Detection of illegal dyes in foods using a polyethersulfone/multi-walled carbon nanotubes composite membrane as a cleanup method
2018-03-07HEYahuiWANGJing
HE Ya-hui, WANG Jing
1 School of Food Science, Xinyang College of Agriculture and Forestry, Xinyang 464000, P.R.China
2 Institute of Quality Standard and Testing Technology for Agro-products, Chinese Academy of Agricultural Sciences, Beijing 100081, P.R.China
1.Introduction
Membrane technology has been widely used in separation because it is simple and effective (Rowe et al.1988).Recently many types of composite membranes were made to take advantage of variation in mechanical properties and antifouling performance; these membranes are used in many different fields (Gestel et al.2002; Yang et al.2011).Up to now, there has been no report on composite membranes applied in detecting illegal dyes in food matrixes.
Food colorants are typically used to enhance the organoleptic properties of food.Industrial dyes could potentially be used as food additives by unscrupulous or ignorant producers because of their low cost and strong coloration ability.However, industrial dyes are potential mutagens and have been linked to an increased risk of cancers in humans, and because of that they are prohibited from use in food (Augustine et al.1980; Lv 2015; Uematsu et al.2017).Neve rtheless, industrial dyes have been found in various foods in many countries (Peiperl et al.1995).Detection methods to identify industrial dyes in food need to be developed and used to maintain food safety.
In order to meet the requirements of the market, a number of analyses are required.At present, there are a number of cleanup techniques for dyes detected in food: microwave pretreatment (Vas 2004); supercritical fluid extraction (Richter et al.1996); stir bar sorptive extraction (Kawaguchi et al.2006); pressurized liquid extraction (Gonzalez et al.2005);and matrix solid-phase dispersion (Beltran et al.2000).The methods used to detect industrial dyes usually cannot detect multiple dyes at the same time; in order to detect multiple dyes, a large number of organic solvents are required, and the process takes a long time.Therefore, the development of simple, fast, and effective analysis and detection methods for industrial dyes is very valuable.
Carbon nanotubes (CNTs) were first described by Iijiama (1991).There are two types of CNTs, according to the principle of carbon atom layers: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) (Iijima 1991).CNTs are reported to have special chemical and physical characteristics(Zhou et al.2006; Wang et al.2007; El-Sheikh et al.2008), including an excellent adsorption capacity due to their large surface area and unique structure.CNTs have been used as sorbents in many fields: CNTs were used in the solid phase extraction (SPE) method to extract pesticides from water samples (Du et al.2008; Ravelo-Pérez et al.2008); a new method using MWCNTs as sorbents for SPE in the determination of benzodiazepine residues in meat was developed by Zhao et al.(2015) and a new analytical method to detect organophosphate pesticides in garlic using MWCNTs in SPE was developed by Zhao et al.(2012).Polyethersulfone (PES) has been widely used as a material for membrane substrates in microfiltration, ultrafiltration, and nanofiltration.PES ultrafiltration membranes can be used in separation, concentration,and purification of food.However, despite these uses of MWCNTs and PES, there are no reports of using PES/MWCNTs composite membranes as an effective detection and cleanup method for illegal dyes in foods.In this paper, the use of PES/MWCNTs composite membranes in detecting illegal dyes in food matrixes is shown to be effective, simple, and rapid.
2.Materials and methods
2.1.Materials
MWCNTs nanoparticles of a quantum size 8-12 nm were obtained with the help of Tianjing Agela Co., Ltd.(China).Dimethylformamide (DMF) and polyethylene glycol (PEG)(with molecular weights of 400), and PES were purchased from Shanghai Chemical Regents Company (Shanghai,China).A total of 15 industrial dye standards were purchased from the company of Dr.Ehrenstorfer GmbH(Germany).The molecular and structural formulas for all standards are shown in
AppendixA.
2.2.Preparation of the composite membrane
The phase-inversion method was used to prepare the PES/MWCNTs composite membrane.The membrane forming solution consisted of DMF (86% weight percentage), PEG(2% weight percentage), and PES (12% weight percentage),and which were dissolved at 65°C for 3 h with constant stirring.MWCNTs were added to the solution after the uniform polymer solution was formed with strong stirring for 2 h.The ratio (w/w) of MWCNTs/PES was 0.3.To remove air bubbles, the solution was kept in the dark for 12 h.Then the membrane was formed by casting the solution using a 100-μm casting knife onto a polyester non-woven fabric.The composite membrane was immersed in a (15±1)°C deionized water coagulation bath after evaporation at (20±1)°C and(60±5)% relative humidity for 1 min.
2.3.Characterization of the membrane
Characterization of PES/MWCNTs composite membrane and MWCNTsThe surface morphology and internal structure of the membrane were observed using a scanning electron microscope (TSM-6700F, TESCAN, Germany).The morphologies of the MWCNTs before and after adsorption of industrial dyes were observed with the TSM-6700F.To observe MWCNTs after adsorption of industrial dyes, a rhodamine B standard (10 μg L-1) was added into a 5-mL Teflon centrifuge tube with 5 mg MWCNTs.The mixture was thoroughly vortexed and then centrifuged with a microcentrifuge at 10 000 r min-1for 3 min.Then the MWCNTs were removed for observation with the TSM-6700F.
Molecular weight (MW) cut-off of the membraneDifferent molecular weight proteins were used to determine the molecular weight cut-off of the composite membrane.The proteins used were: lysozyme (MW=14.7 kDa); chymotrypsin (MW=24.5 kDa); α-amylase (MW=45 kDa); and bovine serum albumin (MW=67 kDa).The protein solution was ultrafiltrated at 20°C and 0.15 MPa.The samples were removed from the feed side and permeate flow side of the composite membrane and were measured with a UV-spectrophotometer (UV-1600) at a wavelength of 280 nm when the process was in a steady state.
The rejection rate of proteins through the membrane was calculated according to eq.(1):

Where, R is defined as the rejection rate, Cperis the concentration of the permeation, and Cfeedis the concentration of the feed.
Pure water fluxMembrane performance was tested using a cross-flow ultrafiltration unit as a flat plate module with an effective membrane area of 2.01×10-2m2.The water flux was calculated as follows:

Where, J is the membrane flux (L m-2h-1), V is the volume permeated (L), A is the area of the membrane (m2), and t is the time (h).
2.4.Sample extraction and cleanup
Blank (free of industrial dye) millet and corn flour were obtained from a local supermarket.A total of 10 g of experimental samples and 10 mL of acetonitrile were added to a 50-mL Teflon centrifuge tube and vortexed vigorously for 1 min.Then, NaCl (1 g) and MgSO4(4 g) were added into the tube and the mixture was vortexed vigorously for 1 min.Finally, the tube was placed into an ice-water bath immediately after vortexing for 5 min, and then centrifuged for 5 min at 3 800 r min-1.
A diagram of the adsorption and elution procedure for industrial dyes in food with the PES/MWCNTs composite membrane is shown in Fig.1.A total of 5 mL of the clarified supernatant was introduced into the PES/MWCNTs composite membrane after centrifugation.The supernatant was filtered by the PES/MWCNTs composite membrane,as shown in the filtration and adsorption step in Fig.1.In this step, the industrial dye was adsorbed onto the composite membrane while other impurities passed through the membrane due to the adsorption of dyes by MWCNTs in the membrane.Then the PES/MWCNTs composite membrane was eluted with 5 mL acetone, acetonitrile,methanol and n-hexane in turn, as shown in the elution step in Fig.1.In this step, the industrial dyes were eluted from the composite membrane.Finally, the eluent was filtered by a 0.22-μm membrane and placed into a liquid chromatograph (LC) vial for chromatographic analysis.
2.5.Apparatus and conditions
Extracts were analyzed using a liquid chromatograph(Waters LC ACQUITY UPLC, Waters, USA) with a mass spectrometric detector (Waters Xevo TQ-S, Waters, USA)in selected ion monitoring (SIM) mode.
Chromatographic separation was carried out on a Waters Quattro Premier XE Mass Spectrometer equipped with a C18 column (2.1 mm×50 cm, 1.7 mm; Waters, USA).Preliminary experiments were carried out to systematically change the strength of the mobile phase and fragmentor voltage in full scan mode using compound standard solutions to find the retention times and the best resolution in the analytic peaks.
Based on the ion suppression produced, no ion-pairing reagent was introduced into the mobile phase, and formic acid was employed in reversed-phase chromatography.CH3CN/H2O (0.05% HCOOH) 10/90 (v/v) was used as the mobile phase.Gradient elution with aqueous acetonitrile-formic acid was used as the mobile phase to get effective and sensitive separation in liquid chromatography.

Fig.1 Diagram of sample cleanup process.1, constant temperature trough; 2, pump; 3, flowmeter; 4, throttle; 5, pressure gauge; 6, waste liquid; 7, eluent; 8, solution for analysis.
A tandem mass detector was used for analysis.The mass spectrometric parameters were operated using full scan and daughter scan for the compounds.The M+ion was chosen as the precursor ion for all analytes.The ion mass spectra of the industrial dyes were obtained using electrospray ionization.The collision energy was optimized for two selective ion transitions for every industrial dye.The most sensitive transition of the multiple reaction monitoring(MRM) transitions was selected for quantification analysis.
2.6.Method performances
Millet and corn flour were selected for validation.The accuracy, precision, limit of quantification (LOQ), and limit of determination (LOD) of the method were determined during validation of the analytical method.The accuracy and precision were evaluated by recovery and reproducibility experiments that were carried out for each sample of millet and corn flour in five replicates each at two fortification levels(0.01 and 0.10 mg kg-1).The LOD was determined as the concentration of analyte giving a signal to noise ratio (S/N)of 3 for the target ion.And the LOQ was determined as the concentration of analyte giving S/N of 10 for the target ion.
3.Results
3.1.Characterization of the composite membrane
Microstructure of the PES/MWCNTs composite membrane and MWCNTsFig.2 shows scanning electron microscopy (SEM) photographs of the surface and cross-section structure of the MWCNTs/PES composite membrane.Most particles of MWCNTs were distributed uniformly in the surface of the membrane.The cross-section of the membrane showed typical asymmetric morphology with finger-like pores.
Fig.3 shows SEM photographs of the MWCNTs before and after adsorption of illegal industrial dyes (rhodamine B).Fig.3-B shows that rhodamine B was adsorbed on MWCNTs.The strong adsorption of MWCNTs, which have a layered hollow structure, for illegal dyes may be caused by the benzene ring structures of the dyes.
Molecular weight cut-offThe molecular weight cut-off of the composite membrane is shown in Fig.4.The cut-off is approximately 20 000, so that carbohydrates, proteins, and other ingredients with a molecular weight greater than 20 000 in food samples will be rejected by the composite membrane.Many sulfur-containing compounds in foods that may cause serious interferences with the matrix during detection will thus be removed by using appropriate molecular weight cut-offs in the membrane.
Water flux and solute rejectionThe membrane was previously filtered by deionized water for 3 h at 50 kPa.The test was operated at 100 kPa and (20±1)°C.The results of water flux are presented in Fig.5, showing that water flux of the composite membrane tends to be stable at about 80 min.The water flux is about 90 L m-2h-1.

Fig.2 Scanning electron microscopy (SEM) micrographs of the surface (A) and cross-section (B) structures of the polyethersulfone(PES)/multi-walled carbon nanotubes (MWCNTs) composite membrane.
3.2.Liquid chromatography-tandem mass spectrometry
Fig.6 shows LC-MS/MS chromatograms of the 15 industrial dyes.The ionization of 15 industrial dyes in the positive mode electrospray ion source was examined.The previous experiment was carried out to optimize the conditions for interfacing the LC system to the MS.The optimized method of LC-MS/MS was highly selective for monitoring specific MRM and was effective in reducing the risk of false positives.The 15 industrial dyes were separated completely by LC-MS/MS.
3.3.Validation of the cleanup method
Recovery and precisionAccuracy was evaluated in terms of recovery.This study was performed using five consecutive extractions (n=5) of spiked matrices at a concentration of 0.1 mg kg-1.The recovery and repeatability data for the 15 industrial dyes in the matrix of millet and corn flour are shown in Table 1.The recoveries of all industrial dyes were in the range of 73-117% (between 75-116% for millet,and between 73-117% for corn flour).Relative standard deviation (RSD) values were all below 15%.

Fig.3 Scanning electron microscopy (SEM) micrographs of the multi-walled carbon nanotubes (MWCNTs) before (A) and after(B) the adsorption of industrial dye (Rhodamine B).
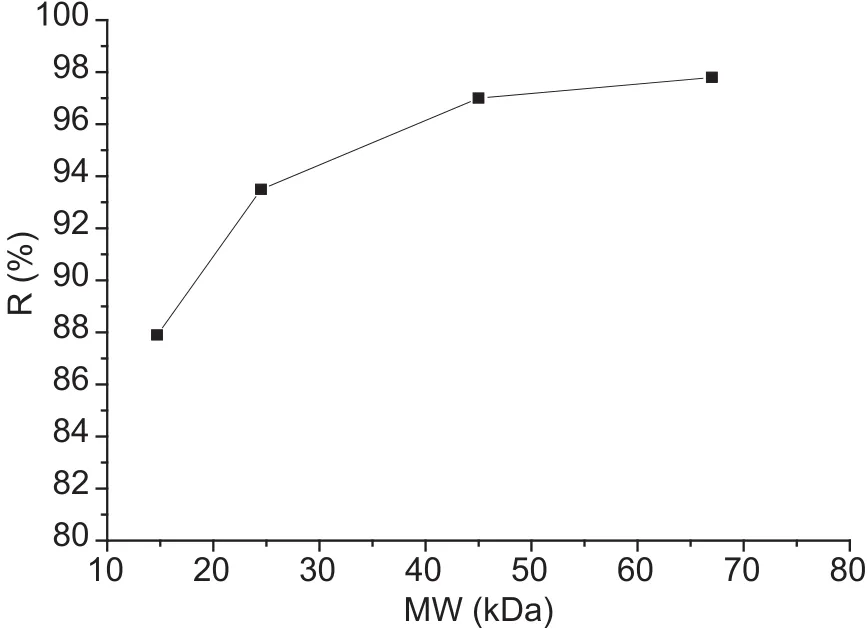
Fig.4 Observed retention of the composite membrane.R,rejection rate.MW, molecular weight.

Fig.5 Water flux of the composite membrane.

Fig.6 LC-MS/MS chromatograms of the 15 industry dyes.MRM, multiple reaction monitor.
LOD and LOQThe LOD and LOQ were determined as the concentration of analyte giving a ratio of S/N of 3 and 10 for the target ion, respectively.The LOD and LOQ values for the 15 industrial dyes in millet and corn flour are shown in Table 2.LOD ranged from 0.01 to 1.57 μg kg-1for millet and 0.01 to 1.01 μg kg-1for corn flour.LOQ ranged from 0.03 to 4.82 μg kg-1for millet and 0.03 to 3.15 μg kg-1for corn flour.
4.Discussion
The use of PES/MWCNTs composite ultrafiltration membrane as a cleanup method led to satisfactory precision,accuracy, selectivity, and recoveries in cleaning up illegal,industrial dyes in food.The following steps occur when using the PES/MWCNTs composite membrane as a cleanup method in the detection of illegal dyes in foods:
First, because there are a variety of pore sizes in the membrane, the composite membrane has the ability to separate and filter certain compounds.When the food matrix passes through the PES/MWCNTs composite membrane,some compounds in foods pass through the membrane,and some are preserved by the membrane.This allows for removal of a large amount of sulfur-containing compounds in foods that may interfere with the matrix in the detection of illegal dyes; see the filtration and adsorption step in Fig.1.
Second, MWCNTs in the PES/MWCNTs composite membrane have a strong selective adsorption effect on the 15 industrial dyes we tested.When the food matrix passes through the composite membrane, the industrial dyes in the food are adsorbed by the composite membrane while other components are removed by the membrane, as shown in filtration and adsorption step in Fig.1.
Third, the membrane was eluted with acetone, acetonitrile, methanol, and n-hexane in turn.A total of 15 kinds of industrial dyes are eluted from the composite membrane by the eluents with different polarities, as shown in the elution step in Fig.1.
Finally, the eluent is detected by the liquid chromatograph with the massspectrometric detector.
5.Conclusion
The PES/MWCNTs composite ultrafiltration membrane was made using the phase-inversion method and represented a new method for the analysis of illegal and industrial dyes in food.A total of 15 industrial dyes in millet and corn flourwere detected using the PES/MWCNTs composite ultrafiltration membrane filtration cleanup method for the adsorption of industrial dyes by MWCNTs.This procedure had satisfactory precision, accuracy, and selectivity, with recoveries ranging from 75-110% and RSD values below 15%.The procedure with the composite membrane used as the cleanup method takes 30 min or less, which is considerably shorter than traditional methods that take around 6-8 h.This cleanup method was proven to be rapid and effective.In conclusion,the PES/MWCNTs composite ultrafiltration membrane is a sensitive method of analysis for industrial dyes in food at trace levels for sample cleanup.
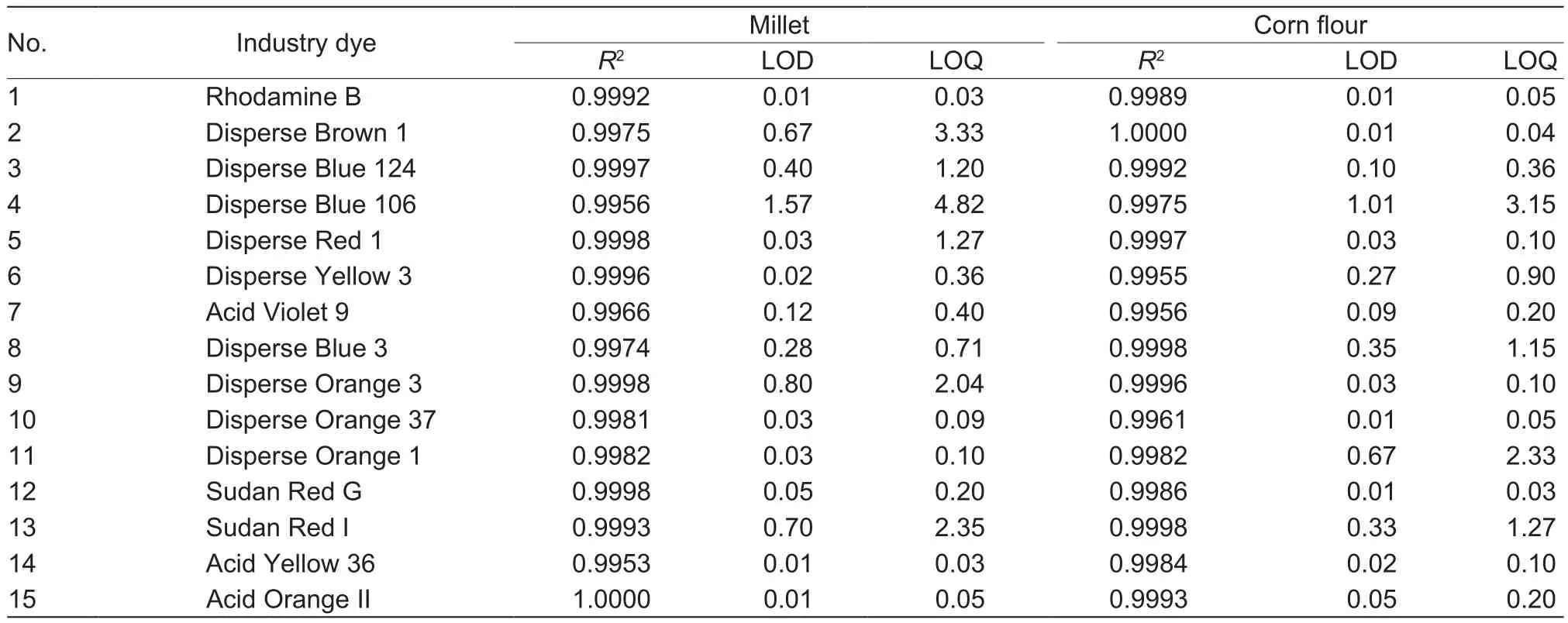
Table 2 Calibration curve coefficients (R2), limits of determination (LOD) (μg kg-1), and limits of quantification (LOQ) (μg kg-1) for 15 industrial dye standards
Acknowledgements
The study was supported by the Fund of Key Projects of Higher Education in Henan Province, China (17A550018)and the Fund of Henan Province Science and Technology Research Project, China (172102310314).
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
Augustine G J, Levitan H.1980.Neurotransmitter release from a vertebrate neuromuscular synapse affected by a food dye.Science, 207, 1489-1490.
Beltran J, López F J, Hernández F.2000.Solid-phase microextraction in pesticide residue analysis.Journal of Chromatography (A), 885, 389-404.
Du D, Wang M, Zhang J, Cai J, Tu H, Zhang A.2008.Application of multiwalled carbon nanotubes for solid-phase extraction of organophosphate pesticide.Electrochemistry Communications, 10, 85-89.
El-Sheikh A H, Sweileh J A, Al-Degs Y S, Insisi A A, Al-Rabady N.2008.Critical evaluation and comparison of enrichment efficiency of multi-walled carbon nanotubes, C18 silica and activated carbon towards some pesticides from environmental waters.Talanta, 74, 1675-1680.
Gestel T V, Vandecasteele C, Buekenhoudt A.2002.Alumina and titania multilayer membranes for nanofiltration preparation, characterization and chemical stability.Journal of Membrrane Science, 207, 73-89.
Gonzalez M, Miglioranza K S, Aizpún de Moreno J E, Moreno V J.2005.Evaluation of conventionally and organically produced vegetables for high lipophilic organochlorine pesticide (OCP) residues.Food and Chemical Toxicology,43, 261-269.
Iijima S.1991.Helical microtubules of graphitic carbon.Nature,354, 56-58.
Kawaguchi M, Ito R S, KNakazawa H.2006.Novel stir bar sorptive extraction methods for environmental and biomedical analysis.Journal of Pharmaceutical &Biomedical Analysis, 40, 500-508.
Lv Z Q.2015.Industrial dyes in food and its hazards.Healthy,32, 25-27.
Peiperl M D, Prival M J, Bell S J.1995.Determination of combined benzidine in FD&C Yellow No.6 (Sunset Yellow FCF).Food and Chemical Toxicology, 10, 829-839.
Ravelo-Pérez L M, Hernández-Borges J, Rodríguez-Delgado M A.2008.Multi-walled carbon nanotubes as efficient solidphase extraction materials of organophosphorus pesticides from apple, grape, orange and pineapple fruit juices.Journal of Chromatography (A), 1211, 33-38.
Richter B E, Jones B A, Ezzell J L, Porter N L.1996.Accelerated solvent extraction: A technique for sample preparation.Analytical Chemistry, 68, 1033-1039.
Rowe K S.1988.Synthetic food colourings and ‘hyperactivity’: A double-blind crossover study.Australian Paediatric Journal,24, 143-147.
Uematsu Y, Mizumachi T, Monma K.2017.Simultaneous analysis of oil-soluble, basic, and acidic illegal dyes in foods using liquid chromatography-diode-array detection.Journal of AOAC International, 100, 1102-1109.
Vas G V.2004.Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis.Journal of Mass Spectrometry, 39, 233-237.
Wang S, Peng Z, Min G, Fang G Z.2007.Multi-residue determination of pesticides in water using multi-walled carbon nanotubes solid-phase extraction and gas chromatographymass spectrometry.Journal of Chromatography (A), 1165,166-171.
Yang C C, Li Y J, Liou T H.2011.Preparation of novelpoly(vinyl alcohol)/SiO2nanocomposite membranes by a solgel process and their application on alkaline DMFC.Desalination, 276, 366-372.
Zhao P, Wang L, Jiang Y, Zhang F, Pan C.2012.Dispersive cleanup of acetonitrile extracts of tea samples by mixed multiwalled carbon nanotubes, primary secondary amine, and graphitized carbon black sorbents.Journal of Agricultural & Food Chemistry, 60, 4026-4033.
Zhao P Y, Huang B Y, Gu K J, Zou N P, Pan C P.2015.Analysis of triallate residue and degradation rate in wheat and soil by liquid chromatography coupled to tandem mass spectroscopy detection with multi-walled carbon nanotubes.International Journal of Environmental Analytical Chemistry,95, 1-11.
Zhou Q X, Xiao J P, Wang W D, Liu G G, Shi Q Z, Wang J H.2006.Determination of atrazine and simazine in environmental water samples using multiwalled carbon nanotubes as the adsorbents for preconcentration prior to high performance liquid chromatography with diode array detector.Talanta, 68, 1309-1315.
杂志排行
Journal of Integrative Agriculture的其它文章
- Complete genome sequences of four isolates of Citrus leaf blotch virus from citrus in China
- The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep)
- Optimal storage temperature and 1-MCP treatment combinations for different marketing times of Korla Xiang pears
- Estimation of irrigation requirements for drip-irrigated maize in a sub-humid climate
- Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize-soybean relay intercropping system
- Effects of Bupleurum extract on blood metabolism, antioxidant status and immune function in heat-stressed dairy cows
