Electroacupuncture and moxibustion promote regeneration of injured sciatic nerve through Schwann cell proliferation and nerve growth factor secretion
2018-04-04LinnaHuJinxinTianWeiGaoJingZhuFangfangMouXiaochunYeYupuLiuPingpingLuShuijinShaoHaidongGuo
Lin-na Hu, Jin-xin Tian, Wei Gao, Jing Zhu Fang-fang Mou Xiao-chun Ye Yu-pu Liu Ping-ping Lu Shui-jin Shao,Hai-dong Guo
1 Department of Anatomy, School of Basic Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
2 Department of Internal Medicine, Shanghai Changhang Hospital, Shanghai, China
3 Department of Gynaecology and Obstetrics, Heze Hospital of Traditional Chinese Medicine, Heze, Shandong Province, China
Introduction
Peripheral nerve injury is a common symptom resulting from accidents or excessive labor. It has a high disability rate and can negatively in fluence the life quality of patients(Faroni et al., 2015). Several studies have shown that neural stimulation can lead to neural regeneration (Shah and Gerasimenko, 2016; Badri et al., 2017). Electrodes that stimulate the peripheral nerves can restore some of functions that are lost in paralyzed or quadriplegic patients (Li et al., 2008).Optogenetic stimulation has been shown to promote the selective regeneration of refractory axons in a living vertebrate(Deisseroth, 2011; Xiao et al., 2015).
Mammalian peripheral nerves can regenerate after injury(Menorca et al., 2013; Scheib and Höke, 2013). The mechanism underlying peripheral nerve regeneration is often considered to be associated with Schwann cell proliferation.In particular, neurotrophic factors secreted by Schwann cells play an important role in peripheral nerve regeneration(Kidd et al., 2013). Nerve growth factor (NGF) is the earliest discovered neurotrophic factor and can provide nutrition for neurons and induce neurite outgrowth (Yu et al., 2014; He et al., 2016). NGF can regulate peripheral nerve development,differentiation, growth, and regeneration. Although exogenous NGF has been utilized in the clinic to improve nerve injury, the optimal administration route, dosage, and side effects of NGF still need to be clearly described. However, this goal is hindered by a complicated puri fication technique and expensive cost (Li et al., 2014). Electroacupuncture (EA) and moxibustion (Mox) in traditional Chinese medicine have been widely accepted and have a long history in the treatment of peripheral nerve injury (Hoang et al., 2012; Chang and Namgung, 2013; Liu et al., 2014), but the mechanism is still unclear.
With respect to the mechanism through which EA and Mox produce their effects, some researchers have proposed the “serum of EA and Mox” theory. The effects of EA and Mox were observed through the changes in function and morphology of targets. The “serum of EA and Mox” theory provides a new approach for studying the mechanisms of EA and Mox. Bioactive substances in serum collected from rats undergoing acupuncture can affect the intracellular Ca2+concentration of cortical neurons, and humoral factors may participate in the effect that acupuncture has on regulating functional activity (Liu et al., 2014). Furthermore, inhibition of TNF-α-mediated chondrocyte inflammation via the Ras-Raf-MEK1/2-ERK1/2 signaling pathway, humoral regulation, improvement of the immunologic function, and anti-aging effects of serum of EA or Mox have been investigated in recent years (Li et al., 2005; Xu et al., 2005; Chen et al., 2017). However, adequate data for understanding the mechanisms through which EA and Mox promote nerve regeneration are lacking. Therefore, this study aimed to explore the effect and mechanism of EA and Mox on nerve regeneration through analyzing Schwann cell proliferation and the effect of “serum of EA and Mox”.
Materials and Methods
Animals
Forty-five 6-week-old male Wistar rats weighing 150—200 g (license No. SYXK[Hu]2014-0008) were kept in a specific-pathogen-free animal experiment center. The rats were maintained at constant temperature (25 ± 2°C) and humidity(50—70%) in a 12-hour light/dark cycle and allowed free access to food. The experimental protocols were approved by the Ethics Committee for Animal Experimentation at Shanghai University of Traditional Chinese Medicine in China (reference No. 2015002) and were performed according to the institution’s Guidelines for Animal Experimentation.
Model establishment and treatment of EA and Mox
Rats were randomly divided into the following three groups one week after adaptive breeding: model group, EA group,and Mox group (15 rats per group). All rats received intraperitoneal anesthesia with 3% pentobarbital sodium 5 mg/kg and were subjected to surgical transection of sciatic nerve as previously described (Horasanli et al., 2017). Briefly, the lateral longitudinal skin of the right hind leg was cut open and the biceps femoris was opened through blunt dissection. The sciatic nerve was exposed and tidily cut off 8 mm away from the edge of the piriformis with a razor blade. The wound was washed with saline and the outer membrane was sutured. Then the muscle and skin were closed by layers. The rats whose sciatic nerve was successfully damaged presented a severe right hind paw drop with deep flexion and inversion of the toes.
In the EA group, three days after the modeling, acupuncture needles (0.25 mm × 13.00 mm, Suzhou Medical Appliance Factory, Suzhou, China) were inserted into the acupoints. A universal pulse therapeutic apparatus (Model G-6805-2; Shanghai Medical Electronic Apparatus, Shanghai, China) with a frequency of 5 Hz was connected and the acupoints were stimulated with intermittent waves with a 20-mA current. The slight hindlimb muscle contraction was an appropriate degree for this type of stimulation. The positive pole was connected to Huantiao (GB30) on the injured side of the rats (posterosuperior border of the hip joint of the hind legs). The negative pole was connected to Zusanli(ST36) of the injured side of (posterolateral side of the knee joint, 5 mm below the capitulum fibulae).
In the Mox group, three days after the modeling, the acupoints Huantiao (GB30) and Zusanli (ST36) on the injured side were treated by warmed moxibustion (18 mm × 200 mm; Nanyang Wolong Hanyi Moxa Factory, Nanyang, China). The moxa stick was held 3—5 cm away from acupuncture points with a supporting device and the skin temperature was maintained at 43 ± 1°C, which contributed to the efficacy of moxibustion. The temperature of the acupuncture point was recorded and controlled with an infrared thermometer (Fluke, Avery De, WA, USA). Each treatment mentioned above was performed for 15 minutes, six times a week for 4 weeks. The rats in the model-only group were handled similarly to those in the EA and Mox groups.
Determination of sciatic functional index (SFI)
The Sfiwas determined using a walking-footprint analysis after 4 weeks of treatment (Badri et al., 2017). The footprints were collected by applying carbon black ink on the rat metapodia. We measured the bilateral print length (maximum distance from heel to toe, represented by PL;), the width of toe (distance between the 1stto 5thtoe, TS), and the inter-toes distance (distance between the 2ndto 5thtoe, IT).The Sfiwas calculated using the Bain formula according to a previous study (Badri et al., 2017). Normal values of Sfiwere 0 and −100, indicating that nerves were completely broken off. The Sfirecovery rate (SFI′) was calculated as:SFI′ = (1 + SFI/100) × 100%.
Measurement of sciatic nerve conduction velocity (NCV)
Rats were laid prone on the procedure table with limbs fixed in place immediately after determining the SFI. Stimulating electrodes (RM6240 Biological Signal Collecting System,Chengdu Instrument Factory, Chengdu, China) were insert-ed on both sides of the sciatic nerve below the ischial tuberosity. Two pairs of recording electrodes were placed on both sides of the nerve at the beginning and end of the gastrocnemius, respectively and the distance between the two pairs of electrodes was measured (mm). Subsequently, an electrical stimulation with an intensity of 5−10 V and a wave width of 0.2 ms was triggered. The time difference between two action potentials (spikes) was observed and recorded. The sciatic NCV was calculated as mm/s by dividing the distance between the electrodes by the time between spikes, and the recovery rate of the NCV was determined by dividing the NCV on the injured side by that for the uninjured side.
Sample collection and hematoxylin-eosin staining
After measuring the sciatic NCV, blood from each group was drawn from the abdominal aortic vein (postcava) under aseptic conditions. After standing at room temperature for 3—4 hours, the blood was centrifuged for 15 minutes at 3,000 r/min, the supernatant was collected and cryopreserved at−80°C. The complete gastrocnemius muscle was dissociated under a microscope, cut into 1 cm3blocks transversely in the center of the muscle, fixed with 4% paraformaldehyde,and embedded in paraffin. The nervous tissue 1 cm from the distal side of the nerve cutoff point was harvested under the microscope (normal tissue was collected in the corresponding position on the uninjured side) and fixed with 4%paraformaldehyde in paraffin sections. Hematoxylin and eosin staining was performed with 5 μm paraffin-embedded sections. Brie fly, the slices were stained with hematoxylin for 5 minutes after being dewaxed and dehydrated, were placed in eosin for 2 minutes, and then underwent conventional dehydration, transparency, and mounting.
Measurement of the gastrocnemius muscle- fiber diameters
The complete gastrocnemius muscle was dissociated under the microscope after 4 weeks of treatment with EA or Mox, then cut into 1 cm3blocks transversely in the center of the muscle.Hematoxylin-eosin-stained fibers were assessed through blind analysis using Image J software (NIH, Bethesda, MD, USA).Fifty randomly captured images from each experimental condition were selected to determine fiber sizes. Fibers were handled manually, and the maximum transverse diameter of each fiber was computed using the software. The quantitative analysis was performed with the mean value used to represent muscle-fiber diameter. The diameter of autologous gastrocnemius muscle fibers on the uninjured side was used as the control. The recovery rate of gastrocnemius muscle fibers was evaluated as the ratio of gastrocnemius muscle- fiber diameter on the injured side to that of the uninjured side.
Immunohistochemistry
After dewaxing and dehydration with gradient alcohol, the tissue sections harvested after 4 weeks of the treatment were washed three times with 0.01 M PBS. After antigen retrieval with 0.01 M sodium citrate buffer solution, the sections were blocked with 10% goat serum for 10 minutes before incubation with the S100 (a speci fic marker for Schwann cells) rabbit monoclonal antibody (1:200; Cell Signaling Technology,Danvers, MA, USA) overnight at 4°C. One drop of reagent B(goat anti-rabbit biotinylated secondary IgG; Zymed Laboratories, San Francisco, CA, USA) was added and incubated for 30 minutes at 37°C after washing with PBS. The reagent C(horseradish peroxidase-labeled streptavidin) was added and incubated for 10 minutes after washing with PBS. Chromogenic reaction with 3,3′-diaminobenzidine was performed to show the S100 positive reaction product. Positive immunoreactive products presented as sepia or brown under a light microscope (Olympus, Tokyo, Japan) at 100× magni fication.The same location of the autologous sciatic nerve on the uninjured side was used as the control. The recovery rate of S100 expression was evaluated as the ratio S100 positive cells on the injured side to those on the uninjured side.
Enzyme linked immunosorbent assay (ELISA)
The expression of NGF in the serum of each group was examined using ELISA after 4 weeks of EA or Mox treatment. The standard substance was diluted according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).Samples were incubated at 37°C for 30 minutes after loading and washed 5 times for 30 seconds. Fifty microliters of enzyme reagent were added to all wells but one and incubated at 37°C for 30 minutes. Subsequently, 50 μL of chromogenic agent was added into each well and incubated at 37°C for 15 minutes. After that, 50 μL of stop buffer was added into each well to terminate the reaction. The optical density values of NGF in each well were measured at a wavelength of 450 nm.
Schwann cell culture and CCK-8
Schwann cells were adaptively cultured for 48 hours in an incubator after acquisition (Shanghai Institutes for Biological Sciences Affiliated to Chinese Academy of Sciences). After one-day culture with Dulbecco’s modified Eagle’s medium(DMEM) without serum, the original culture solution was discarded and washed three times with 0.01 M PBS. Schwann cells were divided into three groups: Model group, EA group,and Mox group. The cells in each group were treated with the serum from Model, EA, or Mox treated rats, respectively. Afterward, the viability and proliferation of Schwann cells were measured using Cell Counting Kit-8 at 1, 2, and 5 days after treatment according to the manufacturer’s instructions.
Statistical analysis
All experimental data were presented as the mean ± SD.Statistical analysis was performed using SPSS 16.0 software(SPSS, Chicago, IL, USA). One-way analysis of variance with Student-Newman-Keuls post hoc test was used to evaluate the statistical differences among multiple groups. A value of P < 0.05 was considered statistically signi ficant.
读后感:加利福尼亚州的丹尼尔·贝莱斯福特说:“读了杜克大学的拒绝信后,我认为我是一个很不错的申请者,而不仅仅是一个未被录取的学生。”
Results
EA and Mox promoted functional recovery of sciatic nerves
Rat activity was normal and limp was improved after treatment for 28 days. The inter-toes distance of the injured limb had increased. Although the non-treated rats had some natural self-healing ability, both EA and Mox treatment promoted signi ficantly more functional recovery of the sci-atic nerves. Sfiin the Mox and EA groups was signi ficantly higher than in the model group (P < 0.05, P < 0.01, respectively). Sfidid not differ between the EA and Mox groups (P> 0.05;Figure 1).
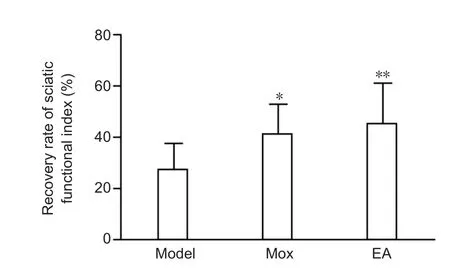
Figure 1 Effects of electroacupuncture (EA) and moxibustion (Mox)on the rat sciatic function index.

Figure 2 Effects of electroacupuncture (EA) and moxibustion (Mox)on the recovery of nerve conduction velocity.
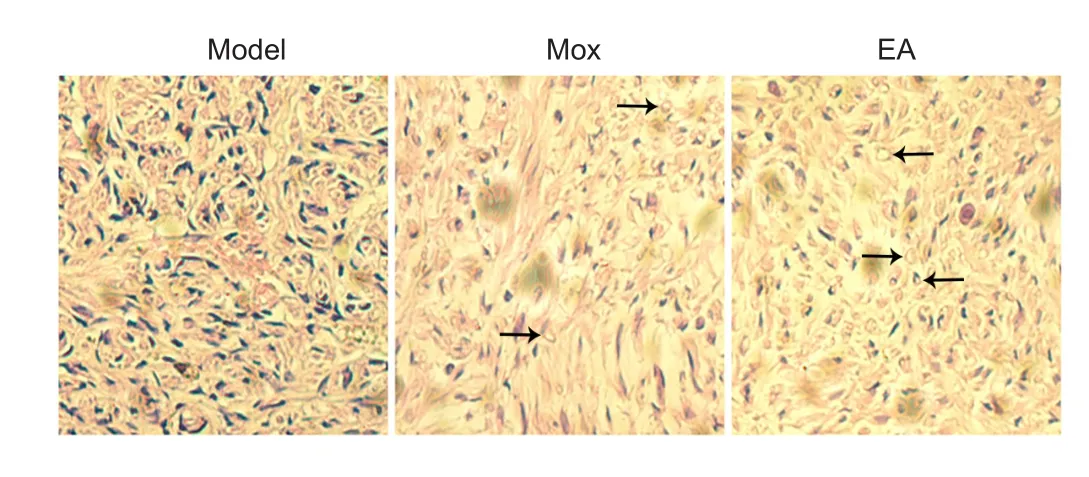
Figure 3 Effects of electroacupuncture (EA) and moxibustion (Mox)on the morphology of injured sciatic nerves (hematoxylin-eosin staining).
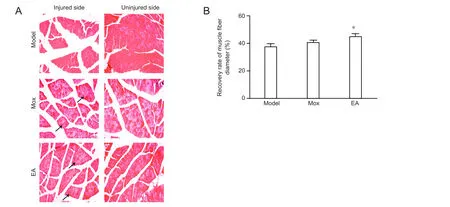
Figure 4 Effects of electroacupuncture (EA) and moxibustion (Mox) on the recovery of muscle fiber diameter.
EA and Mox improved the NCV recovery rate
NCV, the speed at which electrical signals propagate along peripheral nerves, can re flect nerve function in the clinic. A decline in peripheral nerve function is associated with numerous nervous system pathologies. The NCV recovery rate was much higher in both EA and Mox groups than in the model group (P< 0.05 and P < 0.01, respectively) and did not differ between the two treatment groups (P > 0.05;Figure 2).
Morphological changes in injured sciatic nerves after Mox and EA treatment
Hematoxylin-eosin staining was performed to compare the morphology among the groups after treatment. We observed that the structure of the neural tissue was loose and that nerve fibers were not arranged systematically in the model group.
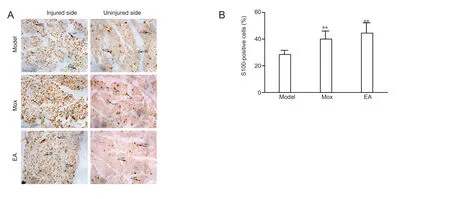
Figure 5 Effects of electroacupuncture (EA) and moxibustion (Mox) on S100 expression at the injured sites.
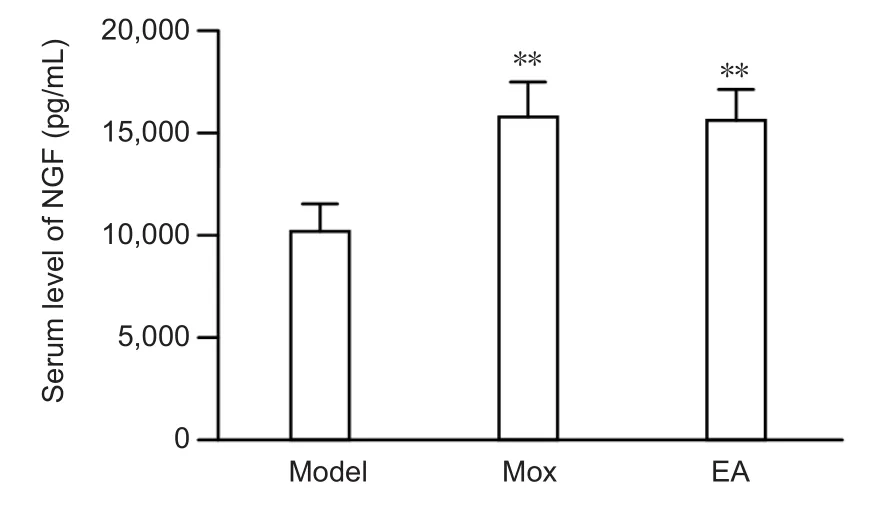
Figure 6 Effects of electroacupuncture (EA) and moxibustion (Mox)on the level of nerve growth factor (NGF) in the serum of sciatic nerve-injured rats in each group (ELISA assay).
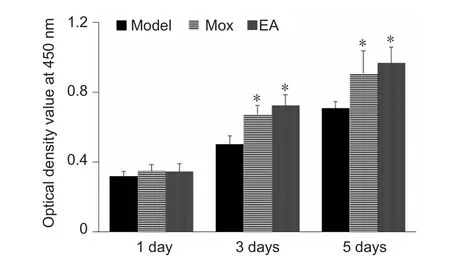
Figure 7 Effects of electroacupuncture (EA) and moxibustion (Mox)on the viability of cultured Schwann cells.
Axonal swellings were obvious in the model group due to the neural lesion. The denatured axons showed loss of myelin,disintegration, and a foam-like shape. The number of regenerating axons in both treatment groups, especially the EA group,was signi ficantly higher than in the model group (Figure 3).
Mox and EA increased the recovery of gastrocnemius muscle fibers
In the model group, skeletal muscle cells presented loss of cytoplasm, decreased diameter, and decreased nuclei under the microscope, which indicated obvious atrophy. The myofibers were sparse and separated by large distances. After both EA and Mox treatment, the atrophy of gastrocnemius muscle fibers was less than what we observed for the model group. The efficiency of EA treatment was higher than that of Mox treatment (Figure 4A). The recovery of gastrocnemius muscle fibers was significant higher in the EA group than in the model group (P < 0.05), but did not differ from that in the Mox group (Figure 4B).
Mox and EA upregulated S100 expression at the injured sites
S100 expression on the uninjured side was used as a control.The number of S100-immunoreactive cells on the injured side was greater than that on the uninjured side. The structure of cells on the injured side was looser in the model group than in the treatment groups (Figure 5A). Both EA and Mox could promote significantly S100 expression in the nerve-injured rats than what was observed in the model group (P < 0.01). S100 expression levels did not differ between the EA and Mox groups (Figure 5B).
EA and Mox enhanced the level of NGF in serum
The level of NGF in the serum of each group was detected using ELISA. NGF is a neurotrophic factor that promotes nerve regeneration after peripheral nerve injury (Tang et al., 2013).The level of NGF in serum was much higher in the Mox and EA groups than in the model group (P < 0.01), but did not differ between the two treatment groups (Figure 6).
Serum of Mox and EA treated rats facilitates proliferation of cultured Schwann cells
Discussion
In this study, the positive pole was connected to GB30 and the negative pole was connected to ST36 because nerves are known to grow toward the negative pole in a weak electrical field and because of the relative function of these acupoints.We found that both EA and Mox promoted sciatic nerve regeneration in rats after injury. Electrical stimulation can promote peripheral nerve regeneration and target reinnervation(Wenjin et al., 2011; Liu et al., 2013; Zhao et al., 2013). Furthermore, the combination of electrical stimulation and polymer composites can enhance neurite outgrowth and nerve regeneration (Koppes et al., 2014; Song et al., 2016). Neurite outgrowth grew faster towards the negative pole under the in fluence of a weak electrical field, but the growth towards the positive pole was inhibited (Song et al., 2004). However, these studies did not examine the effects of acupoint stimulation. A previous study showed that acupuncture at GB30 enhanced axonal regeneration in injured sciatic nerves (Chang and Namgung, 2013) and specifically improved motor recovery(Hoang et al., 2012). EA stimulation at ST36 in rats has been shown to upregulate the expression of axonal growth-associated protein 43 in neurons of the dorsal root ganglion (Kim et al., 2012). Stimulation via EA and Mox has been shown to have direct effects on local areas and could promote blood circulation, decrease the edema of nerves, and improve the nutrition of muscle, thereby promoting functional recovery.Mox increases local skin temperature, which might be part of the mechanism through which Mox therapy works. The spinal cords and brains of juvenile turtles acclimated to warm temperature revealed higher cell proliferation than those acclimated to a cooler environment, which indicates that a high temperature could promote nerve regeneration in juvenile turtles (Radmilovich et al., 2003). Although the environment can affect neuronal proliferation, speci fic acupoint activation may play the key role in nerve regeneration during treatment with EA or Mox after peripheral nerve injury.
The ultimate goal of studying peripheral nerve injury is to achieve functional recovery of injured nerves. Sfihas been shown to be accurate and reliable for evaluating sciatic nerve regeneration and functional recovery (Zencirci et al., 2010).EA stimulation at ST36 and GB30 has been shown to alleviate muscle atrophy induced by sciatic nerve injury (Yu et al.,2017). According to our data, even untreated injury-model rats had some nerve self-healing ability. However, both EA and Mox treatment could promote signi ficantly more functional recovery of the sciatic nerve, recovery of NCV, and less gastrocnemius muscle atrophy than this natural level of self-healing. Although EA seems to have a better effect on sciatic nerve-injury repair than Mox, the current study demonstrated a possible overlap in the mechanism through which EA and Mox act. One of the possibilities is that the same acupoints were stimulated and the main outcome of EA and Mox treatment is derived from the specific acupoints which were stimulated, and not the method of stimulation. However, it appears that combining EA and Mox might exert an even greater effect than EA or Mox alone.Therefore, the combined effect of EA and Mox on peripheral nerve injury deserves to be explored in the future.
Schwann cells have been shown to secrete neurotrophic factors and produce extracellular matrix and cell adhesion molecules, which can then induce, stimulate, and regulate axon regeneration and the formation of myelin sheaths(Madduri and Gander, 2010; Kidd et al., 2013). During the regeneration, Schwann cells quickly migrated to distal regions and only partial Schwann cells positively expressed S100. However, S100 protein was positively expressed in the majority of Schwann cells formed in the distal part of the injury. Based on this finding, a theory was proposed in which peripheral nerve regeneration was regulated by distal parts of the nerve, which could assist in the extension of neurites and in reestablishing innervation through the recognition and interaction between neurons and neurogliocytes. Li et al. (2007) found that EA could promote S100 expression of Schwann cells in the reparative stage after sciatic nerve cutoff.Low frequency EA (5 Hz) exerted an optimal effect for peripheral nerve injury and the effect was most obvious at the early stage. Our study con firmed that both EA and Mox could promote Schwann cell proliferation after injury, which may play an important role in nerve regeneration.
Inspired by the theories of “serum pharmacology” and“Traditional Chinese medicine serology”, some researchers proposed a “serum of EA and Mox” theory (Li et al., 2004,2005; Xu et al., 2005; Chen et al., 2017). After nerve injury, the expression of NGF was increased, which provided an appropriate environment for promoting axon regeneration, reestablishing the connection between axons and Schwann cells,and the reinnervation of targeting areas for improving nerve growth (Tang et al., 2013). We found that the expression of NGF in the serum of the Mox and EA groups was much higher than that in the model group. Further, the serum of EA or Mox treated rats could promote the proliferation of Schwann cells in vitro. Optical density values determined by Cell Counting Kit-8 in both EA and Mox groups were much higher than those in the model group at 3 and 5 days. EA or Mox may augment new axonal connections after peripheral nerve injury, and ultimately accelerate the transport of NGF. Moreover, EA or Mox were able to enhance the proliferation of Schwann cells, which secreted much more NGF. Therefore, the mechanism through which EA and Mox lead to regeneration and repair after sciatic nerve injury might be related to Schwann cell proliferation and enhanced NGF secretion. Critically, EA and Mox are safer and more convenient methods for elevating NGF expression than exogenous NGF administration. It would be interesting to in-vestigate the levels of other neurotrophic factors, such as brain derived neurotrophic factor and glial cell line-derived neurotrophic factor, after EA and Mox treatment.
This study had some limitations. First, we did not include sham EA and sham Mox groups, although the effects of sham EA or sham Mox were not obvious according to our previous studies. Additionally, data from groups that receive real EA and Mox, but at unrelated acupoints, should also be investigated to con firm the importance of these speci fic acupoints. Second, the expression of NGF in the local injured nerve tissue was not determined.
In conclusion, both EA and Mox promoted nerve regeneration and functional recovery after sciatic nerve injury and improved skeletal muscle atrophy. The mechanism underlying this phenomenon might be associated with proliferation of Schwann cells and secretion of NGF.
Author contributions:SJS and HDG participated in study conception and design. SJS was in charge of study supervision. HDG and JXT drafted the paper. LNH, JXT, WG and PPL performed the experiments. LNH, WG,XCY, YPL, FFM and PPL participated in acquisition of data. LNH, WG,XCY, YPL and JZ parcipated in statistical analysis. LNH, WG, XCY, SJS and HDG were responsible for data analysis and interpretation. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81373754, 81102670. The funding sources played no role in the study conception and design, collection, analysis and interpretation of data,in the writing of the paper, and in the decision to submit the paper for publication.
Research ethics:The study protocol was approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine in China (approval number: 2015002).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Badri O, Shahabi P, Abdolalizadeh J, Alipour MR, Veladi H, Farhoudi M,Zak MS (2017) Combination therapy using evening primrose oil and electrical stimulation to improve nerve function following a crush injury of sciatic nerve in male rats. Neural Regen Res 12:458-463.
Chang IA, Namgung U (2013) Induction of regenerative responses of injured sciatic nerve by pharmacopuncture therapy in rats. J Acupunct Meridian Stud 6:89-97.
Chen H, Shao X, Li L, Zheng C, Xu X, Hong X, Li X, Wu M (2017) Electroacupuncture serum inhibits TNF-α-mediated chondrocyte in flammation via the Ras-Raf-MEK1/2-ERK1/2-signaling pathway. Mol Med Rep 16:5807-5814.
Deisseroth K (2011) Optogenetics. Nat Methods 8:26-29.
Faroni A, Mobasseri SA, Kingham PJ, Reid AJ (2015) Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev 82-83:160-167.
He XZ, Wang W, Hu TM, Ma JJ, Yu CY, Gao YF, Cheng XL, Wang P (2016)Peripheral nerve repair; theory and technology application. Zhongguo Zuzhi Gongcheng Yanjiu 20:1044-1050.
Hoang NS, Sar C, Valmier J, Sieso V, Scamps F (2012) Electro-acupuncture on functional peripheral nerve regeneration in mice: a behavioural study.BMC Complement Altern Med 12:141.
Horasanli B, Hasturk AE, Arikan M, Togral G, Helvacioglu F, Dagdeviren A, Mut S, Harman F, Argun G (2017) Comparative evaluation of the electrophysiological, functional and ultrastructural effects of alpha lipoic acid and cyanocobalamin administration in a rat model of sciatic nerve injury. J Back Musculoskelet Rehabil 30:967-974.
Kidd GJ, Ohno N, Trapp BD (2013) Biology of Schwann cells. Handb Clin Neurol 115:55-79.
Kim MH, Park YC, Namgung U (2012) Acupuncture-stimulated activation of sensory neurons. J Acupunct Meridian Stud 5:148-155.
Koppes AN, Zaccor NW, Rivet CJ, Williams LA, Piselli JM, Gilbert RJ,Thompson DM (2014) Neurite outgrowth on electrospun PLLA fibers is enhanced by exogenous electrical stimulation. J Neural Eng 11:046002.
Li L, Yan H, Campbell G, Lineaweaver WC, Akdemir O, Zhang F (2008) Implanted electrodes in peripheral nerve stimulation and recording: prospects of their application in electronic prosthesis design. J Long Term Eff Med Implants 18:227-237.
Li QW (2007) Effect of electro-acupuncture on s-100 protein expression of nerve fibers in the repair process of transected sciatic nerve in rats. Journal of Acupuncture and Tuina Science 5:10-13.
Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L (2014) Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys 68:449-454.
Li RW, Zhang JL, Guo Y, Li CH (2004) Effect of acupuncture serum on intracellular Ca2+concentration in cultured neurons. Zhong Xi Yi Jie He Xue Bao 2:453-455.
Li RW, Zhang JL, Guo Y, Li CH (2005) Preliminary study on effect of acupuncture serum on Ca2+content in cultured neurons of cerebral cortex.Zhongguo Zhen Jiu 25:351-354.
Liu Y, Grumbles RM, Thomas CK (2013) Electrical stimulation of embryonic neurons for 1 hour improves axon regeneration and the number of reinnervated muscles that function. J Neuropathol Exp Neurol 72:697-707.
Liu YL, Li Y, Ren L, Dai LL, Bai ZH, Bai R, Ma TM (2014) Effect of deep electroacupuncture stimulation of “Huantiao” (GB 30) on changes of function and nerve growth factor expression of the injured sciatic nerve in rats.Zhen Ci Yan Jiu 39:93-99.
Madduri S, Gander B (2010) Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst 15:93-103.
Menorca RM, Fussell TS, Elfar JC (2013) Nerve physiology: mechanisms of injury and recovery. Hand Clin 29:317-330.
Radmilovich M, Fernández A, Trujillo-Cenóz O (2003) Environment temperature affects cell proliferation in the spinal cord and brain of juvenile turtles. J Exp Biol 206:3085-3093.
Scheib J, Höke A (2013) Advances in peripheral nerve regeneration. Nat Rev Neurol 9:668-676.
Shah PK, Gerasimenko Y (2016) Multi-site spinal stimulation strategies to enhance locomotion after paralysis. Neural Regen Res 11:1926-1927.
Song B, Zhao M, Forrester J, McCaig C (2004) Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J Cell Sci 117:4681-4690.
Song J, Sun B, Liu S, Chen W, Zhang Y, Wang C, Mo X, Che J, Ouyang Y,Yuan W, Fan C (2016) Polymerizing pyrrole coated poly (l-lactic acid-co-ε-caprolactone) (PLCL) conductive nano fibrous conduit combined with electric stimulation for long-range peripheral nerve regeneration.Front Mol Neurosci 9:117.
Tang S, Zhu J, Xu Y, Xiang AP, Jiang MH, Quan D (2013) The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials 34:7086-7096.
Wenjin W, Wenchao L, Hao Z, Feng L, Yan W, Wodong S, Xianqun F, Wenlong D (2011) Electrical stimulation promotes BDNF expression in spinal cord neurons through Ca(2+)- and Erk-dependent signaling pathways. Cell Mol Neurobiol 31:459-467.
Xiao Y, Tian W, López-Schier H (2015) Optogenetic stimulation of neuronal repair. Curr Biol 25:R1068-R1069.
Xu HM, Ma SL, Yang YQ, Zhang YY (2005) Effects of different segments of acupuncture serum on eosinophil counts in the rat with eosinophilia.Zhongguo Zhen Jiu 25:272-274.
Yu H, Liu J, Ma J, Xiang L (2014) Local delivery of controlled released nerve growth factor promotes sciatic nerve regeneration after crush injury. Neurosci Lett 566:177-181.
Yu J, Wang M, Liu J, Zhang X, Yang S (2017) Effect of electroacupuncture on the expression of agrin and acetylcholine receptor subtypes in rats with tibialis anterior muscular atrophy induced by sciatic nerve injection injury.Acupunct Med 35:268-275.
Zencirci SG, Bilgin MD, Yaraneri H (2010) Electrophysiological and theoretical analysis of melatonin in peripheral nerve crush injury. J Neurosci Methods 191:277-282.
Zhao F, He W, Zhang Y, Tian D, Zhao H, Yu K, Bai J (2013) Electric stimulation and decimeter wave therapy improve the recovery of injured sciatic nerves. Neural Regen Res 8:1974-1984.
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Optic radiation injury in patients with aneurismal subarachnoid hemorrhage: a preliminary diffusion tensor imaging report
- Regulatory role of calpain in neuronal death
- The ROCK pathway inhibitor Y-27632 mitigates hypoxia and oxidative stress-induced injury to retinal Müller cells
- Combined acupuncture and HuangDiSan treatment affects behavior and synaptophysin levels in the hippocampus of senescence-accelerated mouse prone 8 after neural stem cell transplantation
- Endoplasmic reticulum stress transducer old astrocyte speci fically induced substance contributes to astrogliosis after spinal cord injury
