Effect of in ovo zinc injection on the embryonic development,tissue zinc contents, antioxidation, and related gene expressions of broiler breeder eggs
2018-03-07SUNXiaomingLlAOXiudongLULinZHANGLiyangMAQiugangXlLinLUOXugang
SUN Xiao-ming, LlAO Xiu-dong, LU Lin ZHANG Li-yang MA Qiu-gang, Xl Lin, LUO Xu-gang
1 Mineral Nutrition Research Division, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193,P.R.China
2 College of Animal Science and Technology, China Agricultural University, Beijing 100193, P.R.China
3 Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA
1.lntroduction
Because of the increased metabolic rate of the embryos of today, the embryonic nutrient reserves are insufficient and might be depleted in the prenatal period (Yair et al.2013).Such nutritional insufficiencies may induce long-lasting adverse consequences on progeny performance (Petry and Hales 2000).Therefore, intervention strategies involving pre-hatch nutrient supplementation have been developed to reduce nutritional restrictions (Oliveira et al.2015).In ovo injection technology provides a practical means to safely introduce external nutrients into developing embryos (Foye et al.2007; Kadam et al.2008; Bello et al.2014).Feeding the embryo before hatch by in ovo administration of external feed components was reported to cause a positive effect on hatchability, development of the digestive tract, body weight and nutritional status of the hatchling (Uni and Ferket 2004).
Zinc (Zn) is an essential trace element for normal growth,bone development, feathering, enzyme structure and function, and appetite regulation for all avian species (Park et al.2004).Zinc plays an essential role in a wide variety of biochemical processes (Vallee and Auld 1990), and it acts as an antioxidant supplement for protecting poultry against oxidative damage.Dietary Zn supplementation or intravenous Zn injection could enhance expression levels of metallothionein (MT) and copper- and Zn-containing superoxide dismutase (CuZnSOD) as free radical scavengers in tissues of broilers (Huang et al.2007, 2009, 2013; Liu et al.2011, 2013, 2015; Liao et al.2013; Shen et al.2013; Li et al.2015; Suo et al.2015).Zinc deficiency in maternal purified or semi-purified diets of laying breeder hens resulted in a decreased hatchability, abnormal embryonic development,and poor growth performance of offspring birds, whereas maternal dietary Zn supplementation could eliminate these negative effects (Kienholz 1961; Zhu 2016; Zhu et al.2017).In ovo injection of a nano form of either Zn (20 μg/egg) or copper or selenium through the amniotic cavity at 18 d of incubation did not affect the embryonic development or hatchability (Joshua et al.2016).Yair and Uni (2011) reported that injecting a mixture of Zn (600 μg/egg) and other minerals into the amniotic cavity at 17.5 d of incubation increased yolk Zn level and Zn consumption of the broiler embryos.In ovo injection of a solution containing Zn (600 μg/egg), vitamins and carbohydrates improved mechanical properties of bone of offspring broilers at 3 and 14 d of age posthatching (Yair et al.2013).Bakyaraj et al.(2012) injected a mixture of Zn(80 μg/egg) and other trace elements into the amniotic cavity of the 18-d-old embryos, and found that the enrichment might modulate the cell-mediated immunity of offspring broilers.In another study, Oliveira et al.(2015) reported that the injection of organic Zn (81.6 μg/egg), manganese and copper into the amniotic cavity at 17 d of incubation had the potential to improve bone mineralization, but negatively affected the hatchability of fertilized eggs.
During incubation, the first 2 weeks are an important period for the formation of embryonic organs and tissues(Macalintal 2012).The yolk sac is mainly responsible for the transfer of nutrients needed for energy and tissue growth(Noble and Cocchi 1990).Yair and Uni (2011) reported that broiler embryo’s consumption of Zn was medium until E 11,and then increased between E11 and E17, suggesting an increased need of Zn during the middle stage of incubation.However, in previous studies (Yair and Uni 2011; Bakyaraj et al.2012; Yair et al.2013; Oliveira et al.2015; Joshua et al.2016), in ovo injections of Zn (20 to 600 μg/egg) and other nutrients mainly focused on the later stage (E17-18)of incubation and their effects on embryos and offspring birds.No studies on the effect of in ovo Zn injection of chick embryos at the early stage of incubation on the embryonic development and antioxidation have been reported before, although maternal dietary Zn supplementation has been shown to play an important role in the embryonic development and antioxidation (Kienholz 1961; Zhu 2016;Zhu et al.2017).We hypothesized that in ovo Zn injection at the early stage of incubation might be able to increase the embryonic Zn content and antioxidation during the prenatal period, which would have a positive effect on the embryonic development.Therefore, the objective of the current study was to investigate the effect of graded levels of in ovo Zn injection at the early stage of incubation on the embryonic development, tissue Zn contents, antioxidation, and related gene expressions of broiler breeder eggs.
2.Materials and methods
2.1.Experimental design and treatments
All experimental procedures were approved by the Animal Management Committee (in charge of animal welfare issue) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS, Beijing, China) and performed in accordance with the guidelines.Ethical approval on animal survival was given by the Animal Ethics Committee of IAS-CAAS.In the current study, a completely randomized design was adopted.Firstly, an optimal embryonic age for the early in ovo injection needed to be determined prior to in ovo Zn injection experiment.Therefore, we designed 4 treatments in experiment 1, which included the non-injected control and 3 time points (3, 6 and 9 d of incubation (E3, E6 and E9)) of early in ovo yolk sac injection according to previous studies on the early in ovo amino acid or selenium injections (Ohta et al.1999;Macalintal 2012).A total of 720 fertilized eggs with similar weights were randomly distributed into 4 treatments with 6 replicates per treatment and 30 eggs per replicate, and injected with 0.1 mL sterilized water into the yolk sacs at E3, E6 and E9 or non-injection, respectively.
Experiment 2 was conducted using the optimal embryonic age for in ovo injection as determined from experiment 1 to investigate the effect of graded levels of in ovo injected Zn on the embryonic development, tissue Zn contents, antioxidant abilities, and gene expressions of CuZnSOD and MT in the embryonic tissues.A total of 672 fertilized eggs with similar weights were randomly divided into 7 treatments with 6 replicates per treatment and 16 eggs per replicate, and injected with 0.1 mL sterilized water (the negative control) or Zn solutions to provide doses of 50, 100, 150, 200, or 250 μg Zn/egg at E9-10 based on the results from experiment 1(see the following results section), or non-injection (the positive control), respectively.
2.2.Eggs and incubation
All of fertilized eggs used in the present two experiments were purchased from Huadu Broiler Company, Beijing, China,and collected within a 24-h period from the same flocks of Arbor Acres (AA) broiler breeder layers fed the commercial complete corn-soybean meal diet containing 100 mg Zn kg-1on an as-fed basis by analysis.The yolks of 8 breeder eggs in either experiment 1 or 2 were analyzed to contain 686 and 715 μg Zn/egg, respectively.All eggs were stored at 21°C for 24 h.Prior to being placed in the incubator (Lantianjiao Electronic Technology Company, Beijing, China), all eggs were weighed, marked and fumigated with formaldehyde gas.
Eggs from each replicate of each treatment were placed on the same egg tray, and incubated at 38.0°C with a relative humidity of 50%.At E18, eggs were candled for viability, and any dead embryos were counted, discarded,and then the remaining eggs were transferred to a hatcher and placed in hatching boxes.At E22, all hatched chicks were counted and then weighed to obtain the chick hatch weight.The numbers of failed pips, healthy and weak chicks were also recorded.The chicks that were not able to stand were classified as weak chicks.Embryonic mortality was calculated as a percentage of dead embryos in the total number of injected eggs for each replicate per treatment.Hatchability was calculated as a percentage of hatched birds in the total number of injected eggs for each replicate per treatment.
2.3.Preparation of Zn solutions and in ovo injection procedure
Zinc sulfate (ZnSO4·7H2O, reagent-grade, Sinopharm Chemical Reagent Co., Shanghai, China) was dissolved in deionized water to make a stock solution containing 10 mg Zn mL-1, which was subsequently diluted with deionized water to make injection solutions containing 500,1 000, 1 500, 2 000 and 2 500 μg Zn mL-1, respectively.All solutions were filtered using a 0.22-μm acetate filter (MSI®,Westborough, MA, USA).The above injection solutions were analyzed to contain 493, 1 004, 1 494, 1 972 and 2 449 μg Zn mL-1, respectively.
Before injections, eggs were removed from the incubator and candled for viability.The eggs that were cracked,infertilized or shown the sign of early embryonic death were discarded.In ovo injection procedure was carried out according to the description by Macalintal (2012).Under the candle, the outline of the air cell was traced using a pencil.The embryo, which appeared as a dark floating silhouette and the head as a dark spot, was located.The egg was turned about a quarter away from the head, to locate the yolk and the target site of injection.After disinfecting the shell with an alcohol swab, a small hole was drilled on the larger end of the egg, above the air cell.Using a needle (0.5 mm×38 mm), 0.1 mL injection solution was injected into the yolks to provide doses of 0, 50, 100, 150, 200 and 250 μg Zn/egg,respectively.The procedure was carried out in a laminar flow system.The pinhole site on the shell was immediately sealed with paraffin wax, and then eggs were returned to the incubator.All eggs were held outside the incubator for less than 30 min while injected.Like those eggs that were injected, the eggs from the non-injected positive control group remained outside the incubator for the same length of time.
2.4.Sample collections and preparations
Samples of the commercial complete corn-soybean meal diet, the yolks of breeder eggs, and Zn injection solutions were taken for Zn analysis.In experiment 2, 2 embryos at E15 and 5 embryos at E20 from each replicate per treatment were randomly selected and were killed by cervical dislocations.Liver and heart were immediately taken and snap-frozen in liquid nitrogen and then stored at -80°C for future analyses.The tibias were removed from both legs of each embryo, and boiled for approximately 10 min in deionized water to remove all soft tissues, and then dried for 12 h at 105°C to obtain dried tibia samples.All samples from 2 or 5 embryos in each replicate were pooled into one sample in equal weight before analyses.
2.5.Sample analyses
Zinc contents in the commercial complete corn-soybean meal diet, the yolks of breeder eggs, Zn injection solutions,and the embryonic tissues were determined by inductively coupled plasma spectroscopy (model IRIS Intrepid II,Thermal Jarrell Ash, Waltham, MA, USA) after wet digestions with HNO3and HClO4as described by Huang et al.(2009).Validation of the mineral analysis was conducted using bovine liver powder (GBW (E) 080193, the National Institute of Standards and Technology, Beijing, China) as a standard reference material.
The CuZnSOD activities in the embryonic tissues were measured by the nitrite method as described by Huang et al.(2007).Malonaldehyde (MDA) levels in the embryonic tissues were determined using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, China).
The total RNA in the embryonic heart and liver were isolated using Trizol reagent (Life Technologies, Carlsbad,CA, USA) according to the manufacturer’s protocols.And cDNA synthesis was performed using PrimeScript RT Reagent Kit with cDNA Eraser (Qiagen, Chatsworth, CA,USA) according to the manufacturer’s instructions.Realtime PCR reactions were performed on an ABI 7500 Real-Time PCR System (Life Technologies, Carlsbad, CA, USA)using SYBR Green PCR Master Mix (Life Technologies,Carlsbad, CA, USA).The protocol of PCR was as follows:denaturation at 95°C for 2 min followed by 40 cycles of 95°C for 60 s, 60°C for 30 s, and 72°C for 30 s.All the primers were listed in Table 1.The geometric means of β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)were used as references to normalize the expressions of the target genes.Relative mRNA expression levels of CuZnSOD and MT were calculated using the 2-ΔΔCtmethod(Ct=threshold cycle) as described by Livak and Schmittgen(2001).
2.6.Statistical analyses
Data of the two experiments were subjected to one-way ANOVA using the GLM procedure of SAS software (version 9.0; SAS Inst.Inc., Cary, NC).The replicate served as the experimental unit.Differences among means were tested by the LSD method.Orthogonal comparisons were applied for linear and quadratic responses of dependent variables to independent variables (Lu et al.2016; Ma et al.2016).The P<0.05 was considered to be statistically significant.
第二,一般情况下抗菌药物可分为2类,一是时间依赖型,二是浓度依赖型。妇产科中常用的抗菌药物多为浓度依赖型,如喹诺酮类、硝基咪唑类、氨基糖苷类等。此类药物应用时,用药间隔时间可适当延长,1-2次/d便可。时间依赖型主要涉及大环内酯类、磺胺类等,其抗菌活性与药物浓度并无直接关系,而与时间有关,建议2-4次/d。[4]对此选择抗菌药物时,需考虑药物的实际特点。
3.Results
3.1.Embryonic mortality and hatchability
In experiment 1, the embryonic mortality and hatchability were affected (P<0.04) by the embryonic age of in ovo injection of sterilized water (Table 2).The embryonic mortality in E6 group was higher (P<0.05) than that in the non-injected control and E9 groups.The E3 and E6 injections decreased (P<0.05) chick hatchabilities compared with the non-injected control, and the E6 injection reduced(P<0.05) chick hatchabilities compared with the E3 and E9 injections.However, there were no significant differences(P>0.05) in embryonic mortality and hatchability between the E9 injection and the non-injected control or between E3 and E9 and in embryonic mortality between E3 and the non-injected control or between E3 and E6.The above results indicated that the E9 could be chosen as the optimal embryonic age of in ovo Zn injection in the experiment 2.
In experiment 2, in ovo Zn injection affected (P<0.04)the embryonic mortality and hatchability, but had no effect(P>0.60) on chick hatch weight (Table 3).There were no differences (P>0.05) in the embryonic mortality andhatchability between the non-injected positive and negative controls or among the negative control, 50, 100 and 250 μg Zn/egg groups or among 150, 200 and 250 μg Zn/egg groups.No differences (P>0.05) were also observed in the embryonic mortality between 50 and 200 μg Zn/egg groups or among the negative control, 50, 100 and 150 μg Zn/egg groups as well as in hatchability among 50, 150 and 200 μg Zn/egg groups.However, the 200 μg Zn/egg injection increased (P<0.05) the embryonic mortality and the 150 and 200 μg Zn/egg injections decreased (P<0.05) hatchabilities compared with the negative control and 100 μg Zn/egg injection.As injected Zn levels increased, hatchability decreased linearly (P<0.03).
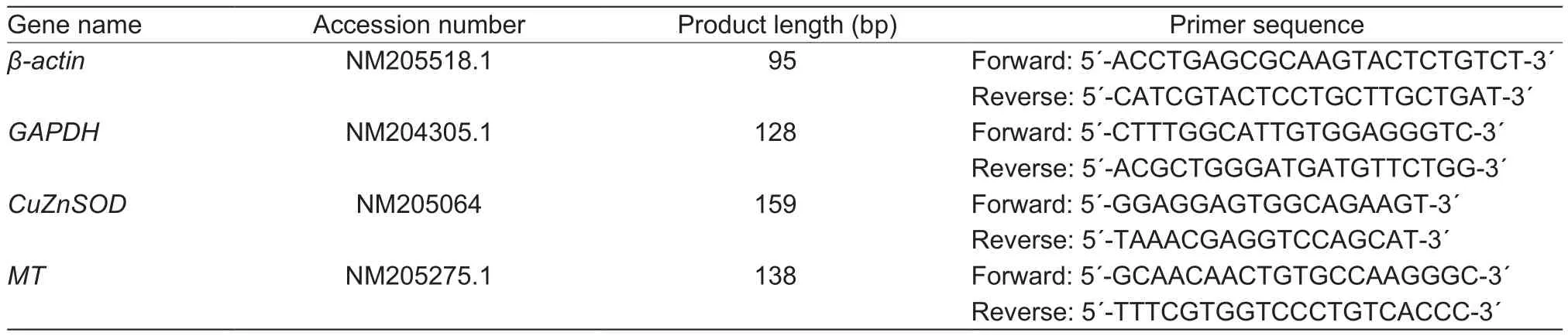
Table 1 Primer sequences of chicken β-actin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), copper- and zinc-containing superoxide dismutase (CuZnSOD) and metallothioneins (MT)
3.2.Zinc contents in the embryonic tibia and liver
In ovo Zn injection affected (P<0.0007) Zn content in thetibia of chick embryos at E20, but had no effect (P>0.50)on Zn content in the embryonic liver (Table 4).There were no differences (P>0.05) in the embryonic tibia Zn content between the non-injected positive and negative controls or among the negative control, 50 and 100 μg Zn/egg groups or among 100, 150 and 200 μg Zn/egg groups or among 150, 200 and 250 μg Zn/egg groups.However, 150, 200 and 250 μg Zn/egg injections increased (P<0.05) the embryonic tibia Zn contents compared with the negative control and 50 μg Zn/egg injection, and the embryonic tibia Zn content was higher (P<0.05) in the 250 μg Zn/egg group than that in the negative control, 50 and 100 μg Zn/egg groups.As injected Zn levels increased, tibia Zn content increased linearly (P<0.05).
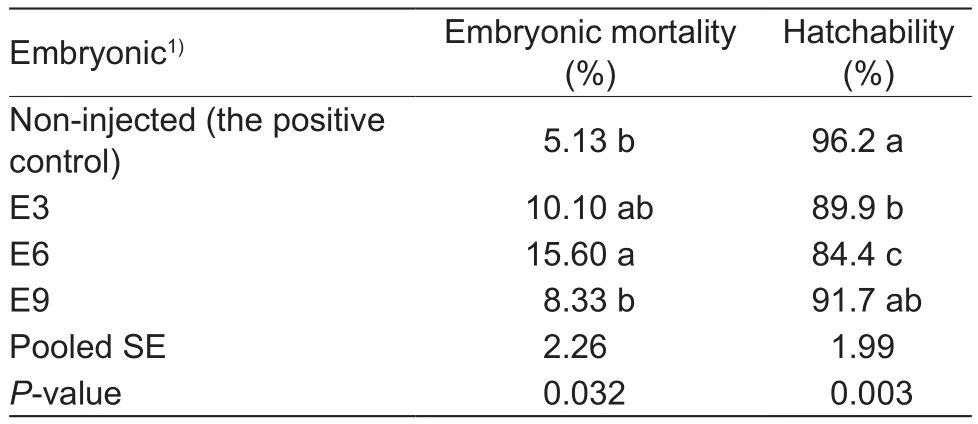
Table 2 Effect of the embryonic age of in ovo sterilized water injection on the embryonic mortality and hatchability(experiment 1)
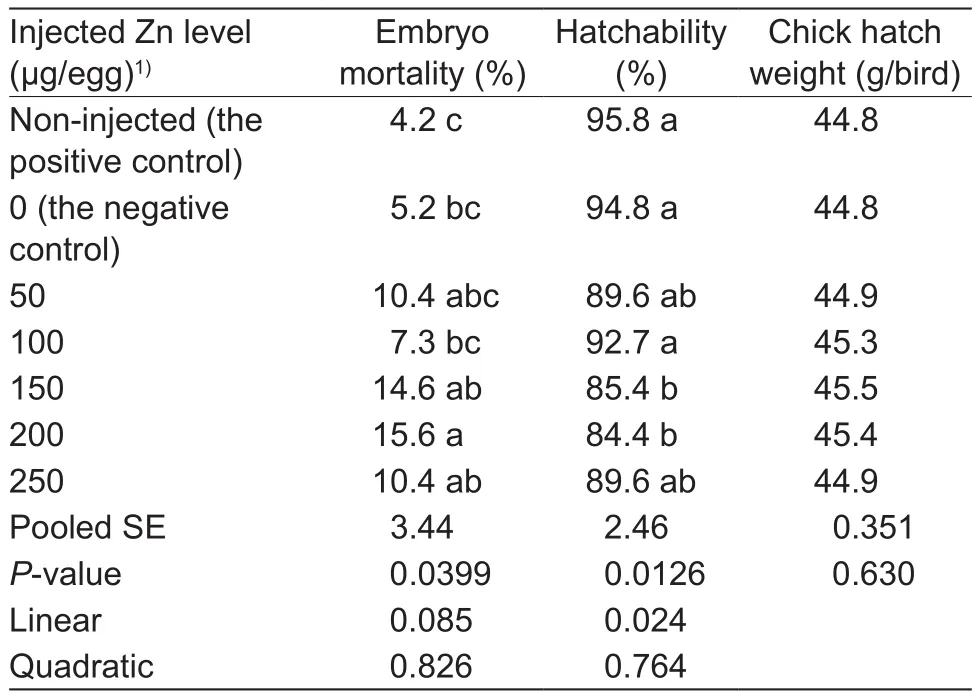
Table 3 Effect of in ovo zinc (Zn) injection on the embryonic mortality, hatchability and chick hatch weight (experiment 2)
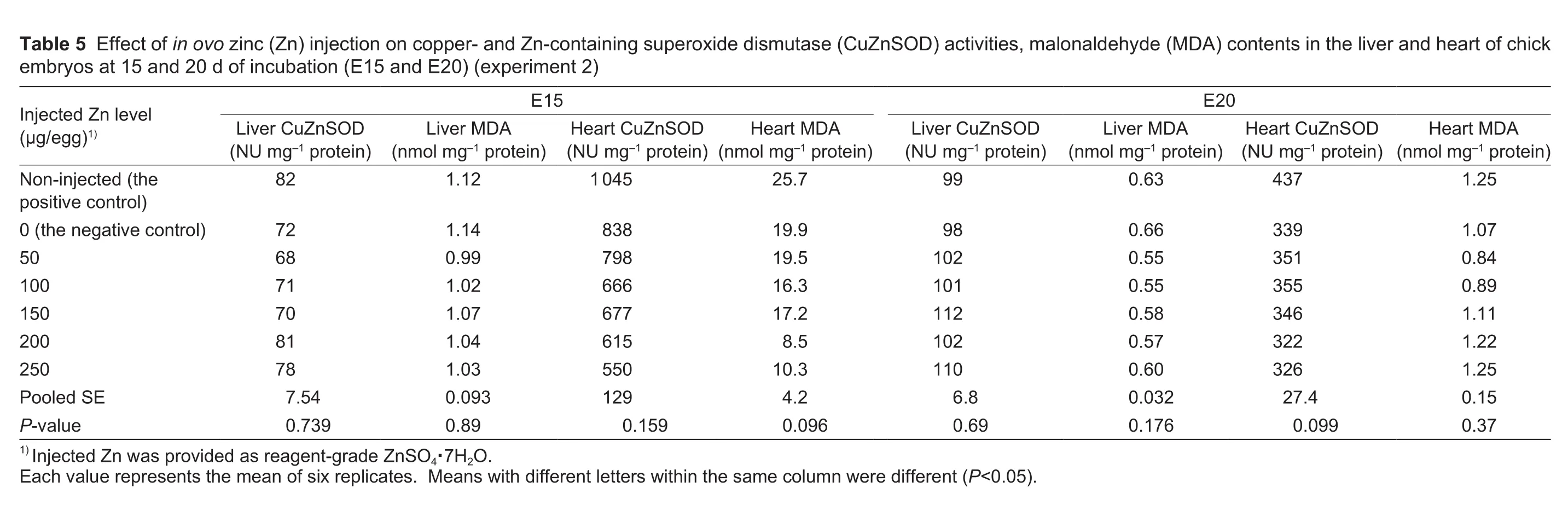
3.3.Tissue antioxidant indices
In ovo Zn injection did not affect (P>0.09) CuZnSOD activities and MDA levels in the liver and heart of chick embryos on both E15 and E20 (Table 5).
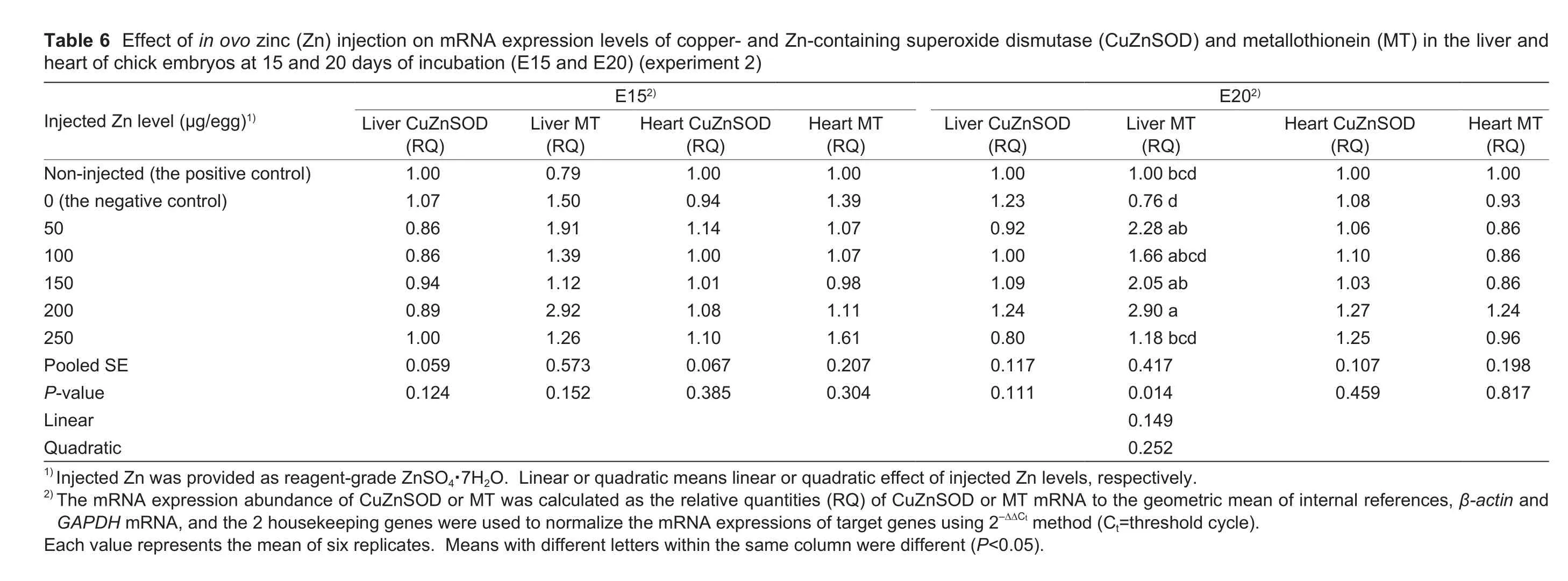
3.4.mRNA levels of CuZnSOD and MT in the embryonic liver and heart
In ovo Zn injection had no effect (P>0.11) on CuZnSOD mRNA expression levels in the liver and heart of embryos at E15 and E20, MT mRNA expression level in the embryonic liver at E15, and MT mRNA levels in the embryonic heart at E15 and E20, but affected (P<0.02) MT mRNA level in the embryonic liver at E20 (Table 6).There were no differences(P>0.05) in MT mRNA level of the embryonic liver at E20 between the positive and negative controls or among thenegative control, 100 and 250 μg Zn/egg groups or among 50, 100, 150 and 200 μg Zn/egg groups or among 50, 100,150 and 250 μg Zn/egg groups.However, the 50, 150 and 200 μg Zn/egg injections up-regulated (P<0.05) MT mRNA level in the embryonic liver at E20 compared with the negative control, and the MT mRNA level in the embryonic liver at E20 was lower (P<0.05) in the 250 μg Zn/egg group than that in the 200 μg Zn/egg group.
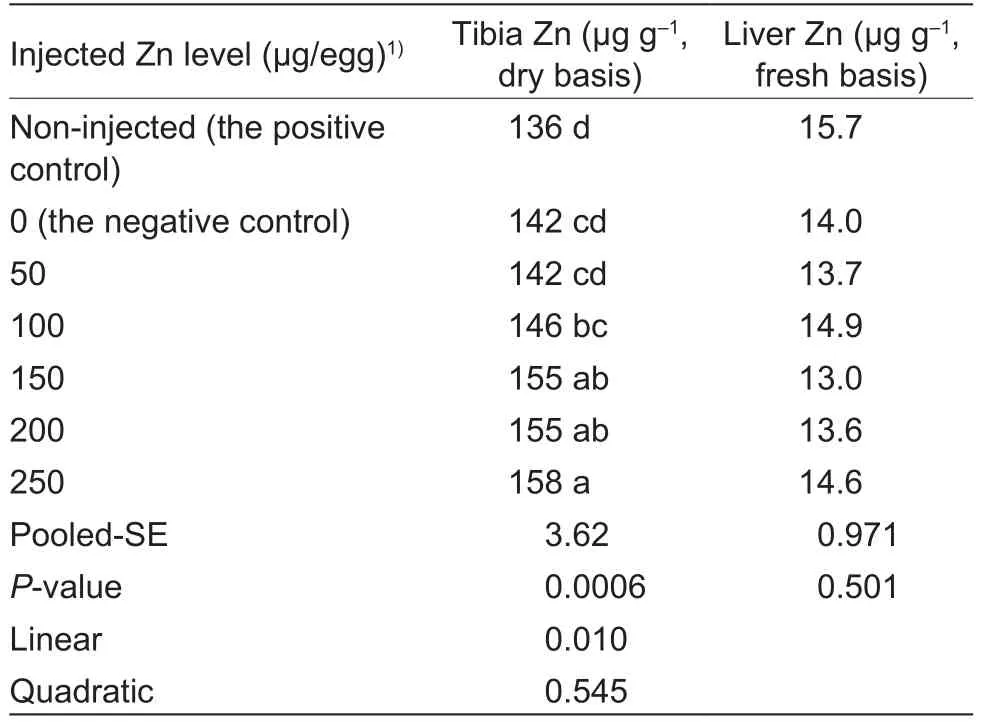
Table 4 Effect of in ovo zinc (Zn) injection on tissue zinc contents of chick embryos at 20 d of incubation (experiment 2)
?
?
4.Discussion
In recent years, in ovo injection has been widely used in the poultry industry as a way to control the incidence of diseases or improve the nutritional status of developing embryos.The results of the experiment 1 in this study showed that in ovo injection at E9 had the minimal effect on embryo viability.The results were similar to those of Macalintal (2012), who reported that the trauma resulting from yolk sac injection at 10 d of incubation was very limited.The early-stage incubation was an essential period for the embryonic differentiations, and the embryonic development was sensitive to the environmental changes (Macalintal 2012).Chick embryos were able to resist to the stimulations from the outside with the increase of embryonic age.Thus,E9 could be chosen as the optimal embryonic age for in ovo injection.
In the present study, unexpectedly, the eggs injected with 150-200 μg Zn/egg had higher embryonic mortalities and lower hatchabilities, indicating that the injections of higher doses of 150-200 μg Zn/egg were harmful to the embryonic development.It has been shown that in ovo injection of nutrients at various concentrations affected hatchabilities (Ebrahimi et al.2012; Bello et al.2013).Oliveira et al.(2015) reported that in ovo injection of higher doses of Zn (81.6 μg Zn/egg), copper and manganese at 17 d of incubation decreased the hatchability of fertilized eggs at 21.5 d of incubation.The results from the current study suggested that the higher doses of 150-250 μg Zn/egg would be toxic for broiler embryos.It might be majorly attributed to the adequate Zn content (715 μg Zn/egg) in the egg yolks, because the eggs used in the present study were collected from the AA broiler breeder layers fed the commercial complete corn-soybean meal diet containing 100 mg Zn kg-1on an as-fed basis by analysis.Therefore,Zn content in the eggs was adequate for the embryonic development.In such circumstance, injecting Zn into the yolk sac might create a mineral imbalance in the yolk sac(Oliveira et al.2015), which would not be beneficial or could be harmful to the embryonic development.
Tissue mineral concentrations are indicators of body mineral storage and status, and have been used to study mineral requirements and bioavailabilities (Huang et al.2007).Microminerals that are important for bone formation and strength include Zn, copper and manganese,which could be greatly reduced in the egg at the 17 d of incubation (Yair and Uni 2011).Zinc participates in important regulatory pathways for bone and cartilage formation, such as collagen synthesis (Starcher et al.1980).Reduced concentrations of Zn might restrict this process during the last days of incubation.In the present study, in ovo injection of Zn increased Zn content in tibia, which might contribute to the improvement of the bone mineralization and development.In contrast, Oliveira et al.(2015) did not observe the increased Zn content in the tibia ash by in ovo Zn injection.These inconsistent results might be due to different embryonic ages of injection and injected Zn source.
Metallothionein is a small, cysteine-rich protein that can bind Zn and other metals with high affinity, thus reducing its potentially harmful effects within the body.It plays a protective role in antioxidant responses by scavenging free radicals, particularly the hydroxyl radical (Ruttkay-Nedecky et al.2013).It was found that tissue MT was positively related to dietary Zn intake in chicks (Huang et al.2007, 2009, 2013; Liu et al.2011, 2013, 2015; Liao et al.2013; Shen et al.2013; Li et al.2015; Suo et al.2015).The results from the present study indicated that in ovo Zn injection up-regulated the mRNA expressions of MT in the embryonic liver at E20 except for the highest dose treatment, suggesting that the increased MT expressions might enhance the embryonic antioxidant ability.
Zinc is also necessary for the structure and function of CuZnSOD, which comprises 90% of the total SOD and protects tissues from oxidative damage (Noor et al.2002).Dietary Zn supplementation was reported to increase liver CuZnSOD activity (Wang et al.2012).Malonaldehyde is a soluble degraded product of lipids and widely used to reflect the extent of lipid oxidation (Raharjo and Sofos 1993).However, the results from the current study indicated that MDA content and CuZnSOD activity as well as mRNA expression of CuZnSOD in the embryonic liver and heart at E15 and E20 failed to reflect the differences among different doses of Zn administration.In contrast, Liu et al.(2015) reported that dietary supplemental Zn increased CuZnSOD activities in the breast and thigh muscles and mRNA expression levels of CuZnSOD in the liver and thigh muscle of broilers.These inconsistent results might be attributed to the different ways of Zn administration and the Zn-adequate eggs used in the present study.
It has been clearly demonstrated in the present study that no beneficial results on the embryonic development and most of related aspects were observed in the yolk sac injections of Zn at the early stage of incubation (at E9-10),which was majorly attributed to the higher Zn content in the egg yolks and also higher injected Zn levels.Therefore,further studies are needed to elucidate the effect of in ovo yolk sac injections of lower Zn levels (below 50 μg Zn/egg) at the early stage of incubation on the embryonic development and related aspects using Zn-low broiler breeder eggs in the future.
5.Conclusion
The results from the current study indicated that in ovo yolk sac injection of sterilized water at E9 had no detrimental effect on the chick embryonic development, and in ovo yolk sac Zn injections at E9-10 increased Zn contents in the embryonic tibia and MT mRNA expression levels in the embryonic liver at E20, but injections of 150-200 μg Zn/egg were harmful to the embryonic development.Further experiments are needed to address the above effects using Zn-low broiler breeder eggs and lower levels of injected Zn in the future.
Acknowledgements
The present study was supported by the Key International Cooperation Program of the National Natural Science Foundation of China (31110103916), the Agricultural Science and Technology Innovation Program, China (ASTIPIAS08), and the earmaked fund for the China Agriculture Research System (CARS-42).
Bakyaraj S, Bhanja S K, Majumdar S, Dash B.2012.Modulation of post-hatch growth and immunity through in ovo supplemented nutrients in broiler chickens.Journal of the Science of Food and Agriculture, 92, 313-320.
Bello A, Hester P Y, Gerard P D, Zhai W, Peebles E D.2014.Effects of commercial in ovo injection of 25-hydroxycholecalciferol on bone development and mineralization in male and female broilers.Poultry Science,93, 2734-2739.
Bello A, Zhai W, Gerard P D, Peebles E D.2013.Effects of the commercial in ovo injection of 25-hydroxycholecalciferol on the hatchability and hatching chick quality of broilers.Poultry Science, 92, 2551-2559.
Ebrahimi M R, Ahangari Y J, Zamiri M J, Akhlaghi A, Atashi H.2012.Does preincubational in ovo injection of buffers or antioxidants improve the quality and hatchability in longterm stored eggs? Poultry Science, 91, 2970-2976.
Foye O T, Ferket P R, Uni Z.2007.The effects of in ovo feeding arginine, beta-hydroxy-beta-methyl-butyrate, and protein on jejunal digestive and absorptive activity in embryonic and neonatal turkey poults.Poultry Science, 86, 2343-2349.
Huang Y L, Lu L, Li S F, Luo X G, Liu B.2009.Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed a conventional cornsoybean meal diet.Journal of Animal Science, 87,2038-2046.
Huang Y L, Lu L, Luo X G, Liu B.2007.An optimal dietary zinc level of broiler chicks fed a corn-soybean meal diet.Poultry Science, 86, 2582-2589.
Huang Y L, Lu L, Xie J J, Li S F, Li X L, Liu S B, Zhang L Y, Xi L, Luo X G.2013.Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed diets with low or high phytate content.Animal Feed Science& Technology, 179, 144-148.
Joshua P P, Valli C, Balakrishnan V.2016.Effect of in ovo supplementation of nano forms of zinc, copper, and selenium on post-hatch performance of broiler chicken.Veterinary World, 9, 287-294.
Kadam M M, Bhanja S K, Mandal A B, Thakur R, Vasan P,Bhattacharyya A, Tyagi J S.2008.Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activities in broiler chickens.British Poultry Science, 49, 736-741.
Kienholz E W.1961.Effects of zinc deficiency in the diets of hens.Journal of Nutrition, 75, 211-221.
Li W X, Ma X Y, Lu L, Zhang L Y, Luo X G.2015.Relative bioavailability of tribasic zinc sulfate for broilers fed a conventional corn-soybean meal diet.Journal of Integrative Agriculture, 14, 2042-2049.
Liao X D, Li A, Lu L, Liu S B, Li S F, Zhang L Y, Wang G Y,Luo X G.2013.Optimal dietary zinc levels of broiler chicks fed a corn-soybean meal diet from 22 to 42 days of age.Animal Production Science, 53, 388-394.
Liu S B, Li S F, Lu L, Xie J J, Zhang L Y, Wang R L, Luo X G.2013.The effectiveness of zinc proteinate for chicks fed a conventional corn-soybean meal diet.Journal of Applied Poultry Research, 22, 396-403.
Liu Z H, Lu L, Li S F, Zhang L Y, Xi L, Zhang K Y, Luo X G.2011.Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers.Poultry Science, 90, 1782-1790.
Liu Z H, Lu L, Wang R L, Lei H L, Li S F, Zhang L Y, Luo X G.2015.Effects of supplemental zinc source and level on antioxidant ability and fat metabolism-related enzymes of broilers.Poultry Science, 94, 2686-2694.
Livak K J, Schmittgen T D.2001.Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method.Methods, 25, 402-408.
Lu L, Chang B, Liao X D, Wang R L, Zhang L Y, Luo X G.2016.Use of molecular biomarkers to estimate manganese requirements for broiler chickens from 22 to 42 d of age.British Journal of Nutrition, 116, 1512-1518.
Ma X Y, Liao X D, Lu L, Li S F, Zhang L Y, Luo X G.2016.Determination of dietary iron requirements by full expression of iron-containing enzymes in various tissues of broilers.Journal of Nutrition, 146, 2267-2273.
Macalintal L M.2012.In ovo selenium (Se) injection of incubating chicken eggs: Effects on embryo viability, tissue Se concentration, lipid peroxidation, immune response and post hatch development.Ph D thesis, University of Kentucky, Lexington.
Noble R C, Cocchi M.1990.Lipid metabolism and the neonatal chicken.Progress in Lipid Research, 29, 107-140.
Noor R, Mittal S, Iqbal J.2002.Superoxide dismutase -applications and relevance to human diseases.Medical Science Monitor, 8, RA210-RA215.
Ohta Y, Tsushima N, Koide K, Kidd M, Ishibashi T.1999.Effect of amino acid injection in broiler breeder eggs on embryonic growth and hatchability of chicks.Poultry Science, 78,1493-1498.
Oliveira T F B, Bertechini A G, Bricka R M, Kim E J, Gerard P D, Peebles E D.2015.Effects of in ovo injection of organic zinc, manganese, and copper on the hatchability and bone parameters of broiler hatchlings.Poultry Science,94, 2488-2494.
Park S Y, Birkhold S G, Kubena L F, Nisbet D J, Ricke S C.2004.Review on the role of dietary zinc in poultry nutrition,immunity, and reproduction.Biological Trace Element Research, 101, 147-163.
Petry C J, Hales C N.2000.Long-term effects on offspring of intrauterine exposure to deficits in nutrition.Human Reproduction Update, 6, 578-586.
Raharjo S, Sofos J N.1993.Methodology for measuring malonaldehyde as a product of lipid peroxidation in muscle tissues: A review.Meat Science, 35, 145-169.
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M,Eckschlager T, Stiborova M, Adam V, Kizek R.2013.The role of metallothionein in oxidative stress.International Journal of Molecular Sciences, 14, 6044-6066.
SAS Institute.2010.SAS/STAT User’s Guide: Statistics.version 9.2.SAS Inst.Inc., Cary, NC.
Shen S F, Wang R L, Lu L, Li S F, Liu S B, Xie J J, Zhang L Y,Wang M L, Luo X G.2013.Effect of intravenously injected zinc on tissue zinc and metallothionein gene expression of broilers.British Poultry Science, 54, 381-390.
Starcher B C, Hill C H, Madaras J G.1980.Effect of zinc deficiency on bone collagenase and collagen turnover.The Journal of Nutrition, 110, 2095-2102.
Suo H Q, Lu L, Zhang L Y, Zhang X Y, Li H, Lu Y F, Luo X G.2015.Relative bioavailability of zinc-methionine chelate for broilers fed a conventional corn-soybean meal diet.Biological Trace Element Research, 165, 206-213.
Uni Z, Ferket R.2004.Methods for early nutrition and their potential.Worlds Poultry Science Journal, 60, 101-111.
Vallee B L, Auld D S.1990.Zinc coordination, function, and structure of zinc enzymes and other proteins.Biochemistry,29, 5647-5659.
Wang M Q, Tao W J, Ye S S, Du Y J, Wang C, Shen S X.2012.Effects of dietary pharmacological zinc on growth,liver metallothionein, Cu, Zn-SOD concentration and serum parameters in piglets.Journal of Animal and Veterinary Advances, 11, 1390-1394.
Yair R, Shahar R, Uni Z.2013.Prenatal nutritional manipulation by in ovo enrichment influences bone structure, composition,and mechanical properties.Journal of Animal Science, 91,2784-2793.
Yair R, Uni Z.2011.Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment.Poultry Science, 90, 1523-1531.
Zhu Y W.2016.Anti-heat stress effects and molecular mechanisms of dietary manganese and zinc in broilers.Ph D thesis, China Agricultural University, Beijing.(in Chinese)
Zhu Y W, Li W X, Lu L, Zhang L Y, Ji C, Lin X, Liu H C, Odle J,Luo X G.2017.Impact of maternal heat stress in conjunction with dietary zinc supplementation on hatchability, embryonic development, and growth performance in offspring broilers.Poultry Science, 96, 2351-2359.
猜你喜欢
杂志排行
Journal of Integrative Agriculture的其它文章
- Detection of illegal dyes in foods using a polyethersulfone/multi-walled carbon nanotubes composite membrane as a cleanup method
- Complete genome sequences of four isolates of Citrus leaf blotch virus from citrus in China
- The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep)
- Optimal storage temperature and 1-MCP treatment combinations for different marketing times of Korla Xiang pears
- Estimation of irrigation requirements for drip-irrigated maize in a sub-humid climate
- Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize-soybean relay intercropping system
