Heart Failure with Preserved Ejection Fraction:Time to Revisit the Stiff Heart
2018-02-28JuanVilaroMDMustafaAhmedMDandJuanArandaJrMD
Juan R. Vilaro, MD , Mustafa Ahmed, MD and Juan M. Aranda Jr. MD
1 Division of Cardiovascular Medicine, University of Florida, Gainesville, FL 32610, USA
Abstract In the current era of cardiovascular disease, the diagnosis of heart failure with preserved ejection fraction (HFpEF) is a well-recognized clinical entity that is equally prevalent but distinctly different from heart failure with reduced ejection fraction (HFrEF). Despite normal EF patients with this disease have similar morbidity and mortality rates compared with HFrEF, as well as a rising rate of hospitalizations. The pathophysiology of HFpEF is incompletely understood. The number of therapies with proven eff cacy at improving longterm cardiovascular outcomes is limited. Women with heart failure syndromes, particularly the elderly, are much more likely to have a HFpEF phenotype at the time of their diagnosis. The purpose of this paper is to review the epidemiology, pathophysiology, clinical features, and current management strategies in HFpEF, especially as it pertains to women.
Keywords: heart failure; sex differences; women; epidemiology; pathophysiology; mortality
Introduction
In the current era of cardiovascular disease, the diagnosis of heart failure with preserved ejection fraction (HFpEF) is a well-recognized distinct clinical entity. It has specif c fundamental epidemiologic,pathophysiologic, and phenotypic dif ferences compared with heart failure with reduced ejection fraction (HFrEF). Multiple studies have demonstrated a rising prevalence of this condition and an increasing number of HFpEF hospitalizations nationwide [ 1, 2].Mortality and hospital readmission rates in HFpEF are comparable to those in HFrEF, and both conditions remain an equally unmet medical and f nancial need in cardiology today [ 2 - 4]. Currently there are no sex-specif c implications regarding the evaluation and management of HFpEF in women compared with men. However, women with heart failure, especially elderly women, are much more likely to have the HFpEF phenotype than the HFrEF phenotype, a notable concern in light of our aging population [ 5]. There are a limited number of evidence-based therapies capable of reducing the morbidity and mortality associated with this condition. We therefore include this article among others in this issue of Cardiovascular Innovations and Applications focused on cardiovascular disease in women. The purpose of this article is to review the epidemiology, pathophysiology, clinical features, and current management strategies in HFpEF, especially as it pertains to women.
Epidemiology/Risk Factors
The most recently published lar ge-scale epidemiologic data sets for HFpEF are relatively outdated[ 6, 7]. However, studies consistently show a steadily increasing trend in the overall prevalence of HFpEF and the number of HFpEF hospitalizations in the United States [ 1, 2]. Moreover, the proportion of admissions of people with HFpEF compared with HFrEF is increasing, and HFpEF is expected to become the more common phenotype of the two. Combined mortality and readmission rates at 60 and 90 days have been shown to be essentially the same for HFpEF and HFrEF, 35.3 and 36.1%,respectively [ 6 ].
There are major sex-specif c differences in the prevalence and incidence of HFpEF. Studies estimate that up to twice as many women have HFpEF compared with men, a statistic that sharply contrasts with the male predominance observed in HFrEF [ 5,8 , 9 ].
The risk of HFpEF increases sharply with age.Additional risk factors such as hypertension, diabetes, obesity, COPD, and chronic renal insuff ciency not only contribute to heart failure hospitalization but also increase stif fness, creating mayor impairments to myocardial relaxation and f lling. HFpEF has been shown to be a disease that coexists with other comorbid conditions more frequently than HFrEF. Up to 50% of patients with HFpEF have f ve or more comorbid conditions. While the allcause mortality rates for both HFpEF and HFrEF are similar, the rate of noncardiovascular death is signif cantly higher in HFpEF compared with HFrEF.Moreover, the greater the number of comorbidities present, the higher the mortality rate in HFpEF.
Diagnosis
Because of the nonspecif c nature of the signs and symptoms of HFpEF, establishment of a true diagnosis presents many challenges. The patient history, physical examination results, serum natriuretic peptide levels, electrocardiogram, chest radiograph, transthoracic echocardiogram, and invasive hemodynamics by right-sided heart catheterization(RHC) can all provide supporting evidence for the diagnosis of HFpEF, but each has its limitations.
Current guidelines recommend that this diagnosis be considered in patients who have a clinical syndrome of heart failure (i.e., signs and symptoms of congestion and/or impaired cardiac output) with a left ventricular ejection fraction (LVEF) of greater than 50% [ 10]. This should be supported by abnormal brain natriuretic peptide levels and evidence of structural heart disease or abnormal diastolic function. The diagnosis of HFpEF should not be made in an asymptomatic patient regardless of abnormalities on other testing (i.e., diastolic dysfunction on transthoracic echocardiogram or elevated natriuretic peptide levels in isolation). The LVEF cutoff justifying a label of HFpEF versus HFrEF has historically been a subject of debate. In earlier proposed def nitions, an L VEF greater than 40% was accepted for the diagnosis of HFpEF. However, there is now a designation known as heart failure with midrange ejection fraction, intended to classify patient with LVEF between 41 and 49% [ 10]. These distinctions are highly relevant as new data continue to emer ge regarding the dif ferent pathophysiology of these subgroups, as well as potential dif ferences in their response to specif c therapies.
There are numerous conditions that can lead to hear t failure syndromes, even in the setting of a preserved ejection fraction, and these must be excluded before a true diagnosis of HFpEF is made. These include constrictive pericarditis, inf ltrative cardiomyopathies such as amyloidosis, genetic disorders of the myocardium such as hypertrophic cardiomyopathy, and any heart failure secondary to severe valvular disease.T able 1 outlines common alternative diagnoses that are clinically similar to HFpEF but that should not be included in the def nition of HFpEF.
Once a clinical syndrome of heart failure is suspected and LVEF is noted to be greater than 50%,and after exclusion of the aforementioned alternative diagnoses, a diagnosis of HFpEF is supported by certain f ndings on a variety of additional laboratory, imaging, or invasive tests. There are multiple pitfalls in every diagnostic modality available, and these can lead to both the overdiagnosis of HFpEF when it is not truly present and the erroneous exclusion of this condition when it is in fact the correct diagnosis. The most relevant to our practice are outlined in the following sections.
Natriuretic Peptide Levels
We now know that a family of hormones called natriuretic peptides are released in response toincreased atrial and ventricular myocardial stretch and wall stress that results from the elevated intracardiac pressures in heart failure patients, with the purpose of restoring sodium and f uid homeostasis [ 11]. The two most commonly used natriuretic peptide assays measure serum levels of B-type natriuretic peptide (BNP) and N-terminal proBNP.Both have established diagnostic and prognostic value in heart failure patients, with higher levels making the diagnosis of heart failure more likely,and in those with an existing heart failure diagnosis, higher levels suggest a worse prognosis [ 12].However, there are some important distinctions in natriuretic peptide interpretation when it comes to HFpEF.
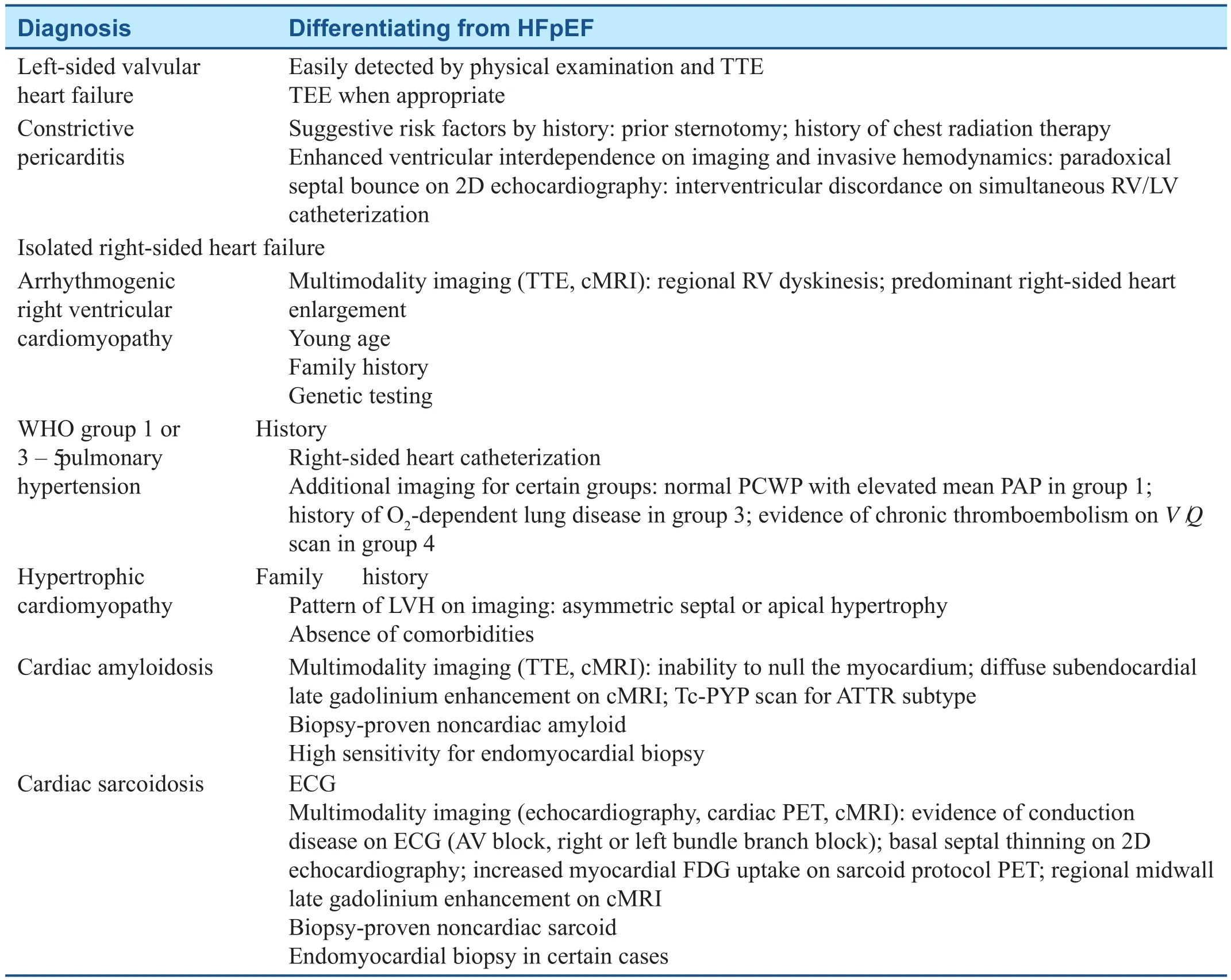
Table 1 Diagnoses that Should not be Included in the Def nition of Heart Failure with Preserved Ejection Fraction (HFpEF).
HFpEF patients have lower average natriuretic peptide levels compared with HFrEF patients, even after adjustment for other markers of congestion and disease severity [ 13]. There are likely several reasons for this. HFpEF patients on average have signif cantly smaller left ventricular dimensions and are more likely to have left ventricular hypertrophy. On the basis of Laplace ’ s principle, the left ventricle in HFpEF is subject to signif cantly lower degrees of left ventricular wall stress, one of the major triggers for natriuretic peptide release [ 14].In addition, natriuretic peptides are highly metabolized in adipose tissue. As a result, obesity, which itself is a major risk factor for HFpEF, is a frequent cause of false negative natriuretic peptide results in these patients [ 15].
Conversely, an elevated natriuretic peptide level is nonspecif c and can be seen in the absence of HFpEF. Alternative causes of elevated natriuretic peptide levels that should be ruled out before a diagnosis of HFpEF is conf rmed include atrial f brillation, chronic renal insuff ciency, hypertensive urgency, pulmonary embolism, sepsis, myocardial infarction, and anemia.
Echocardiography
Transthoracic echocardiography provides an integral comprehensive assessment of cardiac structure and function that extends far beyond estimation of LVEF. Like most diagnostic tools, it can identify numerous f ndings that are suggestive, but not unequivocally diagnostic, of HFpEF. Left ventricular hypertrophy and left atrial enlargement are the most common structural abnormalities in HFpEF but are not always present, and image quality often limits accurate measurements [ 16].
Diastolic function assessment is essential but complex, and often leads to indeterminate results because of discrepancies between the di fferent parameters that are measured. A comprehensive review of diastolic function assessment with echocardiography is beyond the scope of this article; however,current society recommendations are readily available and should be used when needed if a diagnosis of HFpEF is suspected [ 17].
Most indices of diastolic function use blood velocity measurements obtained with Doppler imaging to provide either quantitative or qualitative estimations of intracardiac pressures. As discussed later, many HFpEF patients have normal hemodynamics at rest and may not demonstrate the expected abnormalities needed to conf rm the diagnosis, especially if they are compensated and euvolemic at the time of the examination. Parameters subject to this limitation include mitral inf ow velocity patterns, E / e ’ ratio for pulmonary capillary wedge pressure (PCWP) estimation, pulmonary artery pressure estimations by tricuspid and pulmonic regur gitation Doppler jets,pulmonary vein Doppler f ow patterns, and inferior vena cava diameter.
Mitral annular velocity measurement by tissue Doppler imaging (TDI) is potentially exempt from this diagnostic limitation, as it is a velocity measurement of actual myocardial relaxation as opposed to blood f ow. In patients with true diastolic dysfunction, mitral annular recoil from the apex to the base after systolic contraction is slow and ineff -cient. Therefore TDI values should be abnormally low in HFpEF regardless of loading conditions or volume status [ 18]. However, a wide range of normal values for TDI are reported, with additional variations def ned depending on age and sex; hence no single cutoff value exists to reliably conf rm or exclude diastolic dysfunction by myocardial annular velocity alone [ 17].
Newer echocardiographic imaging techniques,specif cally myocardial strain imaging with speckle tracking software, may increase the sensitivity of echocardiography for diagnosing HFpEF. Evaluation of myocardial function with this parameter shows that longitudinal and circumferential systolic strain is signif cantly impaired in HFpEF patients compared with healthy controls, despite no dif ferences in LVEF [ 19]. Additionally, myocardial contraction fraction, which is the ratio of left ventricular stroke volume to myocardial volume, has also been shown to be a more sensitive index of systolic function that becomes impaired in HFpEF [ 20, 21].
The Role of Right-Sided Heart Catheterization
From a hemodynamic standpoint, all heart failure syndromes are characterized by an abnormally reduced cardiac output, elevations in diastolic f lling pressures leading to systemic and pulmonary venous congestion, or a combination of both.Invasive hemodynamic assessment by RHC remains an invaluable test in the evaluation of patients with suspected HFpEF. In patients with symptoms of heart failure and a preserved ejection fraction, an elevated PCWP and/or the presence of pulmonary venous hypertension as assessed by RHC strongly supports a diagnosis of HFpEF. Invasive RHC is the only test that can def nitively distinguish pulmonary hypertension secondary to HFpEF (i.e., World Health Organization group 2 pulmonary hypertension) from other World Health Organization classif cations that should not be included in the de fnition of HFpEF.
There is, however, a fundamental limitation to routine RHC, in that all measurements are typically obtained with the patient at rest, and ideally when the patient is clinically euvolemic. We now know that in many patients with HFpEF pulmonary artery pressure and PCWP may appear normal at rest,but increase dramatically with a moderate exer-cise challenge [ 22]. This hemodynamic response to exertion allows us to understand the profound activity intolerance that is so characteristic of this condition. Therefore in patients with suspected HFpEF who have normal f ndings on RHC at rest, the next diagnostic step should be exercise hemodynamics so as to maximize the sensitivity of hemodynamic testing.
Pathogenesis
The pathobiology of HFpEF is complex and incompletely understood. Initially, proposed mechanisms emphasized the concept of myocardial afterload excess secondary to arterial hypertension being the main insult by which HFpEF develops [ 23].This theory was supported by the extremely high prevalence of systemic hypertension in the HFpEF population and numerous studies suggesting hypertension is a leading risk factor for HFpEF. In addition to this, pathways demonstrating the adverse myocardial dysfunction and remodeling secondary to hypertensive heart disease are well described.
There are signif cant sex-based dif ferences in myocardial remodeling patterns that may explain why women are more likely to develop HFpEF.Compared with men, women exhibit a more signif -cant degree of concentric hypertrophy in response to long-term increases in afterload, whereas men are more likely to develop eccentric hypertrophy in response to similar myocardial load alterations [ 24,25]. Men also demonstrate increases in left ventricular chamber size with aging, whereas women do not [ 26]. The distinct left ventricular structural changes observed in women compared with men may create greater impairments in both systolic and diastolic function as well as more limitations to cardiac reserve during stress [ 27].
In the last 5 years a new paradigm for the development of HFpEF has been proposed, which identif es a systemic proinf ammatory state created by comorbidities leading to coronary microvascular endothelial dysfunction as the underlying cause, as opposed to excessive afterload from hypertension ( Figure 1 )[ 28 ]. Paulus and Tsch ö pe [ 28 ] highlighted several compelling points supporting this pathway. They noted that lar ge registry data show HFpEF to be more strongly associated with several comorbidities than with arterial hypertension [ 29]. Additionally,in HFpEF patients cardiomyocyte stif fness is not restricted to the left ventricle, but rather involves all four chambers, and in many cases is seen even in the absence of signif cant arterial hypertension [ 30].

Figure 1 Comorbidities Drive Myocardial Dysfunction and Remodeling in Heart Failure with Preserved Ejection Fraction.
This “ multimorbidity” theory, as it has been labeled by leading authors in the f eld [ 2 ], f ts well with what data have shown in the HFpEF population. There is an overwhelmingly high prevalence of comorbidities in HFpEF, and a consistent observation in the literature that an increasing number of comorbidities identif es the highest-risk patients[ 31]. However, the specif c comorbidity prof le for each patient differs, and while most data analyses generally adjust the data for the presence or absence of certain conditions, this is mostly done in a binary fashion to facilitate statistical analyses. Most of these comorbidities, including COPD, renal insuff ciency, diabetes, and obesity, have a severity spectrum, and each of them independently correlates with long-term risk of death, and it is extremely diff cult to adjust the data appropriately when statistical analyses are performed. Understanding the magnitude of this heterogeneity, we can view HFpEF as a common phenotypic end result for patients with overlapping comorbidities who are otherwise quite different from each other. From this perspective, we might understand the relative inability to identify single therapies with proven benef t when applied to the HFpEF population as a whole.
Pathophysiology
The pathophysiology of HFpEF is related to multifactorial issues [ 32]. During systolic contraction,left ventricular twist forces mitral annular motion toward the apex. During early diastole the untwisting process and recoil generate negative intraventricular pressure, gradient, or suction, causing rapid motion of the annulus back to the base of the heart.This aids ventricular f lling by moving the annulus around the incoming column of blood.
Patients with HFpEF develop arterial stiffness,diabetes, and left ventricular hypertrophy, all of which contribute to myocardial f brosis. Although overall ventricular function is normal, there are regional systolic issues that affect strain and relaxation. There is delayed left ventricular untwisting that leads to decreased ventricular suction, causing less recoil of the annulus. This affects early diastolic f lling, requiring increased late diastolic f lling from atrial contraction. With exercise, diastole shortens further, increasing end-diastolic pressure, contributing to breathlessness on exercise.
An increasing number of studies now implicate a more global degree of cardiac dysfunction in HFpEF that extends beyond sole diastolic impairments [ 33]. Chronotropic incompetence, impaired vasodilation, and ineff cient ventriculoarterial coupling are all associated with HFpEF, each of which can contribute to the profound exertional limitations classically described by these patients [ 34 - 36 ].
Management
In addition to all of the diagnostic uncertainties that must be reconciled in HFpEF before an accurate diagnosis is made, the management of these patients can be equally challenging. The goals of care are fundamentally similar to those in patients with HFrEF: reduce symptoms, improve quality of life, and prevent adverse events such as death and all-cause hospitalizations.
Acute HFpEF Management
In HFpEF there are distinct derangements in car-diac pressure-volume relationships that must be understood so as to safely achieve cardiopulmonary decongestion and hemodynamic optimization.One of the central dif ferences between HFpEF and HFrEF is the hemodynamic responses observed with vasodilator therapy. Although HFpEF patients have signif cantly higher arterial blood pressure compared with HFrEF patients, the pressure-volume loops in HFpEF show only a modest increase in stroke volume in response to acute vasodilation, whereas in HFrEF stroke volume response to vasodilation is much more robust [ 37]. It is crucial to understand this to minimize the risk of iatrogenic hypotension and kidney injury in acute HFpEF management, as the risk of postdischarge death increases when hospitalizations are complicated by these events [ 38].
This iatrogenic risk is also present when diuretics are used to achieve optimal volume status in hospitalized HFpEF patients. Achieving clinical euvolemia remains one of the major treatment goals and most ef fective ways to alleviate symptoms.However, the mechanisms of cardiopulmonary f uid overload in HFpEF are complex, and they are not merely a result of increased total blood volume.Decreased splanchnic circulation capacitance and impaired baroreceptor function both contribute to sudden mobilizations of blood volume from the splanchnic circulation into the cardiopulmonary system in HFpEF patients. This inappropriate and exaggerated volume redistribution can occur with any trigger of sympathetic activation, such as exercise or transient hypoxia. Combined with reduced cardiac chamber and pulmonary vascular compliance, this volume of blood redirected from the splanchnic vascular bed to the heart and lungs is a setup for overload and clinical decompensation[ 39]. While afterload reduction and diuresis are necessary in this scenario, it is important to recognize the inherent risk of overdiuresis given many HFpEF patients have only modest total body hypervolemia.Moreover, understanding the optimal rate of diuresis in these patients is equally important. Many clinicians use rising serum blood urea nitrogen and creatinine levels as the main end point for determining when patients have been diuresed to euvolemia.In HFpEF this is an oversimplif ed approach with many pitfalls. Various degrees of preload dependence, plasma ref ll rates, and degrees of renal vein congestion can all cause transient worsening in renal function in HFpEF patients, even when there is persistent congestion [ 40]. Given that persistent congestion on the day of dischar ge identif es one of the highest-risk subgroups among heart failure patients, every effort must be made to conf rm that euvolemia has been truly achieved. When bedside assessment of volume status is in question, intracardiac pressure estimation by Doppler echocardiography or invasive RHC can better characterize hemodynamics and verify euvolemia.
Managing the Chronic HFpEF Patient
The wealth of data that exist demonstrating improved outcomes in HFrEF with aggressive neurohormonal blockade of the renin-angiotensin-aldosterone and sympathetic nervous systems is almost nonexistent in HFpEF. Primary outcomes in numerous clinical trials testing the eff cacy of beta-blockers, ACE inhibitors, and angiotensin receptor blockers have all had negative primary end points [ 5, 41, 42].
There is one notable exception to the long-term trend of negative clinical trial results for pharmacotherapy in HFpEF: the TOPCAT trial [ 43]. This was a randomized, double-blinded multicenter trial testing the eff cacy of spironolactone versus placebo in HFpEF. The signif cance of this trial ’ s f ndings are subject to major individual interpretation, especially with subsequent data that recently emerged regarding signif cant regional variability in the results of the study [ 44]. The original trial reported no signif -cant differences in the primary composite end point of cardiac death, cardiac arrest, or hospitalization for heart failure. However, the secondary end point of heart failure hospitalizations was signif cantly less in the spironolactone arm. This established a potential role for this spironolactone in HFpEF considering that reduction in HFpEF admissions is a large unmet need in clinical practice.
The TOPCAT investigators noted, however, that for patients enrolled in Russia and Georgia, the primary outcome rates were approximately four times lower than for patients enrolled in sites in the United States, Canada, Brazil, and Argentina (the Americas). Additionally, patients enrolled in Russia and Georgia were younger, had less atrial f brillation and diabetes, and were more often enrolled on the basis of a prior hospitalization for heart failure,as opposed to having an elevated serum natriuretic peptide level. The unusually low event rate in Russia and Geor gia, coupled with subsequent data showing undetectable spironolactone metabolites in a signif cant portion of patients enrolled in these sites [ 45], has led many to question not only whether HFpEF was truly present in these patients but also whether the drug was appropriately administered. Post hoc analysis demonstrated that if trial data from the sites in Russia and Georgia sites were excluded, there was in fact a signif cantly lower rate of the primary outcome in the spironolactone group for patients enrolled in the Americas. The extensive data analyses from the TOPCAT trial created the basis by which current guidelines formally recommend use of spironolactone in HFpEF [ 46].
It is crucial to remember, however, that the risk of hyperkalemia increases with spironolactone use[ 47], and spironolactone should not be used if serum potassium levels are above 5 mEq/L or if creatinine levels are above 2.5 mg/dL. Moreover, serum potassium and creatinine levels should be rechecked 48 h and 1 week after initiation of spironolactone therapy to monitor the patient for these complications.
The role of combined angiotensin receptor blockade and neprilysin inhibition has not been fully explored in HFpEF. The combination of sacubitril and valsartan appears to be safe in this population, and produces greater reduction in natriuretic peptide levels than does valsartan alone [ 48]. The PARAGON-HF trial is ongoing and aims to prospectively determine whether this ef fect translates into signif cant reductions in adverse cardiovascular outcomes in HFpEF [ 49].
Invasive Hemodynamic Monitoring
A major challenge in the management of all heart failure patients, including HFpEF patients, is early detection of cardiopulmonary congestion, which is the most common cause of symptoms and hospital admissions. There are now data demonstrating that right-sided heart pressures begin to increase for up to 5 days before the onset of the congestive symptoms seen in clinical decompensation [ 50].This has led to the investigation of invasive hemodynamic monitoring devices that can prompt an earlier adjustment of therapy to prevent symptom worsening and hospitalizations.
The most promising data with this strategy were demonstrated in the CHAMPION trial by Abraham et al. [ 51]. In this trial heart failure patients,regardless of LVEF, were randomized to receive physician-guided clinic-based management alone or physician-guided clinic-based management in combination with a wireless implantable hemodynamic monitor called CardioMEMS. This is a permanent device implanted by a percutaneous approach into the main pulmonary artery that continuously monitors pulmonary artery pressure.In addition to routine clinic visits, patients in the device arm also performed periodic transmissions of the device recording, which were used to guide adjustments in their medical therapy according to prespecif ed algorithms. Most adjustments that occurred were changes in diuretic dosing, although vasodilator therapy was sometimes adjusted as well. The trial demonstrated that hospitalization rates were signif cantly reduced in the CardioMEMS group, without a signif cant increase in procedural complication rates. Subgroup analysis of the patients with L VEF greater than 40% (1 19 of 550 total patients) showed the hospitalization rate was signif cantly lower in the CardioMEMS-guided treatment arm [ 52].
The CHAMPION trial identif ed an opportunity to adjust therapy in HFpEF patients before the onset of clinical decompensation; however, it is important to understand the trial design if we are to extrapolate this therapy to our practice and expect similar results. The key to optimal use of hemodynamic monitoring is ensuring that the data collected can be responded to reliably and in real time. This can be accomplished only in disease management programs with suff cient infrastructure to assign a provider a dedicated role for this purpose. Without this,the window to provide timely recommendations to the patient closes, and the frequency of hospitalizations or emergency department visits will not likely change. In addition, while there was in fact no signif cant increase in adverse events related to the device implant itself, there is a learning curve for every invasive procedure. Complications rates are ultimately operator dependent and might still be higher than that published for the trial.
Targeting Comorbidities: The Future of HFpEF Management
As previously mentioned, the overwhelming majority of HFpEF patients have several comorbid conditions that complicate their management and constitute a major obstacle to achieving signif cant reductions in the rates of adverse events such as death or hospitalization. The comorbidity prof le of each patient and the severity of each comorbid condition differ widely and therefore create signif -cant heterogeneity within the HFpEF population. It is reasonable therefore to consider an individualized approach to the HFpEF patient, tar geting the comorbidities that seem severest or most clinically relevant. Despite the lack of randomized multicenter trials supporting certain treatment options,there is likely still a role for symptom management and improving outcomes in HFpEF subgroups with a particularly disabling comorbidity.
There is an extensive physiologic rationale for pursuing a rhythm control strategy as opposed to rate control for atrial f brillation in HFpEF.Tachycardia, rhythm irregularity, and the absence of atrial contraction all exacerbate the intrinsic impairment in diastolic f lling and can worsen congestion and exertional limitations. Data show that right ventricular function, which itself is a powerful prognostic marker in HFpEF, is signif cantly better in HFpEF patients in sinus rhythm compared with those with atrial f brillation [ 53 , 54 ].
Coronary revascularization is also an option to consider if HFpEF coexists with obstructive coronary artery disease. Single-center data have shown that complete revascularization in HFpEF patients is associated with less deterioration of cardiac function, and possibly reduced mortality, than in unrevascularized or incompletely revascularized HFpEF patients. Retrospective analysis showed mortality rates with complete revascularization did not signif cantly differ from those in patients who had no obstructive coronary artery disease [ 55].
Diabetes is very common in HFpEF, and recent trials suggest that certain strategies for glycemic control, specif cally with sodium/glucose cotransporter 2 (SGLT2) inhibitors, may improve heart failure outcomes in these patients [ 56, 57]. The two largest trials reporting reduction in heart failure events with SGLT2 inhibitors did not specify whether the patients had HFrEF or HFpEF. However, a potential benef t from these agents, specif cally in HFpEF patients, is still hypothesized given the high rates of HFpEF observed in the diabetic population [ 58].
Obstructive sleep apnea (OSA) is very common in HFpEF, and while no randomized trial has yet demonstrated a reduction in outcomes in HFpEF with nocturnal positive pressure use, there are data suggesting the presence of OSA identif es a higherrisk HFpEF subgroup. In addition, the deleterious cardiovascular effects of untreated OSA are extensive, and very well described. Therefore we recommend screening all HFpEF patients for OSA, formal sleep studies when indicated, and if needed, referral to sleep medicine specialists for optimal treatment.
The 2017 heart failure guidelines for HFpEF focus on treatment of comorbid conditions. Treatment of hypertension, diuretics for volume overload, treatment of symptoms of coronary artery disease, and control of atrial f brillation are emphasized. Use of aldosterone antagonists along with angiotensin receptor blockers in this patient population is associated with reduction in heart failure hospitalizations. Of note, the use of nutritional supplements and phosphodiesterase inhibitors has no benef t in HFpEF [ 46 ].
Conclusion
HFpEF is a disabling condition with rising prevalence yet incompletely understood disease mechanisms, pathophysiology, and treatment. Ef forts to better characterize underlying causes and optimal management strategies are evolving rapidly, and must continue to reduce the staggering morbidity and mortality rates created by this disease. While there are a few options with evidence suggesting benef t in every patient, it seems that HFpEF is truly an entity where an individualized approach to the comorbidity prof le of each patient promises the highest likelihood of treatment success.
Conflict of Interest
The authors declare no conf ict of interest.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Challenges in Cardiovascular Risk Prediction and Stratification in Women
- Nonobstructive Coronary Artery Disease in Women: Risk Factors and Noninvasive Diagnostic Assessment
- Antiplatelet Therapy Considerations in Women
- Novel Imaging Approaches for the Diagnosis of Stable Ischemic Heart Disease in Women
- Psychosocial Stress, the Unpredictability Schema, and Cardiovascular Disease in Women
- Heart Disease in Pregnancy: A Special Look at Peripartum Cardiomyopathy