Endoplasmic reticulum stress transducer old astrocyte speci fically induced substance contributes to astrogliosis after spinal cord injury
2018-04-04AtsushiTakazawaNaosukeKameiNobuoAdachiMitsuoOchi
Atsushi Takazawa, Naosuke Kamei,, Nobuo Adachi, Mitsuo Ochi
1 Department of Orthopaedic Surgery, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan
2 Medical Center for Translational & Clinical Research, Hiroshima University Hospital, Hiroshima, Japan
Introduction
The endoplasmic reticulum (ER) is a cellular organelle that regulates the synthesis, folding, and post-translational modi fication of secreted proteins. Various cellular stress conditions,including oxidative stress, ischemic insult, and expression of mutated genes, can lead to ER stress or the accumulation of unfolded or misfolded proteins in the ER. Notably, prolonged or severe ER stress induces cellular apoptosis.
ER stress transducers initiate signals that maintain ER homeostasis through a regulatory system called the unfolded protein response (UPR). In mammalian cells, inositol-requiring enzyme 1 (IRE1), protein kinase R-like endoplasmic reticulum kinase (PERK), and activating transcription factor(ATF)6 are well-known ubiquitous cellular ER transducers that sense ER stress and regulate the UPR. However, other ER stress transducers have been identi fied, including old astrocyte speci fically induced substance (OASIS), a member of the CREB/ATF protein family which is strongly expressed in astrocytes and osteoblasts. Importantly, the OASIS-mediated ER stress response has a stimulatory effect on astrocyte differentiation (Saito et al., 2012).
Mechanical spinal cord injuries (SCIs) caused by an external force involve secondary damage, such as inflammation,hypoxia, ischemia, demyelination, and disruption of the blood-brain barrier, which leads to an expansion of the injury space (Oyinbo, 2011). Following the injury, reactive astrocytes appear around the space and cause astrogliosis, which can have both beneficial and detrimental effects on injury repair. Beneficial effects include the formation of a barrier across the injury space that blocks inflammatory leukocyte accumulation and repairs the blood-brain barrier (Sofroniew,2005; Okada et al., 2006; Barres, 2008), whereas detrimental effects include the prevention of neural growth via glial scar formation (Bradbury et al., 2002; Yiu and He, 2006). Accordingly, regulation of astrogliosis has become a major target of research in the field of SCI treatment.
The present study aimed to demonstrate the role for OASIS in astrogliosis following SCI in a mouse model of SCI.
Materials and Methods
Spinal cord injury mouse model
C57BL/6 female mice (age: 10 weeks, body weight: 21—25 g)were anesthetized via sustained inhalation of 2.0% sevoflurane in air at a rate of 2.0 L/min. The dorsal surface of the dura mater was exposed via microscopic laminectomy at the T10level of the spine. Contusion SCI was induced using an Infinite Horizon Impactor (Precision Systems and Instrumentation, Lexington, KY, USA) with a force-de fined impact of 70 kdyne. Mice in the sham group received only laminectomy without spinal cord contusion.
All research methods were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and all animal-use protocols were approved by the ethical committee of Hiroshima University.
Real-time polymerase chain reaction (PCR)
Real-time PCR was used to analyze the expression of messenger RNAs (mRNAs) relevant to OASIS. The 5-mm-long sections of the spinal cord from the center of the injury site were obtained. Prior to SCI and on post-injury days 1, 3, 7,and 14 (n = 5 per time point), RNA was isolated from excised mouse spinal cords using TRIZOL (Invitrogen, Carlsbad,CA, USA) according to the manufacturer’s protocol. For complementary DNA (cDNA) synthesis, 1.0 μg of RNA was reverse-transcribed using the Super Script VILO Master Mix (Invitrogen) to facilitate first-strand cDNA synthesis.Subsequently, 10 ng of cDNA were used per PCR reaction.Real-time PCR was performed using the ABI Step One Plus kit (Applied Biosystems, Foster City, CA, USA), SYBR Green Master Mix reagent (Invitrogen), and the following OASIS gene-specific primer pair: OASIS-fwd 5′-CCT TGT GCC TGT CAA GAT GGA G-3′ and OASIS-rev 5′-GCA GCA GCC ATG GCA GAG GAG-3′ (Murakami et al., 2009). The relative expression of OASIS mRNA was normalized to that of β-actin mRNA, which was determined using the following primers: β-actin-fwd 5′-TCC TCC CTG GAG AAG AGC TAC-3′ and β-actin-rev 5′-TCC TGC TTG CTG ATC CAC AT-3′ (Murakami et al., 2009).
Western blot analysis
The expression of proteins relevant to OASIS was evaluated using Western blot analysis. A 5-mm-long section of affected spinal cord was lysed prior to SCI and on post-injury days 1,3, 7, and 14 (n = 5 per group) using tissue perfusion extraction reagent (T-PER) (Thermo Fisher Scientific, Waltham, MA,USA), after which the lysates were centrifuged at 10,000 × g for 5 minutes. Supernatant proteins were separated using 10%sodium dodecyl sulfate-polyacrylamide gel electrophoresis(SDS-PAGE) and subsequently transferred electrophoretically to polyvinylidene difluoride membranes. The protein-containing membranes were blocked for 30 minutes at room temperature in a 5% skim milk solution and incubated with either primary polyclonal rabbit anti-CREB3L1 (1:800;Abcam, Cambridge, UK) or polyclonal goat anti-actin C-11(1:200; Santa Cruz Biotechnology, Dallas, TX, USA). Membranes were subsequently incubated with a secondary horse radish peroxidase-conjugated anti-rabbit or anti-goat IgG antibody, as appropriate. Labeled proteins were visualized using Image Quant™ LAS 4000 and results were analyzed with Image Quant TL software (GE healthcare, Arlington Heights,IL, USA). The relative expression of OASIS protein was normalized to that of β-actin in each sample.
Immunohistochemistry
Immunohistochemical (IHC) analysis was performed to evaluate the expression of OASIS in the injured spinal cord. At 7 and 14 days after SCI, mice were anesthetized and transcardially perfused with 4% paraformaldehyde in phosphate-buff-ered saline (PBS). Each spinal cord was resected in 10-mm sections and frozen. Serial sagittal cryostat sections (10 μm)from each spinal cord were mounted on silane-coated glass slides and air-dried, followed by treatment with a serum-free protein block (Dako, Agilent Technologies, Glostrup, Denmark) and mouse-on-mouse IgG blocking reagent (Vector Laboratories, Burlingame, CA, USA) for 60 minutes at room temperature. The slides were then stained overnight at 4ºC with a primary rabbit anti-CREB3L1 (1:100; Abcam) to detect OASIS and a goat anti-glial fibrillary acidic protein (GFAP;1:250; Santa Cruz Biotechnology, Santa Cruz, CA, USA) to detect astrocytes. Subsequently, the slides were treated for 60 min at room temperature with the following secondary antibodies: Alexa Fluor 488-conjugated donkey anti-goat (1:500;Life Technologies, Grand Island, NY, USA) and Alexa Fluor 568-conjugated donkey anti-rabbit (1:500, Life Technologies). The stained sections were observed with a fluorescence microscope (BZ-9000; Keyence Corporation, Osaka, Japan).
Injection of OASIS siRNA
Five days after SCI, animals were anesthetized via sustained inhalation of 2.0% sevo flurane and 2.0 L/min air prior to the injection of siRNA (12.5 μM) into the injured spinal cord. After exposing the T10level of the spinal cord, 2 μL of anti-OASIS siRNA 5′-GAA AUG AGC CAG UUU CUC AdTdT-3′ (sense)and 5′-UGA GAA ACU GGC UCA UUU CdTdT-3′ (antisense)(Kondo et al., 2005) (OASIS siRNA group) or scrambled SiRNA (scrambled siRNA group) mixed with peptide transduction domain-double strand RNA binding domain (PTDDRBD) were injected (2 μL/min) into the center and periphery of the injured lesion using a 32-gauge needle and stereotaxic injector (KDS310; Muromachi Kikai, Tokyo, Japan). Two days later, mice were anesthetized using sustained inhalation of 2.0%sevo flurane and 2.0 L/min air prior to harvesting the injured spinal cords. The spinal cord samples were also harvested from untreated SCI mice (control group) at day 7 after SCI. The mRNA and protein expression of OASIS was assessed using the above mentioned real-time PCR and western blot analysis(n = 5 per group).
Recovery of hind limb motor function
Recovery of hind limb motor function was assessed using the Basso mouse scale (BMS) at 1, 3, 5, 7, 14, 21, 28, 35, and 42 days after SCI (Basso et al., 2006). Injured mice were compared with control SCI models, as well as SCI models injected with anti-OASIS SiRNA and scrambled siRNA (n = 5 per group).
Statistical analyses
Student’s unpaired t-tests were used for comparisons between two samples. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests were used to compare multiple groups.In addition, repeated measures ANOVA followed by Tukey’s post hoc test was used to assess BMS scores. The two-sided P <0.05 was considered statistically signi ficant. Measured values are expressed as the mean ± standard deviation (SD).
Results
Increased expression of OASIS in the spinal cord after SCI
In order to assess changes in the temporal expression of OASIS in the spinal cord after SCI, the expression of OASIS at 1,3, 7, and 14 days after SCI was examined using real-time PCR and western blot. OASIS mRNA expression was signi ficantly higher in injured spinal cords than in uninjured (sham) cords only on day 7 after SCI (Figure 1). On the other hand, OASIS protein expression was signi ficantly upregulated in the injured spinal cords on days 7 and 14 after SCI (Figure 2). These re-sults suggest a temporary increase in OASIS mRNA expression at 7 days after SCI, followed by a subsequent increase in OASIS protein expression for at least an additional 7 days.
Selective expression of OASIS in astrocytes at the periphery of the injured spinal cord
Seven days after SCI, IHC analysis indicated obvious OASIS and GFAP immunoreactivity and co-localization at the peripheries of injury sites(Figure 3). This finding suggests strong expression of OASIS in reactive astrocytes at the peripheral zone surrounding the injury site.
Inhibition of astrogliosis and functional recovery after SCI via OASIS suppression
In order to elucidate the function of OASIS in SCI, anti-OASIS siRNA was injected into the injured spinal cords at 5 days after SCI. As a preliminary analysis, three different concentrations of anti-OASIS siRNA (12.5, 25, and 50 μM) were injected. OASIS mRNA expression was similarly suppressed following the injections of these three different concentrations of anti-OASIS. Therefore, 12.5 μM anti-OASIS siRNA was used for all OASIS suppression experiments. No signi ficant changes in OASIS mRNA and protein expression were observed in injured spinal cords injected with scrambled siRNA. In contrast, mice injected with anti-OASIS siRNA exhibited signi ficant downregulation of both OASIS mRNA (Figure 4) and protein expression (Figure 5). Similarly, in mice injected with scrambled siRNA, IHC analysis revealed the progression of astrogliosis, demonstrating an accumulation of GFAP-positive astrocytes around the injury site at days 7 and 14 after SCI.In contrast, IHC analysis of injured spinal cords from mice injected with anti-OASIS siRNA revealed fewer astrocytes at the periphery of the injury site at day 7 after SCI, as well as a lack of an enclosure containing astrocytes around the site of injury on day 14 after SCI (Figure 6).
The BMS scores for hind limb motor function improved overtime in all treatment groups. However, the scores at 7 and 14 days after SCI were signi ficantly lower in the scrambled siRNA and OASIS siRNA groups than in the control group because of the additive injury due to siRNA injection.Subsequently, BMS scores on or after day 21 were similar to those of the scrambled siRNA and control groups, whereas the BMS scores of the OASIS siRNA group remained signi ficantly lower than those of the control group (Figure 7). These findings indicate that temporary downregulation of OASIS expression in the injured spinal cord inhibits astrogliosis and hind limb motor function recovery after SCI.

Figure 1 Real-time polymerase chain reaction (PCR) analysis of old astrocyte speci fically induced substance (OASIS) mRNA expression after spinal cord injury in C57BL/6 mice.
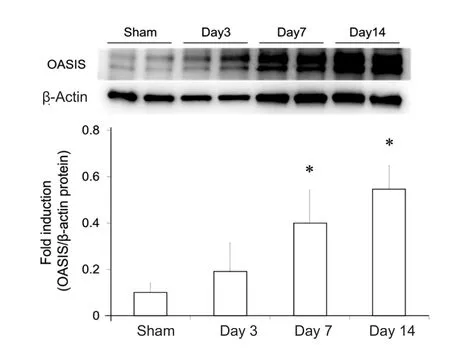
Figure 2 Western blot analysis of old astrocyte speci fically induced substance (OASIS) protein expression after spinal cord injury (SCI)in C57BL/6 mice.

Figure 3 Immunohistochemistry of glial fibrillary acidic protein (GFAP) and old astrocyte speci fically induced substance (OASIS).

Figure 4 Real-time polymerase chain reaction (PCR) analysis of old astrocyte speci fically induced substance (OASIS) mRNA expression at 7 days after spinal cord injury in mice treated with scrambled or OASIS small interfering RNA (siRNA) and control mice.
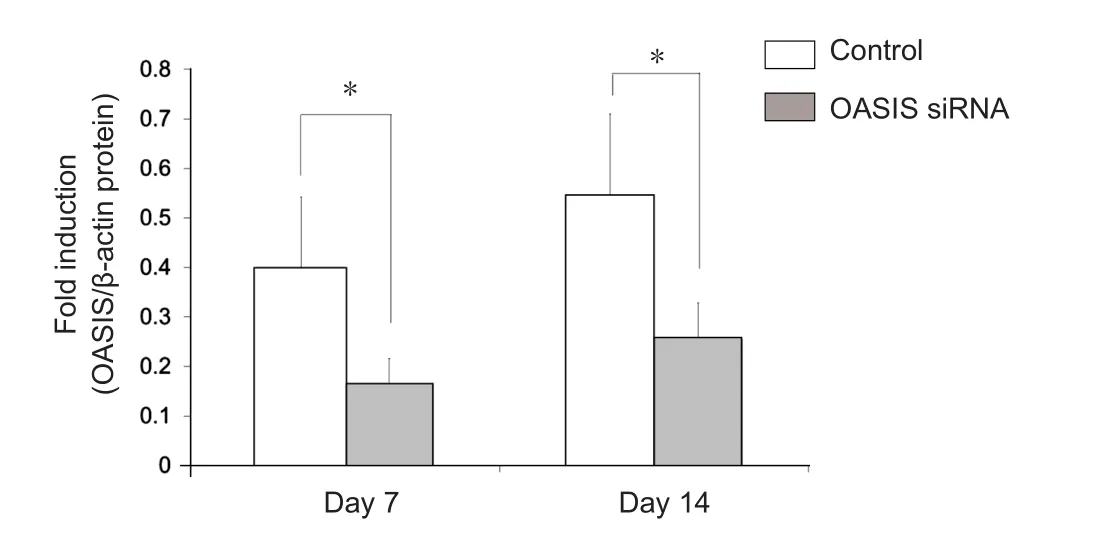
Figure 5 Western blot analysis of old astrocyte speci fically induced substance (OASIS) protein expression in mice from the control and OASIS siRNA groups at 7 days and 14 days after spinal cord injury.
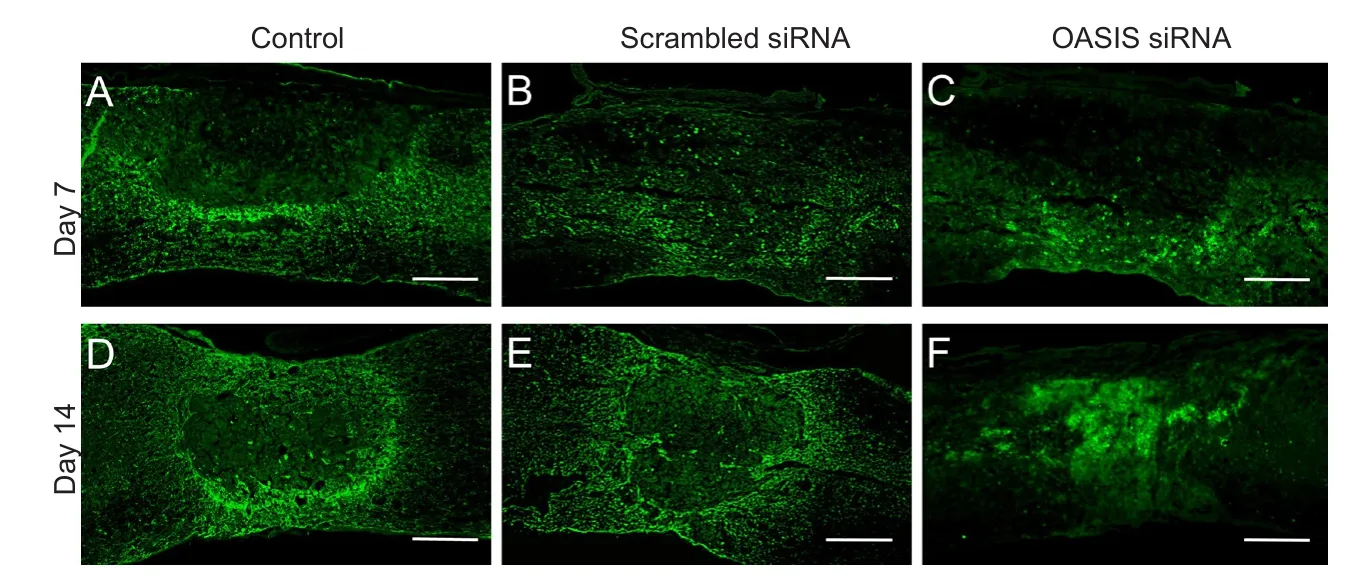
Figure 6 Immunohistochemistry of glial fibrillary acidic protein (GFAP).

Figure 7 Functional recovery after spinal cord injury in the control,scrambled siRNA, and old astrocyte speci fically induced substance(OASIS) siRNA groups.
Discussion
The present study demonstrated that OASIS expression synchronized both temporally and spatially with astrogliosis, and that temporary suppression of OASIS inhibited astrogliosis and the recovery of hind limb motor function following SCI.In the present study, the expression of both OASIS mRNA and protein began to increase 7-days after SCI, whereas in our previous study involving mouse models of SCI, a disappearance of astrocytes around the damaged area of the spinal cord was observed on day 3 after injury, followed by a reappearance and clustering of astrocytes at the periphery of the injury site on day 7 (Kamei et al., 2012). Together, these findings indicate that the timing of increased OASIS-expression coincided with the timing of astrogliosis initiation after SCI. Additionally,results of the IHC analysis conducted in the present study revealed strong OASIS protein expression in the periphery of the SCI, which co-localized with GFAP positive astrocytes on day 7.These findings lead us to conclude that OASIS expression and astrogliosis occur along a similar time line after SCI.
In the healthy central nervous system, astrocytes play crucial roles in the provision of energy, regulation of blood flow,homeostasis of extracellular fluids, ions, and transmitters, and regulation of synaptic remodelling (Fawcett and Asher, 1999;Gordon et al., 2007). In contrast, these cells cause astrogliosis in the injured central nervous system. The effects of astrogliosis on spinal cord functionrecovery may be either bene ficial or detrimental, depending on the timing after injury (Pekny et al., 2014). In the acute post-injury phase, astrogliosis produces bene ficial effects that include the formation of a barrier across the injured area to reduce the tissue damage and lesion size,blocking the migration of in flammatory leukocytes to reduce neuronal loss and demyelination, and restoration of the bloodbrain barrier (Sofroniew, 2005; Okada et al., 2006; Barres,2008). However, in the chronic phase, astrogliosis leads to the formation of a type of high-density scar tissue (or glial scar)that prevents neural growth via chondroitin sulfate proteoglycans (CSPGs), and leads to the creation of a physical barrier(Bradbury et al., 2002; Yiu and He, 2006). In the present study,temporary siRNA-mediated suppression of OASIS inhibited astrogliosis and the recovery of hind limb motor function at or beyond 7 days post-injury. This inhibition of astrogliosis during the acute post-SCI phase might have led to an unsuccessful recovery of hind limb motor function. Taken together,these findings suggest that OASIS contributes to astrogliosis after SCI and plays a crucial role in the recovery of hind limb motor function. Furthermore, although previous studies have reported a relationship between ER stress response and SCI(Ohri et al., 2011; Kuroiwa et al., 2014; Zhang et al., 2014;Xue et al., 2017), nearly all these studies demonstrated their findings using ubiquitous cellular ER transducer involved in cell apoptosis. In contrast, to the best of our knowledge, the present study is the first to demonstrate the contribution of an astrocyte-speci fic ER transducer to post-SCI astrogliosis.
The control of astrogliosis might help in improving functional recovery after a SCI, and according to our findings,OASIS is a candidate for the targeted control of astrogliosis.Although OASIS is expressed at the ER membrane under normal conditions, it is cleaved at the transmembrane region under ER stress, and the resulting fragment, which contains a bZIP domain, is processed and translocated into the nucleus to promote the transcription of the target genes (Kondo et al.,2005; Murakami et al., 2006; Saito et al., 2007). Thus, OASIS has been reported to promote astrocyte differentiation (Saito et al., 2012), and its expression is highly upregulated in astrocytes after brain injury (Kondo et al., 2005). The role of OASIS in astrocyte differentiation is further supported by findings of previous studies in which fewer reactive astrocytes were observed in the hippocampi of Oasis−/−mice, relative to wild-type mice, following kainic acid-induced brain injury (Chihara et al., 2009). Interestingly, the transcription factor Gcm1, which is required for astrocyte differentiation in Drosophila, has been shown to be a target of OASIS. Furthermore, OASIS expression is modulated by the UPR and thus controls astrocyte differentiation (Saito et al., 2012).
Taken together, our results demonstrate that OASIS is closely involved in the kinetics of astrocytes within the injured central nervous system. Bene ficial regulation of astrogliosis, by controlling OASIS expression, may represent a novel approach for the treatment of SCI.
Author contributions:NK, NA and MO were in charge of study conception and design, data interpretation. AT and NK wrote the main manuscript. AT and NK were responsible for data collection and analysis. All authors approved the final version of this paper.Con flicts of interest:None declared.
Financial support:This study was supported by MEXT/JSPS KAKENHI Grant-in-Aid for Scienti fic Research (C) to NK (Grant No. 17K10931).
Research ethics:The study protocol was approved by the ethical committee of Hiroshima University (approval number: A15-77). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1985).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-Shar-eAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430-440.
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23:635-659.
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636-640.
Chihara K, Saito A, Murakami T, Hino S, Aoki Y, Sekiya H, Aikawa Y,Wanaka A, Imaizumi K (2009) Increased vulnerability of hippocampal pyramidal neurons to the toxicity of kainic acid in OASIS-de ficient mice. J Neurochem 110:956-965.
Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49:377-391.
Gordon GR, Mulligan SJ, MacVicar BA (2007) Astrocyte control of the cerebrovasculature. Glia 55:1214-1221.
Kamei N, Kwon SM, Kawamoto A, Ii M, Ishikawa M, Ochi M, Asahara T(2012) Contribution of bone marrow-derived endothelial progenitor cells to neovascularization and astrogliosis following spinal cord injury. J Neurosci Res 90:2281-2292.
Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, Otori K, Iseki K, Wanaka A, Imaizumi K (2005) OASIS, a CREB/ATF-family member,modulates UPR signalling in astrocytes. Nat Cell Biol 7:186-194.
Kuroiwa M, Watanabe M, Katoh H, Suyama K, Matsuyama D, Imai T,Mochida J (2014) Effect of amiloride on endoplasmic reticulum stress response in the injured spinal cord of rats. Eur J Neurosci 40:3120-3127.
Murakami T, Kondo S, Ogata M, Kanemoto S, Saito A, Wanaka A, Imaizumi K (2006) Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem 96:1090-1100.
Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H,Tsumagari K, Ochiai K, Yoshinaga K, Saitoh M, Nishimura R, Yoneda T,Kou I, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Wanaka A, Imaizumi K(2009) Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol 11:1205-1211.
Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR (2011)Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia 59:1489-1502.
Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829-834.
Oyinbo CA (2011) Secondary injury mechanisms in traumatic spinal cord injury:a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 71:281-299.
Pekny M, Wilhelmsson U, Pekna M (2014) The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565:30-38.
Saito A, Hino S, Murakami T, Kondo S, Imaizumi K (2007) A novel ER stress transducer, OASIS, expressed in astrocytes. Antioxid Redox Signal 9:563-571.
Saito A, Kanemoto S, Kawasaki N, Asada R, Iwamoto H, Oki M, Miyagi H,Izumi S, Sanosaka T, Nakashima K, Imaizumi K (2012) Unfolded protein response, activated by OASIS family transcription factors, promotes astrocyte differentiation. Nat Commun 3:967.
Sofroniew MV (2005) Reactive astrocytes in neural repair and protection.Neuroscientist 11:400-407.
Xue LX, Liu HY, Cui Y, Dong Y, Wang JQ, Ji QY, He JT, Yao M, Wang YY,Shao YK, Mang J, Xu ZX (2017) Neuroprotective effects of Activin A on endoplasmic reticulum stress-mediated apoptotic and autophagic PC12 cell death. Neural Regen Res 12:779-786.
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617-627.
Zhang H, Wu F, Kong X, Yang J, Chen H, Deng L, Cheng Y, Ye L, Zhu S,Zhang X, Wang Z, Shi H, Fu X, Li X, Xu H, Lin L, Xiao J (2014) Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury.J Transl Med 12:130.
杂志排行
中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Cerebral ischemia and neuroregeneration
- SNARE complex in axonal guidance and neuroregeneration
- Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage
- The relaxin peptide family – potential future hope for neuroprotective therapy? A short review
- Roles of neural regeneration in memory pharmacology
