Post-irradiation pericardial malignant mesothelioma with deletion of p16: a case report
2018-03-08YaldaNaeiniRamirArcegaSharonHirschowitzNageshRaoHaodongXu
Yalda B. Naeini, Ramir Arcega, Sharon Hirschowitz, Nagesh Rao, Haodong Xu
Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1732, USA
Introduction
Malignant mesothelioma is a rather uncommon malignancy.The pathogenesis in most cases is chiefly related to asbestos,but other potential causes of mesothelioma, although very rare, include radiation, organic chemicals, viruses, and chronic inflammation1. Numerous reports have demonstrated the development of malignant mesothelioma in organs close to areas of delivery of ionizing radiation therapy2. In addition, radiation has been reported to induce localized benign mesothelial proliferation3. Studies have reported an association between pleural malignant mesothelioma and chest radiation for lymphoma and have shown that mesothelioma with this etiology generally occurs in patients who are younger, more likely to have unusual histologic features, and have a longer overall survival compared to patients with asbestos-related mesothelioma4. Pericardial mesothelioma, on the other hand, bears a poor prognosis as a result of its late manifestation, challenging ante-mortem diagnosis, and limited treatment modalities5. Homozygous deletion of 9p21, the locus harboring the p16 gene, has been reported as the most common genetic alteration in malignant mesotheliomas. This genetic alteration is a useful marker to distinguish malignant mesothelial cells from benign reactive/hyperplastic cells6. Loss of p16/CDKN2A is associated with more aggressive clinical behavior in pleural mesothelioma and may have potential therapeutic applications7; however, this issue has not been studied in pericardial mesothelioma. Here we report the first case of pericardial mesothelioma showing homozygous deletion of 9p21.
Case report
Clinical course
We report the case of a 48-year-old man with no history of prior asbestos exposure who developed pericardial mesothelioma. The patient’s past medical history was significant for Hodgkin's lymphoma (HL) at age 16 (no pathology records available at UCLA for review), for which he received radiation therapy, lymph node excision, and splenectomy. He was in remission from HL when he presented to the UCLA cardiology unit with flu-like symptoms and progressive dyspnea. The patient was found to have a large pericardial effusion with signs of possible early tamponade. He underwent urgent bedside pericardiocentesis,with the fluid sent for cytologic examination. In addition,biopsy was performed of the mediastinal fat and pericardium and samples were sent to surgical pathology for histologic review. Preoperatively, the patient showed signs of multisystem organ failure including acute liver and renal failure, progressing to respiratory failure requiring mechanical ventilation. Upon consultation, the family decided on palliative comfort care. The patient expired eight days after the initial diagnosis.
Cytopathology findings
Pericardiocentesis was performed and a 20 mL blood-mixed fluid sample was sent to the cytology laboratory. The cytology smears were markedly cellular, composed of many tubulopapillary and complex clusters, as well as single atypical epithelioid cells. Few flat monolayered sheets demonstrated slit-like intercellular windows. The atypical cells showed round to ovoid, centrally located, atypical nuclei; dark chromatin with occasional small, single to multiple, conspicuous nucleoli; and a moderate amount of dense to delicate cytoplasm. Scattered foamy histiocytes,lymphocytes, and neutrophils were present in the background (Figure 1A). Immunocytochemical analysis was performed on cell blocks, which revealed nuclear positivity for calretinin (Figure 1B) and WT1 (Figure 1C). The atypical cells were negative for MOC31 (Figure 1D).
Histological, immunohistochemical, and fluorescence in situ hybridization (FISH)findings

Figure 1 Cytology and immunocytochemistry of pericardial epithelioid mesothelioma. (A) Diff-Quik shows clusters of atypical epithelioid cell proliferation (Diff-Quick staining, 40 x). Immunocytochemical stains show that the tumor cells are positive for calretinin (B) and WT1 (C)and they are negative for MOC31 (D). (B, C, D immunocytochemical staining, 20 x). WT1: Wilms tumor protein; MOC31: epithelial associated monoclonal antibody.
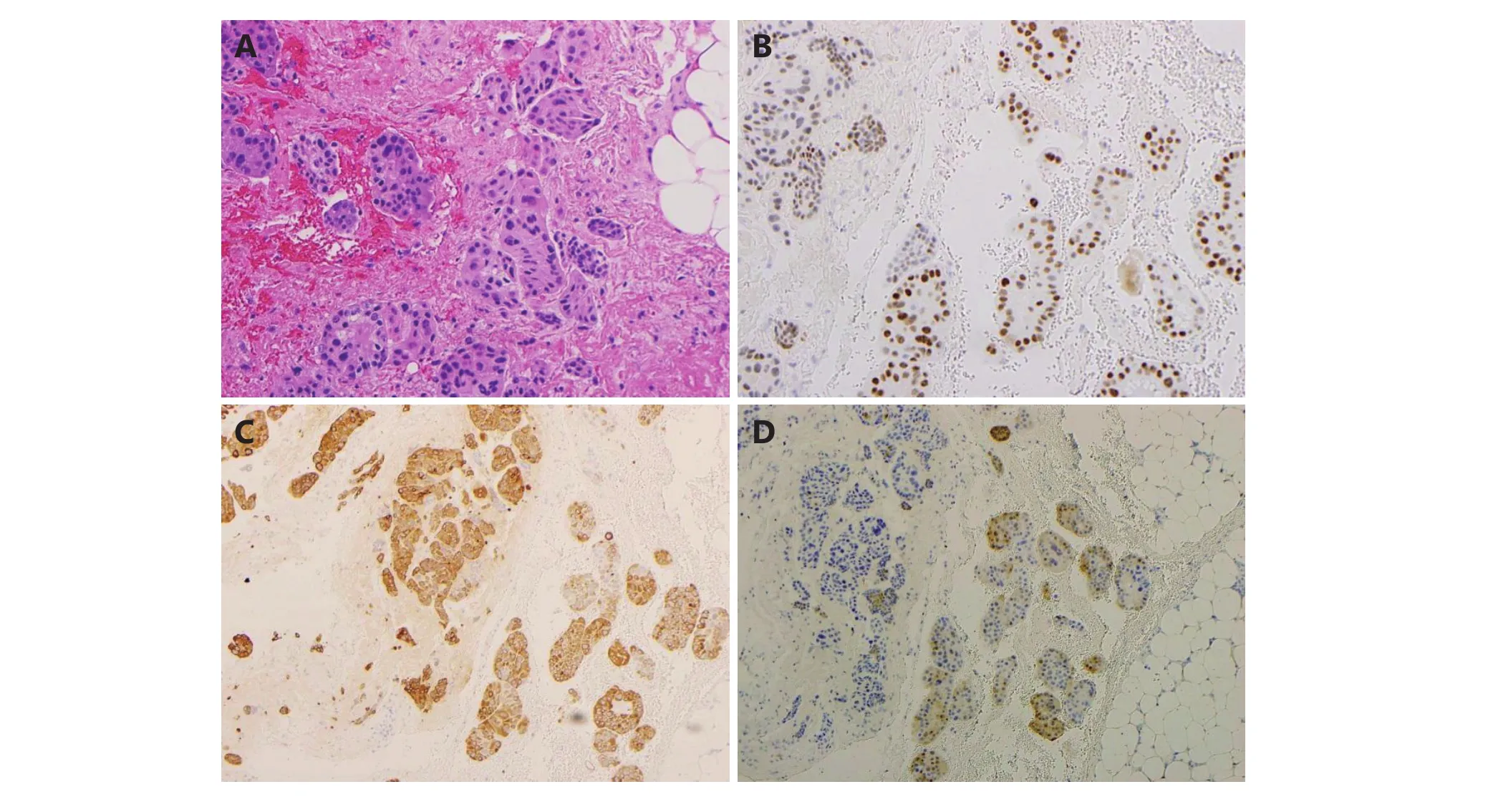
Figure 2 Pericardial epitheliod mesothelioma (IHC staining, 20 x). (A) The pericardial biopsy shows nested malignant epithelioid cells invading into fibroadipose tissue. Immunohistochemical stains show the malignant epitheliod cells are positive for WT1 (B), CK 5/6 (C) and calretinin (D). WT1: Wilms tumor protein; CK: cytokeratin.
Diagnosis of a malignant mesothelioma of the epithelioid type was made on the basis of histological and immunohistochemical examinations. Histologic examination of the excisional biopsy revealed malignant epithelioid cells arranged in small clusters to nests infiltrating the fibroadipose tissue. Morphologically, the malignant epithelioid cells displayed indistinct cellular borders, enlarged hyperchromatic nuclei with occasional small distinct nucleoli, and abundant eosinophilic cytoplasm (Figure 2A).The differential diagnosis included metastatic carcinoma and reactive mesothelial hyperplasia versus malignant mesothelioma; the immunohistochemical stains supported the mesothelial origin of the malignant cells, showing positivity for WT1, CK5/6, and calretinin (Figure 2B, 2C,and 2D), and negativity for BerEP4, B72.3, and MOC31 (not shown). Interphase FISH analysis with the fluorescently labeled dual colored probe set Vysis LSI CDKN2A spectrum orange probe (red signals) (Abbott Molecular Inc., Des Plaines, IL, USA) (~222kb; chromosome 9: 21, 802, 635-22,032, 985; includes MTAP, DMSMFH, CDKN2A, MTS1, P16,MLM, and CMM2 genes, respectively); and a control probe from the centromere of chromosome 9 labeled with spectrum green (green signals) demonstrated abnormal signal patterns with increased copies of chromosome 9 and homozygous loss of the 9p21 signals (Figure 3).Hybridization was performed according to the manufacturer’s protocols (Abbott Molecular Inc.). FISH analyses were performed on 300 nuclei exclusively from previously marked abnormal regions using adjacent hematoxylin and eosin-stained slides. Normal cells exhibit two red and two green signals consistent with two normal copies of chromosome 9. The presence of chromosome 9 polysomy (extra centromere signals) is suggestive of an underlying abnormal karyotype, often a marker of complex and heterogeneous chromosomal abnormalities, commonly described in the cytogenetics of multiple myeloma (MM).Molecular changes in mesothelioma with an impact on prognosis and treatment. The invasive nature of this neoplasm, in addition to p16 deletion, supported the diagnosis of malignant mesothelioma over mesothelial hyperplasia.
Discussion
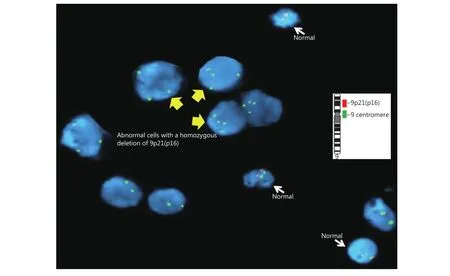
Figure 3 Homozygous deletion in the CDKN2A in pericardial malignant epithelioid mesothelioma. Interphase FISH analysis was performed with dual color chromosome 9 probe, centromere 9 (green)/9p21- CDKN2A (red) (inset), showing bi-allelic deletion (loss of red signals) of 9p21 in the tumor cells.
Malignant mesotheliomas are rare neoplasms associated primarily with asbestos exposure. However, these neoplasms may also arise as a second primary malignancy following radiation therapy, with a latency period of 15–25 years8.Ionizing radiation is a well-known risk factor for several different types of malignancies. Other contributing factors,however, may include genetic predisposition to cancer development and immunocompromised status following treatment of lymphoma, in addition to the carcinogenic nature of radiation and chemotherapeutic agents. The absence of simultaneous pleural disease, as seen in the present case, would argue against previous inhalation exposure to asbestos. Diagnosis of pericardial mesotheliomas can be very challenging, as these lesions show a wide range of histologic patterns; in particular, epithelioid mesotheliomas may closely mimic metastatic carcinomas. In addition,malignant mesothelioma must be distinguished from reactive mesothelial hyperplasia, which requires adequate biopsy material to demonstrate invasion of structures adjacent to the pleura. Therefore, Immunohistochemical stains and other ancillary studies play a pivotal role in the diagnosis of these lesions. Primary pericardial tumors are very uncommon,with a reported incidence of 0.0022% in a large autopsy series, showing a male predominance9. Here we present the first case of post-radiation pericardial mesothelioma with deletion of the p16 gene. Thomason et al.10presented data on 28 patients with primary pericardial mesothelioma including biphasic, epithelioid, and sarcomatoid variants, described in the literature from 1972 to 1992. In their case series, the effusion cytology revealed malignant cells in only 2 of 10(20%) cases. The authors also noted that in contrast to pleural mesothelioma, asbestos exposure as a causative factor is not definitive in pericardial mesothelioma, although it had been documented in a few of the patients in their series10.Post-irradiation mesotheliomas located in the pericardium have so far been demonstrated in only three published cases(Table 1)8,11,12.
Bendek et al.11reported a case of malignant pericardial mesothelioma of the epithelioid type in a 39-year-old man with a history of HL treated with radiation 24 years previously. Examination detected hemorrhagic pericardial fluid, which was followed by tamponade and circulatory arrest. The diagnosis was made at the time of autopsy11.
Velissaris et al.12reported a case of a 49-year-old woman who presented with persistent pericardial effusion following radiotherapy for HL. Histologic examination demonstrated mesothelioma, which had not been previously documented as a consequence of irradiation. Small et al.8reported a case of pericardial malignant epithelioid mesothelioma in a 62-year-old woman who presented with pericardial effusion.The patient had been treated with chemo- and radiotherapy for breast cancer approximately 14 years before the diagnosis of pericardial mesothelioma; however, in this case it was unclear whether the patient may have been exposed to asbestos through her spouse’s occupation. The authors also noted that experience from cancer centers suggests that the risk of developing pleural mesothelioma following radiotherapy for breast cancer is 0.3%.
What makes the present study unique is the significant finding of an abnormal signal pattern with a homozygous(biallelic) deletion of 9p21. This deletion is very common inmalignant pleural and peritoneal mesotheliomas, and results in the loss of p16/CDKN2A, its splice variant p14, p15/CDKN2B, and MTAP13. This genetic alteration is reported in up to 70% of primary epithelioid and 90%–100% of sarcomatoid pleural mesotheliomas14. Functionally, the p16 protein is a tumor suppressor that is important in cell cycle regulation, specifically controlling the G1 to S transition15.Destabilization of this regulatory mechanism via mutations or deletions in the p16 gene can be seen in a wide variety of tumors. A recent study suggested that CDKN2A may play a role in inherited predisposition to malignant mesothelioma and melanoma, leading to the rare familial cancer syndromes16. Detection of homozygous deletion of p16 by FISH is currently the most reliable way to distinguish benign/reactive from malignant mesothelial proliferations,and this deletion is a significant independent adverse prognostic factor in series of pleural and peritoneal cases;Walter et al.19reported that CDKN2A gene expression appeared to be a predictive marker for response to platinbased chemotherapy in patients in their study who received adjuvant treatment17-19.
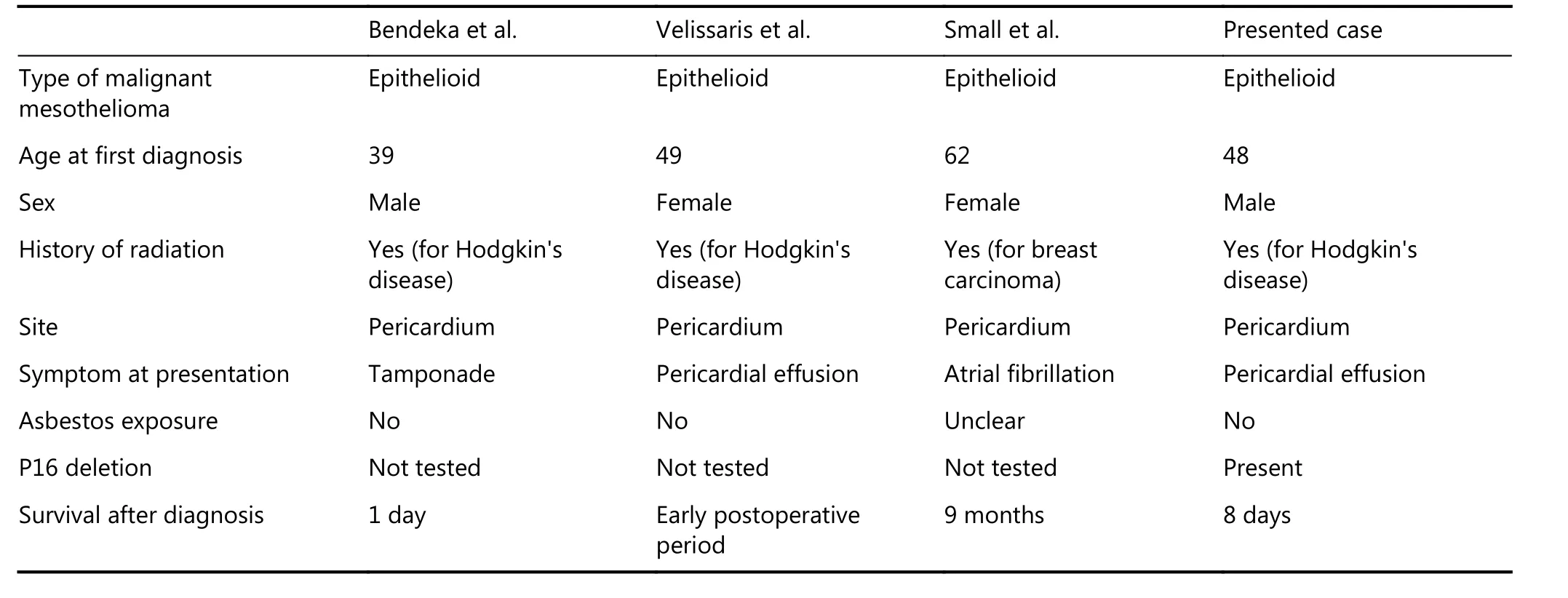
Table 1 Clinicopathologic findings of post irradiation pericardial malignant mesotheliomas
Conclusions
To our knowledge, only three cases of post-irradiation pericardial mesothelioma have been reported in the literature; however, this is the first case of radiation-induced mesothelioma showing p16 gene deletion. This finding may have potential therapeutic application as clinical trials remain important for patients with pericardial mesothelioma, for whom treatment options are extremely limited.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Rizzardi C, Barresi E, Brollo A, Cassetti P, Schneider M, Melato M.Primary pericardial mesothelioma in an asbestos-exposed patient with previous heart surgery. Anticancer Res. 2010; 30: 1323-5.
2.Witherby SM, Butnor KJ, Grunberg SM. Malignant mesothelioma following thoracic radiotherapy for lung cancer. Lung Cancer.2007; 57: 410-3.
3.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989; 13: 640-58.
4.Chirieac LR, Barletta JA, Yeap BY, Richards WG, Tilleman T,Bueno R, et al. Clinicopathologic characteristics of malignant mesotheliomas arising in patients with a history of radiation for Hodgkin and non-Hodgkin lymphoma. J Clin Oncol. 2013; 31:4544-9.
5.Ashinuma H, Shingyoji M, Yoshida Y, Itakura M, Ishibashi F,Tamura H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in a patient with pericardial mesothelioma. Intern Med. 2015; 54: 43-8.
6.Chiosea S, Krasinskas A, Cagle PT, Mitchell KA, Zander DS, Dacic S. Diagnostic importance of 9p21 homozygous deletion in malignant mesotheliomas. Mod Pathol. 2008; 21: 742-7.
7.Ladanyi M. Implications of P16/CDKN2A deletion in pleural mesotheliomas. Lung Cancer. 2005; 49: S95-8.
8.Small GR, Nicolson M, Buchan K, Broadhurst P. Pericardial malignant mesothelioma: a latent complication of radiotherapy?Eur J Cardiothorac Surg. 2008; 33: 745-7.
9.Lagrotteria DD, Tsang B, Elavathil LJ, Tomlinson CW. A case of primary malignant pericardial mesothelioma. Can J Cardiol. 2005;21: 185-7.
10.Thomason R, Schlegel W, Lucca M, Cummings S, Lee S. Primary malignant mesothelioma of the pericardium. Case report and literature review. Tex Heart Inst J. 1994; 21: 170-4.
11.Bendek M, Ferenc M, Freudenberg N. Post-irradiation pericardial malignant mesothelioma: an autopsy case and review of the literature. Cardiovasc Pathol. 2010; 9: 377-9.
12.Velissaris TJ, Tang AT, Millward-Sadler GH, Morgan JM, Tsang GM. Pericardial mesothelioma following mantle field radiotherapy.J Cardiovasc Surg. 2001; 42: 425-7.
13.Churg A, Sheffield BS, Galateau-Salle F. New markers for separating benign from malignant mesothelial proliferations: Are we there yet? Arch Pathol Lab Med. 2015; 140: 318-21.
14.Arif Q, Husain AN. Malignant mesothelioma diagnosis. Arch Pathol Lab Med. 2015; 139: 978-80.
15.Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012; 130: 1715-25.
16.Betti M, Aspesi A, Biasi A, Casalone E, Ferrante D, Ogliara P, et al.CDKN2A and BAP1 germline mutations predispose to melanoma and mesothelioma. Cancer Lett. 2016; 378: 120-30.
17.Monaco ES, Shuai YL, Bansal M, Krasinskas AM, Dacic S. The diagnostic utility of p16 FISH and GLUT-1 immunohistochemical analysis in mesothelial proliferations. Am J Clin Pathol. 2011; 135:619-27.
18.López-Ríos F, Chuai SN, Flores R, Shimizu S, Ohno T, Wakahara K, et al. Global gene expression profiling of pleural mesotheliomas:overexpression of aurora kinases and P16/CDKN2A deletion as prognostic factors and critical evaluation of microarray-based prognostic prediction. Cancer Res. 2006; 66: 2970-9.
19.Walter RFHW, Vollbrecht C, Werner R, Mairinger T, Schmeller J,Flom E, et al. Screening of pleural mesotheliomas for DNA-damage repair players by digital gene expression analysis can enhance clinical management of patients receiving platin-based chemotherapy. J Cancer. 2016; 7: 1915-25.
杂志排行
Cancer Biology & Medicine的其它文章
- The evolution of Epstein-Barr virus detection in nasopharyngeal carcinoma
- Dual-specificity phosphatase 6 (DUSP6): a review of its molecular characteristics and clinical relevance in cancer
- Silencing of syndecan-binding protein enhances the inhibitory effect of tamoxifen and increases cellular sensitivity to estrogen
- EGFR tyrosine kinase inhibitor HS-10182 increases radiation sensitivity in non-small cell lung cancers with EGFR T790M mutation
- Calcium channel α2δ1 subunit as a novel biomarker for diagnosis of hepatocellular carcinoma
- Parkin protein expression and its impact on survival of patients with advanced colorectal cancer
