水溶性碳纳米管对人胚肾和肝癌细胞毒性的研究
2018-03-15于世平苏旭东杜晶磊王军丽高宇端杨永珍刘旭光
于世平, 苏旭东, 杜晶磊, 王军丽, 高宇端, 张 利, 陈 琳, 杨永珍, 刘旭光
(1.山西医科大学第二医院 介入治疗科,山西 太原030001; 2.太原理工大学 新材料界面与工程教育部重点实验室,山西 太原030024; 3.山西大医院 血管外科,山西 太原030032; 4.太原理工大学 新材料工程技术研究中心,山西 太原030024; 5.山西大医院 眼科,山西 太原030032; 6.山西大医院 泌尿外科,山西 太原 030032)
1 Introduction
At present, carbon nanomaterials with a high stability and good biocompatibility have had extensive application prospects in biomedical fields including biosensors, drug and vaccine delivery system, composite bone materials, tumor targeted therapy and novel biological materials[1-2]. Carbon nanotubes (CNTs) have become a hotspot of study in drug carrier because of their hollow structure and large specific surface area allowing the accommodation of specific biomolecules and drug molecules, and excellent cell penetrability[3-4]. However, they produce a strong immunogenicity in the body because of their insolubility in physiological buffer, causing great limitations in the application of CNTs to a large extent[5].
Polyethylene glycol (PEG) with a superior biocompatibility was used to modify CNTs to prepare water-soluble CNTs (PEG-CNTs), which can improve the biocompatibility, solubility and drug delivery of CNTs[6-8]. As a drug carrier, some characteristics, including non-toxic, non-antigenic and excretion ability, are necessary besides good biocompatibility. Our previous study[9]shows that the functionalized CNTs introduced into living rabbit were mainly distributed in lung, heart, liver, spleen and kidney, and they had not deposited in the body because they were gradually excreted through urinary system with the passing of time. But the study also confirms that CNTs had a lightly longer retention time in liver and kidney than in other organs.
Based on this phenomenon, this paper aims at evaluating the toxicity of PEG-CNTs in tumor cells and normal cells in order to verify that PEG-CNTs can be used as a drug carrier to inhibit tumor cells without the damage of PEG-CNTs themselves towards tumor cells. Therefore, an in vitro experiment was conducted, where hepatoma cells (HepG2) were chosen as the experimental groups and normal cells of human embryonic kidney cells (293T) as the control groups. Functionalized PEG-CNTs with different concentrations were introduced into the two classes of cells. The relative survival rates of 293T cells and HepG2cells were measured with a water-soluble tetrazolium salt (MTT assay), which can indirectly reflect the influence on the cell survival rate. The actual survival of cells was measured by a flow cytometer (FCM) after 24 h of continuous exposure. Finally, the in vitro cytotoxicity of PEG-CNTs in 293T cells and HepG2cells was studied. This research article gives a strong support for CNTs as a drug carrier in biomedical field.
2 Experimental
2.1 Materials
CNTs, with an inner diameter of 8-15 nm, length of 0.5-2 μm, specific surface area of 233 m2/g and purity of 95 wt%, were purchased from Chengdu Organic Chemistry Co. Ltd., Chinese Academy of Sciences. Human embryonic kidney cells (293T) were purchased from Shanxi Medical University Parasites Laboratory. Human liver cancer cells (HepG2) were purchased from the Cell Center of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College. DMEM/High Glucose (1×) medium was purchased from Thermo Fisher Scientific Chemical Co., Ltd. Fetal bovine serum (FBS) was purchased from Hangzhou Sijiqing Co. Trypsin-EDTA digestion solution, green streptomycin mixture (100×), polyethylene glycol 800, thionyl chloride, chloroform, dimethyl formamide (DMF) and dimethyl sulfoxide (DMSO) were purchased from Beijing Solarbio Technology Co., Ltd. (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) was purchased from Sigma, USA. Propidium iodide (PI) staining kit (containing 10×BufferA) was purchased from KGI. Phosphate buffer (PBS, 1×) was prepared in laboratory.
2.2 CNTs modified by PEG
First, carboxyl functional groups were introduced onto CNTs by a mixed acid treatment. CNTs (0.2 g) were added to 2.5 mol/L concentrated HNO3under sonification for 24 h, then reacted in the mixed acid consisting of concentrated HNO3and H2SO4(v/v=3∶1) for 4 h. After the reaction, black powder deposited in the lower layer after standing. The supernatant was removed, and the black powder were thoroughly rinsed with deionized water, filtered and dried under vacuum for 8 h to obtain CNTs containing-COOH, which is denoted as CNTs-COOH.
Then, the surface of CNTs-COOH was grafted with hydrophilic groups of polyethylene glycol with the following methods. CNTs-COOH (200 mg) was added to a 50 mL round bottom flask, and then a mixture of sulfoxide chloride (15 mL) and 3 drops of DMF was added. The reaction system was heated at 60 ℃ with reflux for 10 h. After the completion of reaction, the system was cooled to room temperature, and the mixtures were filtered by a 0.48 μm filter membrane, followed by removal of sulfoxide chloride residues through chloroform washing, and drying under vacuum at room temperature to obtain acyl chlorinated CNTs. The mixture of 15 mL of chloroform, 0.1 mL of polyethylene glycol 800 and 3 drops of DMF were added to the acyl chlorinated CNTs in a 50 mL round bottom flask, followed by heating and reflux at 50 ℃ for 10 h. After the completion of the reaction, the reaction system was cooled to room temperature, and then the mixture was filtered by a filter membrane, washed with chloroform and dried under vacuum at room temperature to obtain PEG-CNTs.
2.3 In vitro cytotoxicity of PEG-CNTs against 293T cells and HepG2 cells
MTT assay was performed to determine the relative cell count and cell viability of 293T cells and HepG2cells against different concentrations of PEG-CNTs, which can indirectly reflect the influence of PEG-CNTs on the cell survival rate. In addition, the actual survival of cells was measured by a FCM detection instrument after continuous exposure to PEG-CNTs. The in vitro cytotoxicity of PEG-CNTs against 293T cells and HepG2cells was explored.
2.3.1 Preparation of PEG-CNTs solutions and reagent
PEG-CNTs (0.01 g) were dispersed into 10 mL of PBS to prepare 1 mg/mL stock solution, which was autoclaved at 121 ℃ for 30 min and then sealed. The solution was ultrasonically dispersed for 30 min before using. The experiment was divided into an experimental group and a control group. The solution was treated to obtain 6.25, 12.5, 25, 50, 100 and 200 μg/mL PEG-CNTs solutions with DMEM/High Glucose (1×) medium.
The concentration of MTT was 5 mg/mL in MTT method. MTT (0.5 g) was dissolved in 100 mL of PBS, filtered with a 0.22 μm filter membrane to remove the bacteria, and then placed at 4 ℃ under the dark condition (keeping out of the sun in the process of preparation and preservation).
Preparation of 1× Buffer A: 10×Buffer A was diluted 10 folds with double distilled water before using.
2.3.2 Cell culture
293T cells and HepG2cells were grown in a DMEM/High Glucose (1×) medium with a 10% (volume) fetal bovine serum inactivated at 56 ℃, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 ℃ in 5% CO2atmosphere. The cells were digested using trypsin-EDTA every 3 days according to the growth condition, until they reached the logarithmic growth phase.
2.3.3 Cytotoxicity detection
(1) The relative survival rate of cells determined by MTT assay
MTT colorimetric method is adopted to detect the cell survival and growth, of which the detection principle is that the succinate dehydrogenase in living cell mitochondrias can reduce exogenous MTT to water-insoluble blue-violet crystal forma (Formazan) depositing in the living cells, but no such reaction occurs in dead cells. DMSO can dissolve formazan in cells, and the absorbance value of formazan can be measured at 490 nm wavelength by an enzyme-linked immunometric meter to indirectly reflect the number of living cells. In a certain number range of cells, the crystal number formed by MTT assay is in proportion to the number of cells, which is one of the most commonly used method of cytotoxicity detection. With the absorbance value the cell, survival rate can also be calculated, so as to reflect the cytotoxicity of the PEG-CNTs solutions to cells. However, MTT assay can only detect the relative number of cells or relative vitality, not the absolute vitality of cells[10].
In present study, both 293T cells and HepG2cells were exposed with PEG-CNTs at concentrations of 6.25, 12.5, 25, 50, 100 and 200 μg/mL. The absorbance values of the experimental group were compared with those of the control group at 24, 48 and 72 h, separately. The absorbance ratio of the experimental group to control group was used to calculate the survival rate, and to draw a conclusion by statistical method.
The detailed detection procedure is as follows. The density of 293T cells and HepG2cells in the logarithmic phase was adjusted to 1×105cells/mL. Then the cells were beat into single cell suspension and carried onto a 96-well flat bottom plate (200 μL/well). The plates were incubated at 37 ℃ in 5% CO2in a humidified incubator for 24 h. The nutrient media was then removed until cells monolayer wall covered the bottom of the hole. The cells of experimental groups were exposed with different concentrations of PEG-CNTs solutions. By contrast, the cells of the control groups were grown in the same volume of DMEM/High Glucose (1×) medium. The plates were incubated at 37 ℃ in 5% CO2in a humidified incubator for 24, 48 and 72 h. Each group had five holes. The cells were observed under an inverted microscope. Aliquots of 20 μL of freshly prepared MTT solution (5 mg/mL, 0.5% MTT) were added to each well. After incubation for 4 h, the nutrient media was removed, and 150 μL of DMSO was added to each well and shaken at a low speed for 10 min on a shaker in order to dissolve the Formazan crystals sufficiently. Absorbance (A value) was read at a primary wavelength of 450 nm and a reference wavelength of 630 nm by a microplate reader.
Cell relative survival rate (RSR) and relative growth rate (RGR) were calculated according to the following equations:
RSR = {absorbance value(experimental group)/ absorbance value(control group)} × 100%[11]
RGR= {average absorbance value(experimental group)/ average absorbance value(control group)} × 100%.
(2) Cell viability measured by FCM
The fluorescent staining is the premise of identifying apoptotic and living cells by FCM. As-used PI can only stain apoptotic cells, not normal cells. Thus, the living and apoptotic cells can be differentiated by confocal microscopy and FCM to further calculate the ratio of apoptotic or living cell in total cells[12].
HepG2in logarithmic growth phase were diluted to a single cell suspension with a density of 1×106/mL and seeded in 6 well plates (2 mL each well). After cell adherence overnight, the as-prepared PEG-CNT solutions with gradient concentrations and DMEM as control were separately added to plate for incubation for 24 h at 37 ℃ in 5% CO2atmosphere. The cell was digested by a trypsin-EDTA solution and collected in labelled centrifuge. After washed once by 1×Buffer A (centrifuge for 5 min at 2 000 rpm/min), cells were resuspended in a moderate 1×Buffer A and tuned to a concentration of 1×106/mL. After that, 5 μL of PI was added into 95 μL of cell suspension for cell staining with gent swirling, followed by sealing onto glass slide for 15 min at room temperature in the dark. Then, they were sealed with cover side. Finally, the images of the treated cells were observed by using a laser confocal scanning microscope, and further the cell survival rate was obtained by FCM.
2.3.4 Evaluation criterion
According to ISO2109932-5 cytotoxicity standard, the cell toxicity is divided into five levels[13].The RGR value of above 100%, between 75% and 99%, between 50% and 74%, between 25% and 49%, and between 1% and 24%, corresponds a toxicity of grade 0, grade I, grade II, grade III, grade IV and grade V, respectively. The grade 0 and I are considered as no toxicity, grade II as a slight toxicity, grade III and IV as a moderate toxicity, and grade IV as an obvious toxicity.
2.4 Statistical analysis
All the datas in the study were analyzed by the statistic package SPSS 16.0. Datas were expressed as mean value ± standard deviation (SD). Multiple comparisons between two groups were conducted by one-way analysis of variance combining with the SNK method. The results were considered to be significant at p<0.05.
3 Results and discussion
3.1 Water-soluble CNTs
PEG, a hydrophilic linear polymer, was used to improve the biocompatibility of CNTs. The morphology of CNTs was characterized by FESEM and TEM (Fig. 1a).

Fig. 1 (a) FESEM and TEM images of CNTs; (b) Photographs of 1 000 μg/mL of original CNTs (left) and PEG-CNTs dispersed in PBS solution (pH=7.4) with concentrations of 1 000 μg/mL (middle) and 2 000 μg/mL (right) for 72 h.
It can be seen that original CNTs have a coiling shape with diameters of 30-60 nm and length of several micron. To investigate the effect of grafting PEG on the dispersibility of CNTs in biological environment, different concentrations of PEG-CNTs (1 000 and 2 000 μg/mL) and original CNTs (1 000 μg/mL) were separately dispersed in the PBS buffer solution (pH=7.4) and held for standing for 72 h. It is observed that the original CNTs aggregate and precipitate in the bottom of container (Fig.1b, left), while the dispersion of PEG-CNTs remain stable (Fig. 1b, middle and right). These demonstrate that PEG-CNTs show an excellent solubility in the physiological solution after CNTs are modified by PEG.
3.2 Cytotoxicity assay of PEG-CNTs on 293T
3.2.1 Cell morphological change
Fig. 2 shows the fluorescence microscopic images of 293T incubated by PEG-CNTs with different concentrations for 24 h. It is can be seen from Fig. 2a that the fusiform cells in the control group grow against wall in a monolayer and mosaic array with a close intercellular space among cells. As shown in Fig. 2b, when the concentration of PEG-CNTs is 6.25 μg/mL, cell morphology is the same as that of the control group. At higher concentrations, PEG-CNTs show aggregate in black precipitate with point or sheet-like distributions (Fig. 2c). With further increasing the concentration of PEG-CNTs to 25.0 μg/mL, cell morphologies have no obvious changes (Fig. 2d). As the concentration of PEG-CNTs increases to 50 μg/mL, the tentacles of some cells become short and cells are in slightly round without an obvious apoptosis, as shown in Fig. 2e. In Fig. 2f and (g), when the concentration of PEG-CNTs is greater than 100 μg/mL, there are more suspended necrotic cells in the culture medium. The lysis, disruption and necrosis of cells are found with an increased intercellular space and loose array, as well as abnormal morphology in the remaining living cells. At the highest concentration of 200 μg/mL, cells show a large necrosis area with an indecipherable morphology and the number of suspended necrosis cells evidently increases.

Fig. 2 Fluorescence microscopic images of 293T incubated with various concentrations of PEG-CNTs for 24 h.
3.2.2 The cell survival rate
293T cells were cultured by PEG-CNTs with different concentrations for 24, 48 and 72 h. The absorbance, cell RSR and RGR and toxicity were measured using the MTT method, as shown in Table 1A, 1B and 1C. The results show that PEG-CNT treatments cause a concentration-dependent death rate of 293T cells. It can be seen that 293T cell RSR decreases as the concentration of PEG-CNTs increases when the concentration of PEG-CNTs is above 12.5 μg/mL. Compared with the control group, the groups with 50, 100 and 200 μg/mL PEG-CNTs are considered to be statistically significant (P values<0.05) in differences in RSR values, while the groups with 6.25, 12.5 and 25 μg/mL PEG-CNTs show no significant difference. Compared with those of the cells contaminated for 24 h, the measured RSR results of the cell contaminated for 48 and 72 h show no significant difference, indicating no further development in cytotoxicity. According to ISO2109932-5 cytotoxicity standard, after incubation for 24 h, the toxicity of all groups (except the control group) is Grade I at the concentration of ≤100 μg/mL and Grade II at the concentration of 200 μg/mL. After incubation for 48 and 72 h, the cell toxicity hardly changes.

Table 1A The effects of the concentration of PEG-CNTs on 293T survival rate for 24 h (n=5, x±s).

Table 1B The effects of the concentration of PEG-CNTs on 293T survival rate for 48 h (n=5, x±s).

Table 1C The effects of the concentration of PEG-CNTs on 293T survival rate for 72 h (n=5, x±s).
Note: *Compared with the control group (PEG-CNTs 0 μg/mL) P<0.05. RSR stands for the cell relative survival rate and RGR stands for the cell relative growth rate
3.3 Cytotoxicity of PEG-CNTs against HepG2
3.3.1 The morphological change of HepG2
As shown in Fig. 3a, HepG2has different shapes, among which the typical HepG2grows in spindle and polygon adhering to the wall. When HepG2is cultivated with PEG-CNTs (<100 μg/mL) for 24 h, HepG2presents similar aggregation and precipitation as 293T. When the concentration of PEG-CNTs is >100 μg/mL (Fig. 3g and f), HepG2shows a large necrosis area, in which a large number of necrosis cells aggregate and suspend in the cell culture medium. The morphology of living cells in the bottom has lost typical morphology of HepG2.
3.3.2 The effect of PEG-CNTs on HepG2cells survival rate
(1) MTT measurement
After HepG2cells were cultured for 24, 48, and 72 h with different concentrations of PEG-CNTs, the absorbance, cell RSR and RGR and toxicity were measured using the MTT method, as shown in Table 2A, 2B and 2C. The results show that the RSR of HepG2depends on the concentration of PEG-CNTs, in the similar way as 293T. HepG2cell RSR decreases as the concentration of PEG-CNTs increases when the concentration of PEG-CNTs is above 25 μg/mL. Compared with the control group, the groups with 50, 100 and 200 μg/mL PEG-CNTs are considered to be statistically significant (P values<0.05) in differences in the survival rate, while the groups with 6.25, 12.5 and 25 μg/mL PEG-CNTs show no obvious significant differences. The cytotoxicity of all groups (except the control group) belongs to Grade I at the concentrations of PEG-CNTs ≤100 μg/mL and Grade II at the concentration of 200 μg/mL. Similar with 293T, HepG2cells show no obvious change in cytotoxicity with prolonging time.
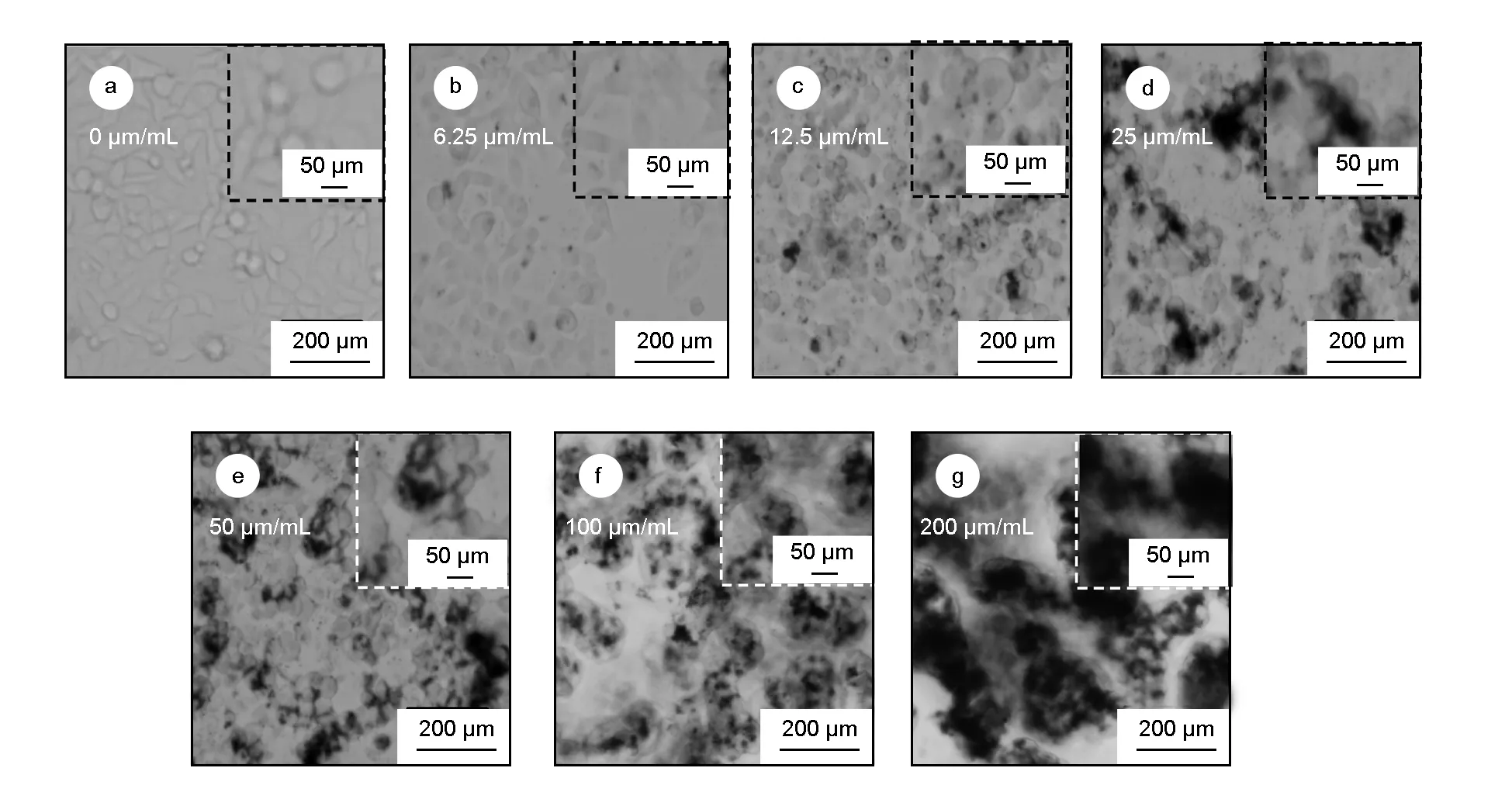
Fig. 3 Morphological changes of HepG2 interacted with PEG-CNTs of different concentrations for 24 h.

Table 2A The effects of the concentration of PEG-CNTs on HepG2 survival rate for 24 h (n=5,
Table 2B The effects of the concentration of PEG-CNTs on HepG2 survival rate for 48 h (n=5,x±s).
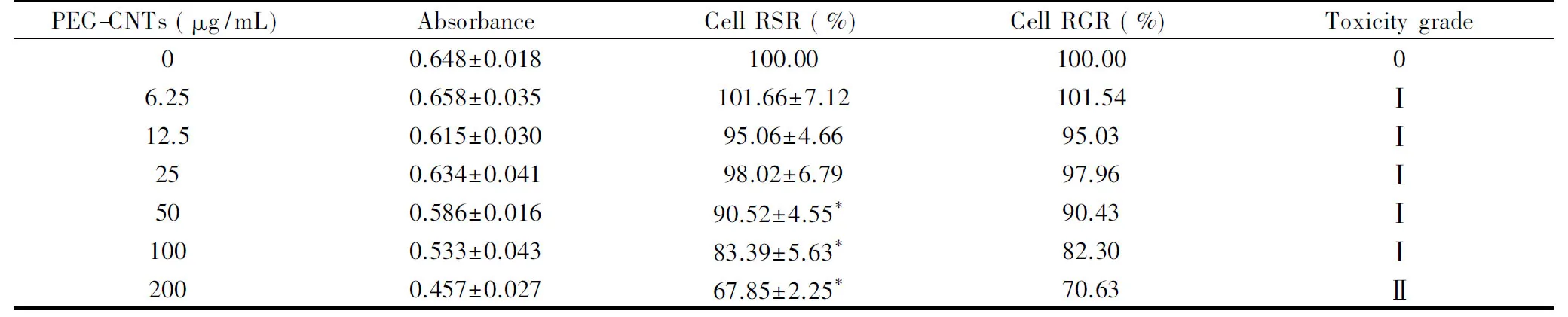
Table 2B The effects of the concentration of PEG-CNTs on HepG2 survival rate for 48 h (n=5,x±s).

Table 2C The effects of the concentration of PEG-CNTs on HepG2 survival rate for 72 h (n=5,
Note: *Compared with the control group (PEG-CNTs 0 μg/mL) P<0.05. RSR stands for the cell relative survival rate and RGR stands for the cell relative growth rate
(2) FCM detection
After treated with PEG-CNTs for 24 h, the morphology of HepG2cells stained with PI were observed by confocal microscopy. Fig. 4 presents the cell morphology of HepG2treated with diverse concentrations (0, 12.5, 100 and 200 μg/mL) of PEG-CNTs for 24 h. It can be seen that normal cells are not stained, while the apoptotic cells exhibit an intense red fluorescence. With the treatment of 12.5 μg/mL PEG-CNTs (Fig. 4b), there are few apoptotic cells in visual field, and no obvious difference exists from the control group without the PEG-CNT treatment (Fig. 4a). When the concentration of PEG-CNTs increases to 100 μg/mL (Fig. 4c), the number of apoptotic cells increases. At a higher concentration of 200 μg/mL, the apoptotic cells have a noticeable increase in number, and some cells swell obviously and lose original shapes, which is attributed to the strong toxicity at a high concentration of PEG-CNTs. The detained cell survival rate was measured by FCM, as shown in Table 3.
FCM test was conducted to measure the cell survival rate of HepG2, as shown in Fig.5, where B1 represents the proportion of apoptotic cells and B3 that of living cells. It can be seen from Fig.5 that when the concentration of PEG-CNTs is 6.25, 12.5, 25 and 50 μg/mL, the apoptotic HepG2cells account for 12.4%, 12.1%, 13.1% and 15.2%, and the living cells account for 86.1%, 83.8%, 86.1% and 82.7%, respectively. There is an obvious difference in comparison with the control group, in which the apoptotic cells account for 10.7% and the living cells account for 88.1%. The results reveal a low cytotoxicity of PEG-CNTs at low concentrations. While the concentration of PEG-CNTs further increases to 100 and 200 μg/mL, the groups display an enhanced rate of apoptosis (25.4% and 37.6%) and a low rate of living cells (72.3% and 61.9%), showing a toxic effect.
Different from the MTT method, where only the cell relative survival rate is obtained[10], FCM test can give the rate of actual living cells in every group. The results from FCM test show that compared with the control group, only the groups with 100 and 200 μg/mL PEG-CNT treatment give a statistically significantly different survival rate (P values<0.05), while there is no statistically significant difference in survival rate for other groups (shown as Table 3).

Fig. 4 Confocal microscopic images of apoptotic cells induced by different concentrations of CNTs-PEG after PI staining.

Table 3 Survival rate of HepG2 cells after interacted with PEG-CNTs for 24 h measured by flow cytometry (n=5, x±s).
Note: *Compared with the control group (PEG-CNTs 0 μg/mL) P<0.05

Fig. 5 Survival rates of HepG2 cells after interacted with PEG-CNTs and PI staining measured by FCM: the B1 quadrant is the proportion of dead cells, and the B3 quadrant is the proportion of viable cells.
3.4 Analysis of cytotoxicity test results of PEG-CNTs
Currently, the impact of materials on cell viability is an indirect expression to evaluate the cytotoxicity of materials towards cells. Cell viability testing methods mainly include PI staining combined with FCM[14], MTT staining combined with UV absorption[15], Trypan blue staining combined with the directly counting method[16], and Bradford method detecting protein concentration for indicating cell viability indirectly[17]. Trypan blue staining combined with the directly counting method has a low precision and Bradford method is complex in testing process, so FCM after PI staining and the MTT method were adopted for rapid, simple and accurate analyses of the effect of PEG-CNTs on the cell survival rate.
Although the MTT method has many advantages such as a high sensitivity and economy, it also has some drawbacks. First, formazan from MTT by mitochondrial reduction needs to be dissolved in organic solvents because it is insoluble in water, which is not only tedious, but also affects the accuracy of results as a result of more operations. DMSO, a commonly used organic reagent, is toxic and harmful to operator’s health. Second, CNTs have a large aspect ratio, large surface area and high chemical activity. Hurt et al. reported that carbon nanomaterials including CNTs can easily realize nonspecific adsorption towards diverse dyes, fluorescent molecules and fluorescent reagents because of their high surface area. Therefore, CNTs are easy to interact with dye molecules, which makes the result deviate from true values[18].
Cytotoxicity analyses of PEG-CNTs by the MTT assay have shown that concentration is the key factor affecting the cytotoxicity of PEG-CNTs, that is, the cytotoxicity increases with increasing concentration. However, the toxicity has no significant change after contaminating for 24 h, which indicates that the cytotoxicity does not increase with a further extension of time. The results are similar as those reported by Liu et al.[19]. They argued that cells have their own regulatory mechanism towards the toxicity of nanomaterials, by which they can resist or reduce the cytotoxicity caused by CNTs after sustained action. In our experiments, we observed that when the concentration exceeds 50 μg/mL, the tangling agglomeration of CNTs is more obvious in the groups with larger concentrations (Fig. 2(c-g)), suggesting that as-generated formazan crystals are hard to be dissolved by DMSO because they are easily entangled with each other by CNTs, resulting in an inaccurate (probably reduced) absorbance value and consequently affecting the estimation (probably underestimation) of the survival rate. Therefore, it is concluded that the aggregation of CNTs themselves has an effect on the cells, which would have a negative impact on experimental results. Sato et al.[20]found that the multi-walled CNTs with an average length of 825 nm were more likely to cause inflammation than the 220 nm-long multi-walled CNTs, after comparing the biological effects of multi-walled CNTs with different lengths. Wick et al[21]found that the CNTs with larger aggregation degrees had greater hardness and volume, leading to more toxicity on cells. Furthermore, the CNTs with large aggregation degrees easily adsorbed dyes and crystals, which affected the absorbance test.
FCM was used to further verify above results. PI, a DNA-tuberculous dye with an excitation at 536 nm and emission at 617 nm, can emit a red fluorescence, and only stain dead cells but not living cells because it cannot penetrate the cell membrane. Therefore, under fluorescence microscope, the dead cells exhibit a strong red fluorescence (Fig.4)[22]. After staining, contaminated cell suspension can be used to detect the survival rate by FCM. This method is fast and simple, but the only disadvantage is the possible adsorption of the dye by PEG-CNTs. Therefore, the cells were washed to remove the free and aggregated PEG-CNTs as far as possible in the experiment to further minimize the effect on the experimental results. The results show that there is no statistical difference between the control group and the experiment group at 50 μg/mL of PEG-CNTs, while there are differences in the MTT assay. The experimental results with 100 and 200 μg/mL of PEG-CNTs are consistent with those obtained from the MTT method, and this is due to the fact that the aggregated PEG-CNTs can absorb formazan to decrease absorbance, and 50 μg/mL might be the critical concentration point of the difference, which results in the inconsistency of two measurement methods. Therefore, it is inferred that the critical concentration for PEG-CNTs to exhibit the toxic reaction should be between 50 and 100 μg/mL. It can be seen that the experimental results in the MTT assay are indeed affected by aggregation of CNTs. Therefore, in order to use PEG-CNTs as a carrier in medicine, functionalized carbon nanotubes with a less toxicity, good water-solubility and low aggregation still need to be further explored.
4 Conclusions
For investigating the in vitro cytotoxicity of water-soluble PEG-CNTs on 293T cells and HepG2cells, MTT and FCM were used to detect the cell survival rate.
The results of MTT show that the cell survival rate of 293T and HepG2cells decreased gradually with increasing the concentration of PEG-CNTs after incubation with water-soluble solutions of CNTs for 24 h in vitro. Compared with the control group, the groups of 50, 100 and 200 μg/mL PEG-CNTs had a statistical difference in the survival rates (P <0.05). These groups (except the control group) showed a toxicity Grade I at the concentrations of ≤ 100 μg/mL, which was considered as no cytotoxicity. The group with 200 μg/mL of PEG-CNTs showed a toxicity Grade II with a mild toxicity. The toxicity did not further change after exposure for 48 and 72 h. The above-mentioned MTT measurement results indicate that the concentration of PEG-CNTs is the main factor affecting the cytotoxicity of PEG-CNTs. The cytotoxicity increases with increasing the concentration, and the toxicity is very low when the concentration of PEG-CNTs is low. At the same time, the toxicity did not increase with time after exposure for 24 h, indicating that the cytotoxicity flattened with the prolongation of time.
PI staining tests show that the dead cells presented a strong red fluorescence while the normal cells were not stained under the confocal microscope. The number of apoptotic cells increases with increasing the concentration of PEG-CNTs. FCM tests indicate that the difference in the cell survival rate between the groups 100 and 200 μg/mL and the control group was statistically significant (P<0.05), and there was no significant difference for other groups. Therefore, we speculate that when at low concentrations, PEG-CNTs is non-toxic to cells.
[1] Li Z X, de Barros A L B, Soares D C F, et al. Functionalized single-walled carbon nanotubes: Cellular uptake, biodistribution and applications in drug delivery[J]. International Journal of Pharmaceutics, 2017, 524(1): 41-54.
[2] Yang K, Feng L Z, Liu Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy[J]. Advanced Drug Delivery Reviews, 2016, 105: 228-241.
[3] Dineshkumar B, Krishnakumar K, Bhatt A R, et al. Single-walled and multi-walled carbon nanotubes based drug delivery system: Cancer therapy: A review[J]. Indian Journal of Cancer, 2015, 52(3): 262-273.
[4] Faraj Al A, Shaik A P, Shaik A S. Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: Noninvasive monitoring using diffusion-weighted magnetic resonance imaging as sensitive imaging biomarker[J]. International Journal of Nanomedicine, 2015, 10: 157-161.
[5] Liu J J, Wang C, Wang X J, et al. Mesoporous silica coated single-walled carbon nanotubes as a multifunctional light-responsive platform for cancer combination therapy[J]. Advanced Functional Materials, 2015, 25(3): 384-392.
[6] Zhang W L, He J L, Liu Z, et al. Biocompatible and pH-responsive triblock copolymer mPEG-b-PCL-b-PDMAEMA: Synthesis, self-assembly, and application[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2010, 48(5): 1079-1091.
[7] Das M, Bandyopadhyay D, Singh R P, et al. Orthogonal bio-functionalization of magnetic nano-particles via “clickable”poly (ethylene glycol) silanes: A “universal ligand”strategy to design stealth and target-specific nano-carriers[J]. Materials Chemistry, 2012, 22(47): 24652-24667.
[8] Moghimi S M, Hunter A C, Murray J C. Long-circulating and target-specific nanoparticles: Theory to practice[J]. Pharmacol Review, 2001, 53(2): 283-318.
[9] Yu S P, Yuan W, Gao Y D, et al. The distribution of intravenously administered functionalized carbon nanotubes in rabbit tissue and their urinary excretion[J]. New Carbon Materials, 2012, 27(6): 421-426.
[10] Almutary A, Sanderson B J S. The MTT and crystal violet assays: Potential confounders in nanoparticle toxicity testing[J]. International Journal of Toxicology, 2016, 35(4): 454-458.
[11] E Z. Tissue Culture and Molecular Cytology[M]. Beijing: Beijing Press, 1997: 108-121.
[12] Niu Y Y, Li S S, Lin Z T, et al. Development of propidium iodide as a fluorescence probe for the on-line screening of non-specific DNA-intercalators in fufang banbianlian injection[J]. Journal of Chromatography A, 2016, 1463: 102-109.
[13] Hao H P. Standard Guide for Biological Evaluation of Medical Devices[M]. Beijing: Standards Press of China, 2002: 100-110.
[14] Nescerecka A, Hammes F, Juhna T. A pipeline for developing and testing staining protocols for flow cytometry, demonstrated with SYBR Green I and propidium iodide viability staining[J]. Journal of Microbiological Methods, 2016, 131: 172-179.
[15] Rezaei A, Noori L, Taghipour M. The Use of ANFIS and RBF to model and predict the inhibitory concentration values determined by MTT assay on cancer cell lines[J]. International Journal of Information Technology and Computer Science (IJITCS), 2016, 8(4): 28-33.
[16] Bottini M, Bruckner S, Nika K, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis[J]. Toxicol Letter, 2006, 160: 121-126.
[17] Carlsson N, Borde A, Wölfel S, et al. Quantification of protein concentration by the bradford method in the presence of pharmaceutical polymers[J]. Analytical Biochemistry, 2011, 411(1): 116-121.
[18] Hurt R H, Monthioux M, Kane A. Toxicology of carbon nanomaterials: Status, trends, and perspectives on the special issue[J]. Carbon, 2006, 44: 1028-1033.
[19] Liu B Z, Zhou B, Wang H Y, et al. Effect of functionalized multi-walled carbon nanotubes on L02 cells[J]. Chinese Medical Sciences Journal, 2010, 32(4): 455-460.
[20] Sato Y, Yokoyama A, Shibata K, et al. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo[J]. Molecular Biosystems, 2005, 1: 176-182.
[21] Wick P, Manser P, Limbach L K, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity[J]. Toxicology Letters, 2007, 168(2): 121-131.
[22] Crowley L C, Scott A P, Marfell B J, et al. Measuring cell death by propidium iodide uptake and flow cytometry[J]. Cold Spring Harbor Protocols, 2016(7): 87-163.
