncorhynchus mykiss(Borquez,Serrano,Dantagnan,Carrasco,&Hernandez,2011),Japanese seabass L.japonicus(Hu et al.,2013),red porgy P.pagrus(García-Romero,Gines,Izquierdo,&Robaina,2014),and Songpu mirror carp Cyprinus carpio(Tian,Lei,&Ji,2016).Most of the diets with high FAs content showed high FAs levels in muscle tissues.However,dietary polyunsaturated fatty acids(PUFAs),such as 18:2n-6 and 18:3n-3,can be converted into LC-PUFAs by freshwater fish(Oboh,Betancor,Tocher,&Monroig,2016;Rasal et al.,2016).Similarly,although both the F1 and F9 diets contained trace amounts of 22:6n-3 in this study,S.haraldi in the F1 group accumulated more 22:6n-3 than those in the F9 group.This was probably due to the more efficient retention of this FA bydiscus in the F1 group,as supported by the higher FCR and subsequent higher 22:6n-3 retention relative to the F9 group.Another possible reason might be that discus fish were able to convert 18:3n-3 to 20:5n-3 and to 22:6n-3,as the F1 diet was richer in 18:3n-3 and 20:5n-3 relative to the F9 diet.Similarly,despite the F7 diet having the lowest levels of 18:2n-6 and 20:4n-6,S.haraldi fed this diet showed the lowest concentrations of these two FAs.Comparable concentrations of 20:4n-6 were recorded in this group relative to the F2,F3,F6,and F8 groups,indicating that discus fish might also have the potential to convert 18:2n-6 to 20:4n-6.These results suggested that S.haraldi has the ability to preferentially accumulate 20:4n-6,20:5n-3,and 22:6n-3 and/or synthesize of these EFAs from corresponding precursors.This hypothesis was supported by the highest incorporation ratios of 20:4n-6 and 22:6n-3 of the F2 groups.The FA compositions of S.haraldi thus depended on both dietary FAs and PUFAs bioconversion.
LC-PUFAs,such as 20:4n-6(ARA),20:5n-3(EPA),and 22:6n-3(DHA),can contribute to the growth of cultured fish(e.g.,Jin et al.,2017;Shahkar et al.,2016).In this study,S.haraldi from the F9 group showed the lowest SGR,LGR,and HGR.Moreover,the F9 group contained the lowest EPA and DHA concentrations,despite the highest ARA content.These results suggested that EPA and DHA were more vital for the growth of S.haraldi.Similarly,Hu et al.(2013)revealed that the growth performance of Japanese seabass L.japonicus was reduced with decreasing dietary n-3 HUFA levels,resulting from dietary fish meal replaced by an animal protein blend.Thus,the relatively high concentrations of EPA and DHA in the F6 diet might account for the highest SGR of S.haraldi fed this diet.However,S.haraldi fed the F1 diet,which was poor in DHA,showed the second highest SGR and the relatively high DHA level,indicating that the requirements for DHA might be met by dietary 18:3n-3.Yet,S.haraldi fed the F7 diet,which were rich in both EPA and DHA,showed significant lower SGR relative to those fed the F6 diet,probably due to the lack of beef heart in the diet F7 and the subsequent nutrient deficiencies,such as certain essential amino acids.Another possible reason might be that S.haraldi could not effectively utilize and absorb of nutritional components,as supported by the relatively high FCR of this group.The poor growth performance of S.haraldi in the F7 group may then be related to the reduced diet digestibility and nutritional imbalance.
In conclusion,after the 56 d feeding trial,most of the FAs profiles in fish body tended to reflect those of corresponding diets.Moreover,in comparison with ARA,EPA and DHA were more vital for the growth of S.haraldi.The replacement of beef heart only with shrimp meat significantly reduced the growth performance and thus shrimp meat might not necessarily be added into moist feed for S.haraldi culture.Dietary inclusion of up to 60%duck heart showed no adverse effect on the growth of S.haraldi,but had some negative influence on survival rates.
Acknowledgements
The study presented in the manuscript was funded by the Key Project of Developing Agriculture through Science and Technology of Shanghai Municipal Agricultural Commission(2015—19),China;the China Postdoctoral Science Foundation(2017M621433);and the Doctoral Scientific Research Foundation of Shanghai Ocean University(A2-0203-17-100306).
AOAC(Association of Official Analytical Chemists).(1995).Official methods of analysis of official analytical chemists international(16th ed.).Arlington,VA,USA:Association of Official Analytical Chemists.
Bligh,E.G.,&Dyer,W.J.(1959).A rapid method of total lipid extraction and Purification.Canadian Journal of Biochemistry and Physiology,37,911—917.
Booth,M.A.,Allan,G.L.,&Anderson,A.J.(2012).Influence of poultry meal,meat meal or soybean meal inclusion on weight gain and production characteristics of Australian snapper Pagrus auratus.Aquaculture International,20,99—115.
Borquez,A.,Serrano,E.,Dantagnan,P.,Carrasco,J.,&Hernandez,A.(2011).Feeding high inclusion of whole grain white lupin(Lupinus albus)to rainbow trout(Oncorhynchus mykiss):Effects on growth,nutrient digestibility,liver and intestine histology and muscle fatty acid composition.Aquaculture Research,42,1067—1078.
Chong,A.S.C.,Hashim,R.,&Ali,A.B.(2000).Dietary protein requirements for discus(Symphysodon spp.).Aquaculture Nutrition,6,275—278.
Chong,A.,Hashim,R.,&Ali,A.B.(2002).Inhibition of protease activities in discus Symphysodon spp.by three plant meals.Aquaculture International,10,433—441.
Chong,A.,Hashim,R.,&Ali,A.B.(2003).Assessment of soybean meal in diets for discus(Symphysodon aequifasciata HECKEL)farming through a fishmeal replacement study.Aquaculture Research,34,913—922.
Ganga,R.B.J.G.,Bell,J.G.,Montero,D.,Robaina,L.,Caballero,M.J.,&Izquierdo,M.S.(2005).Effect of dietary lipids on plasma fatty acid pro files and prostaglandin and leptin production in gilthead seabream(Sparus aurata).Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,142,410—418.
Gao,Q.F.,Shin,P.K.S.,Lin,G.H.,Chen,S.P.,&Cheung,S.G.(2006).Stable isotope and fatty acid evidence for uptake of organic waste by green-lipped mussels Perna viridis in a polyculture fish farm system.Marine Ecology Progress Series,317,273—283.
García-Romero,J.,Gines,R.,Izquierdo,M.S.,Haroun,R.,Badilla,R.,&Robaina,L.(2014).Effect of dietary substitution of fish meal for marine crab and echinoderm meals on growth performance,ammonia excretion,skin colour,and flesh quality and oxidation of red porgy(Pagrus pagrus).Aquaculture,422,239—248.
García-Romero,J.,Gines,R.,Izquierdo,M.,&Robaina,L.(2014).Marine and freshwater crab meals in diets for red porgy(Pagrus pagrus):Effect on fi llet fatty acid pro fi le and fl esh quality parameters.Aquaculture,420,231—239.
García,J.R.,Kalinowski,C.T.H.,Izquierdo,M.S.L.,&Robaina,L.E.R.(2010).Marine and freshwater crab meals in diets for red porgy(Pagrus pagrus):Effect on growth, fish composition and skin colour.Aquaculture Research,41,1759—1769.
Goda,A.M.,El-Haroun,E.R.,&Chowdhury,K.(2007).Effect of totally or partially replacing fish meal by alternative protein sources on growth of African cat fish Clarias gariepinus(Burchell,1822)reared in concrete tanks.Aquaculture Research,38,279—287.
Henchion,M.,McCarthy,M.,&O'Callaghan,J.(2016).Transforming beef by-products into valuable ingredients:Which spell/recipe to use?Frontiers in Nutrition.https://doi.org/10.3389/fnut.2016.00053.
Herath,S.S.,Haga,Y.,&Satoh,S.(2016).Effects of long-term feeding of corn coproduct-based diets on growth, fi llet color,and fatty acid and amino acid composition of Nile tilapia,Oreochromis niloticus.Aquaculture,464,205—212.
Hu,L.,Yun,B.,Xue,M.,Wang,J.,Wu,X.,Zheng,Y.,et al.(2013).Effects of fish meal quality and fish meal substitution by animal protein blend on growth performance, flesh quality and liver histology of Japanese seabass(Lateolabrax japonicus).Aquaculture,372,52—61.
Jin,M.,Lu,Y.,Yuan,Y.,Li,Y.,Qiu,H.,Sun,P.,et al.(2017).Regulation of growth,antioxidant capacity,fatty acid pro fi les,hematological characteristics and expression of lipid related genes by different dietary n-3 highly unsaturated fatty acids in juvenile black seabream(Acanthopagrus schlegelii).Aquaculture,471,55—65.
Lee,J.,Choi,I.C.,Kim,K.T.,Cho,S.H.,&Yoo,J.Y.(2012).Response of dietary substitution of fi shmeal with various protein sources on growth,body composition and blood chemistry of olive flounder(Paralichthys olivaceus,Temminck&Schlegel,1846).Fish Physiology and Biochemistry,38,735—744.
Liu,R.,Xing,L.,Zhou,G.,&Zhang,W.(2017).What is meat in China?Animal Frontiers,7,53—56.
Livengood,E.J.,Ohs,C.L.,&Chapman,F.A.(2009).Candidate species for Florida aquaculture:Discus Symphysodon spp.,a pro fi table but challenging species for Florida aquaculture.
Li,J.,Zhang,L.,Mai,K.S.,Ai,Q.H.,Zhang,C.X.,Li,H.T.,et al.(2010).Potential of several protein sources as fish meal substitutes in diets for large yellow croaker,Pseudosciaena crocea R.Journal of the World Aquaculture Society,41,278—283.
Millamena,O.M.(2002).Replacement of fish meal by animal by-product meals in a practical diet for grow-out culture of grouper Epinephelus coioides.Aquaculture,204,75—84.
Moutinho,S.,Martínez-Llorens,S.,Tomas-Vidal,A.,Jover-Cerda,M.,Oliva-Teles,A.,&Peres,H.(2017).Meat and bone meal as partial replacement for fish meal in diets for gilthead seabream(Sparus aurata)juveniles:Growth,feed efficiency,amino acid utilization,and economic efficiency.Aquaculture,468,271—277.
Oboh,A.,Betancor,M.B.,Tocher,D.R.,&Monroig,O.(2016).Biosynthesis of longchain polyunsaturated fatty acids in the African cat fish Clarias gariepinus:Molecular cloning and functional characterisation of fatty acyl desaturase(fads2)and elongase(elovl2)cDNAs7.Aquaculture,462,70—79.
Paulsen,M.,Clemmesen,C.,&Malzahn,A.M.(2014).Essential fatty acid(docosahexaenoic acid,DHA)availability affects growth of larval herring in the field.Marine Biology,161,239—244.
Rasal,A.,Roy,S.,Rana,R.S.,Murali,S.,Krishna,G.,Gupta,S.,et al.(2016).Molecular cloning and nutritional regulation of putativeΔ6 desaturase mRNA from striped cat fish(Pangasianodon hypophthalmus).Aquaculture,451,413—420.
Shahkar,E.,Yun,H.,Lee,S.,Kim,D.J.,Kim,S.K.,Lee,B.I.,et al.(2016).Evaluation of the optimum dietary arachidonic acid level and its essentiality based on growth and non-specific immune responses in Japanese eel,Anguilla japonica.Aquaculture,452,209—216.
Song,X.,Wang,L.,Li,X.,Chen,Z.,Liang,G.,&Leng,X.(2016).Dietary astaxanthin improved the body pigmentation and antioxidant function,but not the growth of discus fish(Symphysodon spp.).Aquaculture Research,48,1359—1367.
Sørensen,M.,Berge,G.M.,Reitan,K.I.,&Ruyter,B.(2016).Microalga Phaeodactylum tricornutum in feed for Atlantic salmon(Salmosalar)—effect on nutrient digestibility,growth and utilization of feed.Aquaculture,460,116—123.
Tian,J.J.,Lei,C.X.,&Ji,H.(2016).Influence of dietary linoleic acid(18:2n-6)andαlinolenic acid(18:3n-3)ratio on fatty acid composition of different tissues in freshwater fish Songpu mirror carp,Cyprinus carpio.Aquaculture Research,47,3811—3825.
Tocher,D.R.(2010).Fatty acid requirements in ontogeny of marine and freshwater fish.Aquaculture Research,41,717—732.
Wang,Y.,Wang,F.,Ji,W.X.,Han,H.,&Li,P.(2015).Optimizing dietary protein sources for Japanese sea bass(Lateolabrax japonicus)with an emphasis on using poultry by-product meal to substitute fish meal.Aquaculture Research,46,874—883.
Zhang,S.,Xie,S.,Zhu,X.,Lei,W.,Yang,Y.,&Zhao,M.(2006).Meat and bone meal replacement in diets for juvenile gibel carp(Carassius auratus gibelio):Effects on growth performance,phosphorus and nitrogen loading.Aquaculture Nutrition,12,353—362.
Zhao,Y.T.,Wu,X.G.,&Chang,G.L.(2013).Effect of dietary DHA level on growth,lipid composition and hypoxia stress of juvenile Chinese mitten crab Eriocheir sinensis.Acta Hydrobiologica Sinica,37,1133—1144(In Chinese with English abstract).
杂志排行
Aquaculture and Fisheries的其它文章
- Immunity,feed,and husbandry in fish health management of cultured Epinephelus fuscoguttatus with reference to Epinephelus coioides
- Characterization of two splice variants of EGFR and their effects on the growth of the razor clam
- Effects of three positively buoyant dietary supplements on the buoyancy of feces,growth and intestinal health of Tilapia,Oreochromis niloticus×O.aureus
- Serum osmolality and ions,and gill Na+/K+-ATPase of spottedtail goby Synechogobius ommaturus(R.)in response to acute salinity changes
- Rotifer community structure and its response to environmental factors in the Backshore Wetland of Expo Garden,Shanghai
