A protocol for the reporting and methodological quality of robotic surgery case reports
2018-04-23ShuguoNiuMingLiuCuncunLuYuanYuanTingtingLuLongGeJinhuiTian
Shuguo Niu,Ming Liu,Cuncun Lu,Yuan Yuan,Tingting Lu,Long Ge,Jinhui Tian
1Affiliated Traditional Chinese Medical Hospital of Wuwei,Wuwei Occupational College,Wuwei,733000,China.2Evidence-Based Medicine Center,Lanzhou University,Lanzhou,730000,China.3Gansu University of Traditional Chinese Medicine,Lanzhou,730000,China.
*Joint First Authors.
Introduction
The conflict of the number of biomedical papers increasing rapidly with limited time of clinician accelerated the generation of evidence-based medicine[1-3]. Well-conducted systematic review of randomized controlled trials is regarded as the best quality evidence in evidence-based medicine[4],and the randomized controlled trial with rigorous methodology is the gold standard for clinical trial[5].But it is impossible to resolve all medical questions using randomized controlled trial[6-7],for example,discovery of a rare disease or adverse drug reactions,test of new medical technology and equipment etc.Though case reports are uncontrolled study design with risk of bias but have profoundly influenced the medicine development[8].There is a good example[9], in 1985, the American Medical Association assessed papers from Journal of American Medical Association and found 5 case reports had significantly improved the development of medicine over the past 150 years.But we need to know faults or bias are not limited to case reports, systematic review and randomized controlled trial are no exception,biased results also because researchers do not to adhere to optimal standards strictly[10].
Robotic surgery is a complex innovation intervention,it is favored by surgeons[11-12].And,the number of robotic surgery case reports is increasing,but there is unclear on its reporting and methodological quality.The detailed and regulatory specification of medical research can help readers and researchers to judge the implementation process,which contributes to the evaluation of reliability of results[13],and any research design needs strict methodology to support.There are a large body of tools or guidelines to regulate medical research, for example, CONSORT(Consolidated Standards of Reporting Trials)[14]and Jadad (Jadad scale) [15] are used to regulate randomized controlled trials, PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)[16]and AMSTAR2(A MeaSurement Tool to Assess systematic Reviews-2) [17] are used to improve quality of rigorous methodology systematic review,AGRR II(Appraisal of Guidelines,Research and Evaluation 2)[18]and RIGHT(Reporting Items for practice Guidelines in HealThcare) [19] can regulate clinical practice guideline. And for case reports,its reporting guideline-CARE(CAse REport)[20],which was published in 2013 by Joel J Gagnier et al,the methodological quality tool[21]was published in 2016 by JBI(Joanna Briggs Institute).To the best of our knowledge, there is no a study to assess the reporting and methodological quality of robotic surgery case reports,and the status quo of reporting and methodological quality is unclear,therefore,we will design a study to fill this gap and provide reference for future case reports not limited to robotic surgery.
Methods
Identification and selection of study
The PubMed database will be searched with hig hspecificity search strategy ("Robotics"[Mesh]AND"Case Reports"[Publication Type])from Jan 1,2012 to Aug 1,2018 to collect relevant records by an author (Cun-cun Lu). All hits will be downloaded with a txt profile from PubMed and the Microsoft Excel 2016 will be used to generate random number.Then,the records will be ranked from the smallest to the largest by assigned random numbers,and the first 100 studies will be selected,if selected case report was not eligible,the next study will be included.
Eligibility criteria
All included case reports must meet the following criteria:first,we will only include case report that sample size is no more than 5 patients;second,the study must be fully published in English from 2012 to2018; Third, the full text should be downloaded from database to allow us to read.
Data extraction
A draft data extraction form will be created using Microsoft Excel 2016,then all authors will be divided into two groups (first group including Shu-guo Niu,Ming Liu,and Long Ge;second group Cun-cun Lu,Yuan Yuan and Jin-hui Tian)to test this data form using randomized 10 samples from included records and according feedbacks to revise the form.The revised data extraction form will be used to extract information by two authors(Shu-guo Niu and Ming Liu) and any disagreement will be resolved through discussion.The extracted information will include:first author,year of publication,journal(including category(SCI or not SCI,Science Citation Index),impact factor),funding,number of authors;page number of studies;number of patients.
Assessment of reporting quality
The present study will assess the reporting quality of robotic surgery case reports using CARE guideline(Table 1 The CARE guidelines items).In this study,the assessment standard for each item will be assigned by one of the following three responses: ‘‘yes’’for total compliance will be given 1 point;‘‘partial’’for partial compliance will be given 0.5 point;‘‘no’’for noncompliance will be given 0 point.And we will define:≤12 points are low quality,13-19 points are moderate quality
and ≥20 points are high quality.The process of assessment will be performed by two authors(Shuguo Niu and Ming Liu)independently and any discrepancy will consult the third author(Long Ge)to reach consensus.

Table 1 The CARE guidelines items
Assessment of methodological quality
The present study will assess the methodological quality of robotic surgery case reports using the tool provided by JBI(Table 2,JBI Critical Appraisal Checklist for Case Report). The assessment standard for each item will be assigned one of the following four responses:Yes, No unclear,Not applicable.And for"yes"will be equal to 1 point,and other responses all will be given 0 point.And we will define:≤3 points are low quality,4-6 points are moderate quality and ≥7 points are high quality.This assessment will be completed by two authors(Shu-guo Niu and Yuan Yuan)independently and any discrepancy will consult the third author(Tingting Lu)to reach consensus.
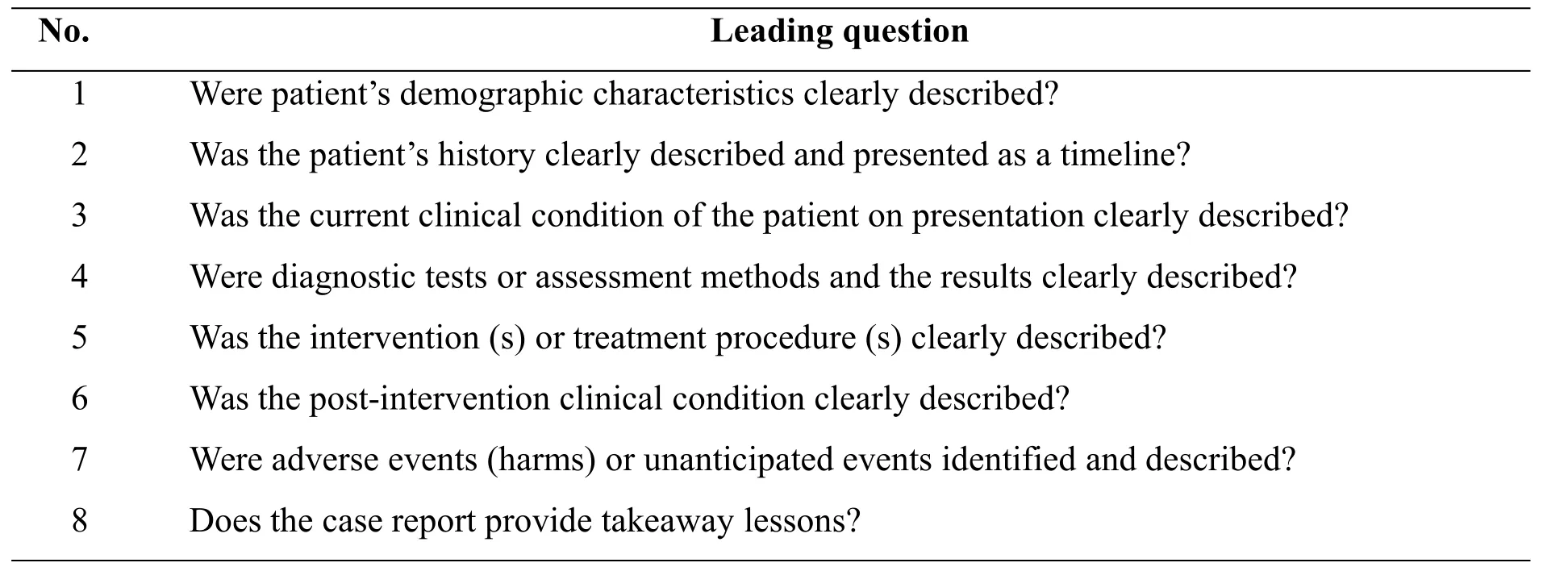
Table 2 JBI Critical Appraisal Checklist for Case Report
Data analysis
The CARE guideline and JBI checklist are used to assess all included studies and we present descriptive results.Meanwhile,stratified analyses of assessment of reporting and methodological quality will be conducted separately based on journal category(SCI or not),funding(yes or no),impact factor of journal(the cutoff value will be median,one group is less than or equal to median,the other group is more than median.The following are the same),number of authors;page number of studies and number of patients to explore potentially influential factors,and every subgroup comparison result will be presented using mean score and SD (Standard Deviation) with 95%CI(95%confidence interval),for example,if 50 studies (funding-group) were supported with funding,other 50 studies(no-funding-group)were not,the mean scores and their SD with 95%CI for the two group will be calculated separately,and Inverse-Variance random-effect model will be used to pool and present result of subgroup comparison using MD(Mean Difference)and 95%CI,if MD>0,the quality of funding-group will be better than no-funding-group.Statistical significance will be set at P<0.05.All data analyses will be performed using Microsoft Excel 2016 and Review Manager 5.3 software.
Discussion
Robotic surgery is a complex innovation intervention and quality of robotic surgery case reports is unclear.Therefore,we design a study to assess the reporting and methodological quality of robotic surgery case reports to fill the gap.As far as we know,this is a first study to assess the quality of surgery case reports using CARE and JBI’s tool.We hope the results of the present study will provide some references to future case reports but not limited to robotic surgery.
1. Evidence-based medicine.A new approach to teaching the practice of medicine. JAMA,1992,268:2420-5.
2. Mulrow C D. Rationale for Systematic Reviews.Bmj British Medical Journal,1994,309:597-599.
3. Bastian H,Glasziou P,Chalmers I.Seventy-Five Trials and Eleven Systematic Reviews a Day:How Will We Ever Keep Up?.PLoS Medicine,2010,7:e1000326.
4. Ge L,Tian J H,Li Y N,et al.Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study.Journal of Clinical Epidemiology,2018,93.
5. Bothwell L E,Greene J A,Podolsky S H,et al.Assessing the Gold Standard--Lessons from the History of RCTs.New England Journal of Medicine,2016,374:2175.
6. Shrier I,Boivin J F,Steele R J,et al.Should Meta-Analyses of Interventions Include Observational Studies in Addition to Randomized Controlled Trials? A Critical Examination of Underlying Principles.American Journal of Epidemiology, 2007,166:1203-1209.
7. Vandenbroucke J P.In defense of case reports and case series.Annals of Internal Medicine,2001,134:330-334.
8. Murad M H, Sultan S, Haffar S, et al.Methodological quality and synthesis of case series and case reports.Bmj Evid Based Med,2018.
9. Meyer H,Lundberg G.Landmark articles in medicine. Chicago: American Medical Association,1985.
10. Foroutan F,Guyatt G,Alba A C,et al.Metaanalysis:mistake or milestone in medicine?.Heart,2018,313042.
11. Wright J D. Robotic-Assisted Surgery:Balancing Evidence and Implementation.JAMA,2017,318:1545.
12. Hirst A,Philippou Y,Blazeby J,et al.No Surgical Innovation Without Evaluation:Evolution and Further Development of the IDEAL Framework and Recommendations.Annals of Surgery,2018:1.
13. Sun F.Interpretation of Standards for Medical reports. Peking University Medical Press,2015.
14. Moher D,Hopewell S,Schulz K F,et al.CONSORT 2010 Explanation and Elaboration:Updated guidelines for reporting parallel group randomised trials.Bmj British Medical Journal,2012,340:698-702.
15. Jadad A R,Moore R A,Carroll D,et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?.Controlled Clinical Trials,1996,17:1-12.
16. Moher D,Liberati A,Tetzlaff J,et al.Research methods and reporting. preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. Plos Medicine,2009,339:332-339.
17. Shea B J, Reeves B C, Wells G, et al.AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions,or both.Bmj,2017,358:j4008.
18. Brouwers M C,Kho M E,Browman G P,et al.For the AGREE Next Steps Consortium.AGREE II:Advancing guideline development,reporting and evaluation in healthcare.Journal of Clinical Epidemiology,2010,63:1308-1311.
19. Chen Y,Yang K,Marušic A,et al.A Reporting Tool for Practice Guidelines in Health Care:The RIGHT Statement. Annals of Internal Medicine,2017,3:127-128.
20. Gagnier J J,Kienle G,Altman D G,et al.The CARE Guidelines:Consensus-based Clinical Case Report Guideline Development.Journal of Nutraceuticals Functional&Medical Foods,2013,10:381-390.
21. Moola S,Munn Z,Tufanaru C,et al.Chapter 7:Systematic reviews of etiology and risk.In:Aromataris E,Munn Z(Editors).Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute,2017.
杂志排行
Clinical Research Communications的其它文章
- Fabry disease combined with acute interstitial nephritis:one case and literature review
- Treatment of refractory H.pylori by Chai Ping Decoction:two case reports
- Research progress of microRNA in prevention and treatment of osteonecrosis of the femoral head
- Research progress of continuous nursing care in patients with percutaneous coronary intervention
