Antiplatelet Therapy Considerations in Women
2018-02-28KhadeejaEsmailMDandDominickAngiolilloMDPhD
Khadeeja Esmail, MD and Dominick J. Angiolillo, MD, PhD
1 University of Florida, College of Medicine, Jacksonville, FL, USA
Abstract Coronary artery disease (CAD) is the leading cause of death worldwide, but because of several factors, one of which is antiplatelet therapy, the mortality rates have steadily declined. However, women continue to experience higher CAD mortality rates than men. This may be explained by differences in comorbidities, increased time to presentation, higher bleeding rates, and differences in management. There are numerous landmark trials in the f eld of antiplatelet therapy;however, women are consistently underrepresented in these trials. The results of these trials reveal that women experience the same benef t as men from antiplatelet therapy but experience higher bleeding rates; therefore bleeding-reduction strategies are imperative in this patient population. This review provides an overview of the available evidence on CAD in women and its implications for antiplatelet medications.
Keywords: antiplatelet therapy ; women ; atherosclerosis
Introduction
Antiplatelet therapy is the mainstay of treatment for patients with manifestations of coronary artery disease (CAD) [ 1 ]( Figure 1 ). CAD is the leading cause of death in men and women worldwide and in the United States [ 2]. In 2010, one in six deaths in the United States was caused by CAD. Cardiovascularrelated health costs were approximately $315.4 billion in 2010. Every 31 seconds an American has a coronary event, and 83 seconds an American dies of CAD [ 3]. Traditionally CAD has been thought of as a “ man’ s disease ” but more women die of
CAD each year. In 2006, there were approximately 432,000 CAD deaths in women and 398,600 CAD deaths in men [ 4]. These statistics illustrate the need to understand potential differences in CAD in women and men. Although research in this f eld is robust, sex disparities still exist. Women are underrepresented in clinical trials, comprising on average 20 - 30% of enrollment [ 5 ]. This underrepresentation has implications for patient management that may potentially impact clinical outcomes. Indeed, differences in both ischemic and bleeding risk prof les according to sex make considerations for antiplatelet therapy of critical importance [ 6]. This review provides an overview of the available evidence on CAD in women and its implications for antiplatelet medications.
Basics of Atherosclerosis
Atherosclerosis is the major disease process of cardiovascular medicine [ 7]. It is a chronic inf ammatory process that begins in childhood and progresses throughout a person ’ s lifetime.Atherosclerotic plaques have two main components: a soft lipid-rich core and a hard collagenrich f brous cap. In stable plaques, the thick f brous cap stabilizes the plaque and prevents plaque rupture. Unstable plaques have a thin f brous cap and thus have a greater risk of rupture. During plaque rupture, the thin f brous cap ruptures, exposing the necrotic plaque core, which forms a blood clot. In plaque erosion, the endothelium lining the vessel is eroded, leaving a raw surface that is a nidus for thrombus formation [ 7]. It was previously believed that female patients with ST-segment-elevation myocardial infarctions (STEMIs) were more likely to have plaque erosion than plaque rupture. This theory was derived from pathology studies but not proven in vivo [ 8]. Optical Coherence Tomography Assessment of Gender Diversity in Primary Angioplasty (OCTAVIA) was a study designed to look at sex differences of culprit plaque using optical coherence tomography (OCT), histopathology,serum biomarkers, and immunochemistry. It was a prospective study of 140 patients with STEMIs,who underwent OCT of the infarct-related artery before percutaneous coronary intervention (PCI),after everolimus-eluting stent implantation, and at follow-up at 9 months. In this study there was no difference in plaque volume and makeup, and with OCT there were no differences in the proportion of ruptured or eroded plaques. On repeated OCT at 9 months, more than 90% of both women and men had fully covered stent struts [ 9]. This study demonstrated that women respond as well to primary PCI as men and should undergo the same treatment for STEMI.
Sex Differences in Platelet Biology
Animal and human studies have revealed d ifferences between men and women regarding platelet reactivity [ 10]. Genetic Study of Aspirin Responsiveness(GeneSTAR) demonstrated higher platelet reactivity among women than men in response to dif ferent concentrations of arachidonic acid, adenosine diphosphate (ADP), or epinephrine after adjustment for age, risk factors, race, menopausal status, and hormone therapy. However, after low-dose aspirin therapy, men and women showed similar degrees of platelet inhibition in response to arachidonic acid.After aspirin therapy, women ’ s platelets remained signif cantly more reactive than those of men in response to collagen or ADP stimulation [ 11]. Sex hormones may play a role in the differences in platelet reactivity. It has been postulated that estrogen has cardioprotective ef fects on the basis of the observation that the number of cardiovascular events increase after menopause and in women with premature ovarian failure [ 12]. Estrogen increases prostacyclin and nitric oxide production, inhibitors of platelet aggregation. On the basis of the cardioprotective benef ts of estrogen discussed, there has been interest in hormone replacement therapy for postmenopausal women. Although observational studies demonstrated a cardioprotective effect with hormone replacement therapy, larger clinical trials such as the Women’ s Heart Initiative trial and the Heart and Estrogen/Progestin Replacement Study did not demonstrate a reduction in CAD [ 13].
Antiplatelet Therapy
Aspirin
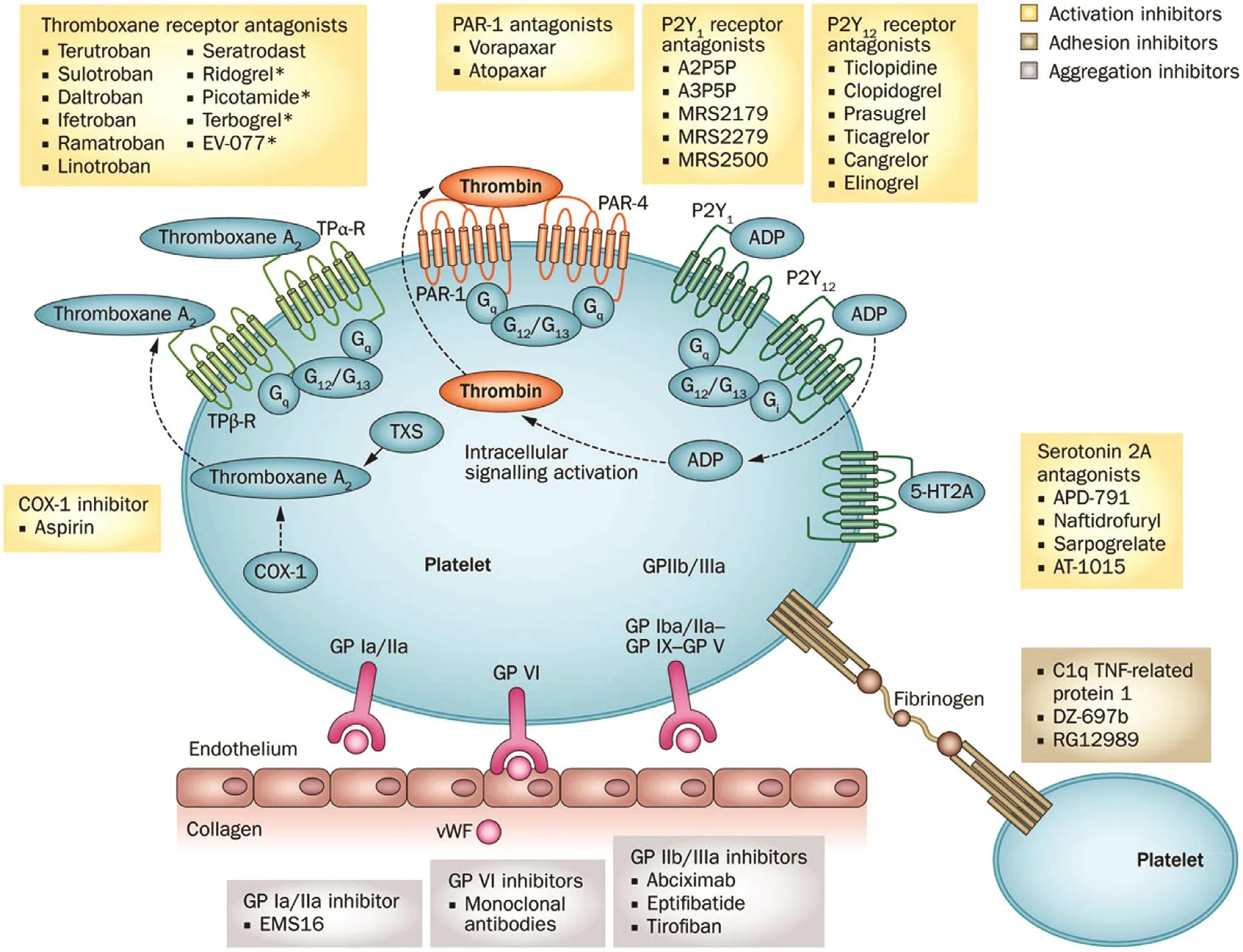
Figure 1 Receptors and Pathways Targeted by Antiplatelet Therapy.
Aspirin is the most broadly prescribed antiplatelet drug. It exerts its effect by selectively and irreversibly acetylating cyclooxygenase 1, blocking the formation of thromboxane A2synthesis in platelets( Figure 1 ). Thromboxane A2stimulates the activation of new platelets and increases platelet aggregation [ 14 ]. The Women ’ s Health Study evaluated the eff cacy and safety of aspirin by a 10-year follow-up for a f rst major cardiovascular event (nonfatal myocardial infarction [MI] or stroke or death from a cardiovascular cause) of 39,876 healthy women aged 45 years or older. Patients were randomly assigned to receive 100 mg of aspirin or placebo. Aspirin did not reduce the overall risk of major cardiovascular events, but the risk of stroke decreased (relative risk[RR] 0.83, 95% conf dence interval [CI] 0.69 - 0.99,P = 0.04). Aspirin had no effect on the risk of fatal or nonfatal MI (RR 1.02, 95% CI 0.84 - 1.25, P = 0.83)or death from cardiovascular causes (RR 0.95, 95%CI 0.74 - 1.22, P = 0.68). Aspirin therapy resulted in a 1.4-fold higher risk of gastrointestinal bleeding requiring transfusion (RR 1.40, 95% CI 1.07 - 1.83,P = 0.02) [ 15]. In a meta-analysis of six randomized trials, with a total enrollment of 95,456 patients,assessing primary prevention with aspirin therapy,there was a signif cant reduction in risk of cardiovascular events independent of sex. Aspirin reduced the odds of cardiovascular events by 12% in women(odds ratio [OR] 0.88, 95% CI 0.79 - 0.99, P = 0.03)and 14% in men (OR 0.86, 95% CI 0.78 - 0.94,P = 0.01). The benef t was driven by reduction of MI in men (OR 0.68, 95% CI 0.54 - 0.86, P = 0.01)and ischemic stroke in women (OR 0.76, 95% CI 1.35 - 2.20, P < 0.001). Aspirin treatment increased the risk of bleeding in women (OR 1.68, 95% CI 1.13 - 2.52, P = 0.01) and in men (OR 1.72, 95% CI 1.35 - 2.20, P < 0.001) [ 16]. On the basis of older research in this f eld, the US Preventive Services Task Force recommends low-dose aspirin use be started for the primary prevention of cardiovascular disease (CVD) in adults aged 50 - 59 years who have a 10% or greater 10-year CVD risk, do not have increased risk of bleeding, have a life expectancy of at least 10 years, and are willing to take low-dose aspirin daily for at least 10 years. The decision to start low-dose aspirin therapy for patients aged 60 -69 years should be individualized [ 17]. However,the current European guidelines, using data from more recent trials, do not endorse aspirin therapy for primary prevention of CVD [ 18]. However, these recommendations may change in light of recent studies on the role of aspirin in primary prevention.
In the Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE) trial, moderate-risk patients were randomized to receive enteric-coated aspirin(100 mg) or placebo tablets once daily. Importantly,the study specif cally excluded patients with diabetes [ 19 ]. The primary eff cacy end point was a composite outcome of the time to the frst occurrence of cardiovascular death, MI, unstable angina, stroke,or transient ischemic attack (TIA) and occurred in 4.29% of patients in the aspirin group versus 4.48%in the placebo group (hazard ratio [HR] 0.96, 95%CI 0.81 - 1.13, P = 0.60). Gastrointestinal bleeding occurred in 0.97% of patients in the aspirin group versus 0.46% in the placebo group (HR 2.1 1, 95%CI 1.36 - 3.28, P = 0.0007). The subgroup analysis revealed results that were consistent with the overall f ndings for the primary end point, with no difference between women and men. Although this trial set out to recruit a moderate-risk population,the event rate was much lower than expected, making the study more representative of a low-risk population. Some explanations for the low event rate include better management of dyslipidemia, blood pressure, and other risk factors [ 19, 20]. A Study of Cardiovascular Events in Diabetes (ASCEND)was a randomized trial to assess the eff cacy and safety of enteric-coated aspirin at a dosage of 100 mg daily, compared with placebo, in persons who had diabetes without manifest CVD at trial entry. The primary eff cacy outcome (MI, stroke,TIA, or death from any vascular cause) occurred in 8.5% of participants in the aspirin group and 9.6% of participants in the placebo group (rate ratio 0.88, 95% CI 0.79 - 0.97, P = 0.01). Major bleeding occurred in 4.1% of participants in the aspirin group and 3.2% of participants in the placebo group (rate ratio 1.29, 95% CI 1.09 - 1.52, P = 0.003). The subgroup analysis revealed results that were consistent with the overall f ndings for the primary end point,with no difference between women and men. The results from this trial show the benef ts of aspirin were largely counterbalanced by the bleeding hazard [ 21 ].
ISIS-2 (a randomized trial of intravenously administered streptokinase, orally administered aspirin,both, or neither among 17,187 cases of suspected acute MI) was the f rst trial to test aspirin in the acute phase of MI. Before ISIS-2 there was only one small randomized trial, which involved just a single aspirin tablet with no further treatment. ISIS-2 revealed a reduction in vascular mortality in women of 15.8% with aspirin versus 13.2% with placebo (OR 0.8, 95% CI 0.75 - 0.9) [ 22]. After ISIS-2, numerous trials continued to show the benef t of aspirin for secondary prevention of major coronary events.In a meta-analysis of 16 secondary prevention trials for aspirin, the proportional reduction in major coronary events was 20% (rate ratio 0.8, 95% CI 0.73 - 0.88, P < 0.00001). The proportional reduction in major coronary events for women was 27% (rate ratio 0.73, 95% CI 0.51 - 1.03) [ 23]. On the basis of numerous clinical trials demonstrating the benef t of aspirin for secondary prevention, all major societal guidelines recommend aspirin for secondary prevention in patients with established CVD, including the American College of Chest Physicians [ 24], the American Heart Association/American College of Cardiology [ 25], and European [ 26] guidelines.
P2Y 12 Inhibitors
P2Y12inhibitors are one of the most broadly studied classes of antiplatelet agents used in patients with CAD, particularly in high-risk patients with acute coronary syndrome (ACS) and those undergoing PCI. These include both oral and intravenous therapies as described in the following sections. A summary of the impact of sex on outcomes in pivotal trials is provided in Table 1 [ 6].
Clopidogrel
Clopidogrel is a prodrug whose active metabolite inhibits platelets by irreversibly binding to the platelet ADP P2Y12receptor [ 27 ]. Clopidogrel ’ s absorption is limited by the drug eff ux transporter P-glycoprotein, which is encoded by the ABCB1 gene. Once absorbed, approximately 15% of the drug is converted to the active metabolite. The formation of the active metabolite is a two-step process, with cytochrome P450 2C19 (CYP2C19),cytochrome P450 1A2, and cytochrome P450 2B6 (CYP2B6) involved in the f rst step and CYP2C19, cytochrome P450 2C9, CYP2B6, and cytochrome P450 3A involved in the second step.Polymorphisms of CYP2C19 contribute to differences in bioavailability between patients [ 28].Plasma levels of the active metabolite are similar in men and women [ 29]. In the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial, which involved patients with unstable angina and non - ST-segment-elevation MI who were randomized to receive clopidogrel plus aspirin compared with aspirin alone, the primary
end point (a composite of death from Cardiovascular causes, nonfatal MI, or stroke) occurred in 9.3%of patients in the clopidogrel group versus 11.4%of patients in the placebo group (RR 0.80, 95%CI 0.72 - 0.9, P < 0.001). Major bleeding was more common in the clopidogrel group (3.7%) than in the placebo group (2.7%; RR 1.38, 95% CI 1.13 - 1.67,P = 0.001). In women the relative risk reduction was 0.9 (95% CI 0.75 - 1.05) compared with 0.77(95% CI 0.65 - 0.85) in men [ 30]. The Clopidogrel as Adjunctive Reperfusion Therapy - Thrombolysis in Myocardial Infarction 28 (CLARITY-TIMI 28)trial, conducted in patients with STEMI, revealed those who were randomized to receive clopidogrel after f brinolytic therapy with aspirin experienced a reduction in the odds of the composite end point of cardiovascular death, MI, and reinfarction leading to ur gent revascularization by 20% compared with those who received placebo (OR 0.8, 95% CI 0.65 - 0.97, P = 0.026). These results remained consistent with respect to sex [ 31]. A meta-analysis of the f ve major clopidogrel trials (CURE, CREDO,CLARITY-TIMI 28, COMMIT, and CHARISMA)showed a 16% relative reduction in the odds of any major cardiovascular event with clopidogrel versus placebo in men (7.8 vs. 9.0%; OR 0.84, 95% CI 0.78- 0.91). The relative reduction in women was 7% but was not signif cant (11.0 vs. 1 1.8%; OR 0.93, 95% CI 0.86 - 1.01). However, the P value for interaction between male and female patients with respect to the outcome did not reach statistical signif cance (P = 0.092), and much of the difference between men and women could be explained by chance [ 32 ].

Table 1 Clinical Trials on Adenosine Diphosphate P2Y12 Receptor Antagonists.
The Dual Antiplatelet Therapy trial investigated the benef ts and risks of continuing dual antiplatelet therapy (DAPT) beyond 1 year of PCI. Continued treatment with a thienopyridine (mostly clopidogrel) as compared with placebo reduced the rates of stent thrombosis (0.4 vs. 1.4%; HR 0.29, 95% CI 0.17 - 0.48, P < 0.001) and major adverse cardiovascular and cerebrovascular events (4.3 vs. 5.9%; HR 0.71, 95% CI 0.59 - 0.85, P < 0.001). The rate of MI was lower with thienopyridine treatment than with placebo (2.1 vs. 4.1%; HR 0.47, P < 0.001). The rate of death from any cause was 2% in the thienopyridine group and 1.5% in the placebo group (HR 1.36, 95% CI 1 - 1.85, P = 0.05). The rate of moderate or severe bleeding was increased with continued thienopyridine treatment compared with placebo(2.5 vs. 1.6%; P = 0.001). The subgroup analysis,however, suggested a lower benef t in women for stent thrombosis reduction with prolonged DAPT(0.6 vs 0.8% in the placebo group; HR 0.73, 95% CI 0.28- 1.91) compared with men (0.3 vs. 1.5% in the placebo group; HR 0.21, 95% CI 0.1 1- 0.39), with P = 0.04 for interaction [ 33 ].
Prasugrel
Prasugrel is a newer-generation, more potent irreversible P2Y12inhibitor [ 1]. The metabolism is very eff cient and not lar gely affected by variations in cytochrome P450 activity. Prasugrel is primarily metabolized by cytochrome P450 3A4 [ 29].The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel - Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) trial revealed the primary end point (death from cardiovascular causes, nonfatal MI, or nonfatal stroke) was signif cantly reduced in favor of prasugrel compared with clopidogrel(HR 0.82, 95% CI 0.73 - 0.93, P = 0.002). There were no signif cant interactions between sex and increased risk of bleeding in both men and women.It is important to note that prasugrel is contraindicated in patients with prior TIA or stoke. Low body weight, less than 60 kg, and age greater than 75 years increase the risk of bleeding, and prasugrel should be used with caution in these populations[ 34]. The information in this subsection reveals similar eff cacy and safety in women with prasugrel therapy, and thus prasugrel should be used in women when indicated.
Ticagrelor
Ticagrelor belongs a new class of antiplatelet agents called cyclopentyltriazolopyrimidines [ 1 ].Ticagrelor directly inhibits the P2Y12receptor without hepatic activation. This leads to rapid platelet inhibition. Another important property of ticagrelor is it a reversible inhibitor, allowing quicker return of platelet function on cessation of medication[ 1]. The Platelet Inhibition and Patient Outcomes(PLATO) trial comparing clopidogrel with ticagrelor in patients with ACS revealed at 12 months the primary end point (death from vascular causes, MI,or stroke) was 9.8% in patients receiving ticagrelor compared with 11.7% in the clopidogrel group (HR 0.84, 95% CI 0.77 - 0.92, P < 0.001). There was no signif cant difference in the rates of major bleeding between patients receiving ticagrelor and patients receiving clopidogrel, 11.6 and 11.2%, respectively(HR 1.04, 95% CI 0.95 - 1.13, P = 0.43). However,ticagrelor was associated with a higher rate of major bleeding not related to coronary artery bypass graft (4.5 vs. 3.8%; HR 1.19, 95% CI 1.02 - 1.38,P = 0.03) [ 35 ]. A prespecif ed analysis of the PLA TO trial assessing the association between sex and the effects of ticagrelor versus clopidogrel showed that ticagrelor was similarly more ef fective than clopidogrel in reducing rates of the primary end point in women (11.2 vs. 13.2%; adjusted HR 0.88, 95% CI 0.74- 1.06) and men (9.4 vs. 1 1.1%; adjusted HR 0.86, 95% CI 0.76 - 0.97, P = 0.78) and all-cause death in women (5.8 vs. 6.8%; adjusted HR 0.9,95% CI 0.69 - 1.16) and men (4.0 vs. 5.7%; adjusted HR 0.80, 95% CI 0.67 - 0.96, P = 0.49). The treatments did not dif fer for PLA TO-def ned overall major bleeding complications in women (adjusted HR 1.01, 95% CI 0.83 - 1.23) or men (adjusted HR 1.10, 95% CI 0.98 - 1.24). Sex had no signif cant association with these outcomes (P = 0.43 - 0.88f or interaction) [ 36]. The information in this subsection reveals similar eff cacy and safety in women with ticagrelor therapy, and thus ticagrelor should be used in women when indicated.
Cangrelor
Cangrelor is an intravenously administered, rapidacting, potent, direct ADP-receptor antagonist that has rapidly reversible ef fects [ 37]. Cangrelor was approved on the basis of the results of the CHAMPION PHOENIX trial, in which 11,145 patients undergoing either urgent or elective PCI were randomized to receive a bolus and infusion of cangrelor or a loading dose of 600 mg or 300 mg of clopidogrel [ 38]. The primary eff cacy end point was a composite of death, MI, ischemiadriven revascularization, or stent thrombosis at 48 hours after randomization; the key secondary end point was stent thrombosis at 48 hours. The primary safety end point was severe bleeding at 48 hours. The study showed the rate of the primary composite eff cacy end point was signif cantly lower in the cangrelor group than in the clopidogrel group (4.7 vs. 5.9%; OR 0.78, 95% CI 0.66 - 0.93, P = 0.005). The rate of the primary safety end point was 0.16% in the cangrelor group and 0.1 1% in the clopidogrel group (OR 1.50,95% CI 0.53 - 4.22, P = 0.44). In women, cangrelor reduced the odds of the primary end point by 35%(adjusted OR 0.65, 95% CI 0.48 - 0.89, P = 0.01)and reduced the odds of stent thrombosis by 61%(adjusted OR 0.39, 95% CI 0.20 - 0.77, P = 0.01) as compared with clopidogrel. In male patients, cangrelor was associated with a 14% reduction in the odds of the primary end point (adjusted OR 0.86,95% CI 0.7 - 1.05, P = 0.14, P = 0.23 for interaction)and a 16% reduction in the odds of stent thrombosis (adjusted OR 0.84, 95% CI 0.53 - 1.33, P = 0.44,P = 0.11 for interaction). Cangrelor increased the odds of Global Use of Strategies to Open Occluded Coronary Arteries (GUST O) moderate bleeding in women when compared with clopidogrel (0.9 vs. 0.3%; adjusted OR 3.63, 95% CI 1.2 - 10.87,P = 0.02). Overall, intravenous ADP-receptor inhibition with cangrelor signif cantly reduced the rate of ischemic events in patients undergoing PCI,with a consistent benef t in all major subgroups[ 38 , 39 ] .
Intravenous Glycoprotein IIb/IIIa Inhibitors
Another widely used group of drugs are the glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors (abciximab,tirof ban, and eptif batide), which block the f nal common pathway leading to platelet aggregation by inhibiting the binding of f brinogen to the GPIIb/IIIa receptor on the platelet surface [ 40]. A meta-analysis of six trials that enrolled 31,402 patients revealed a 9%reduction in the odds of death or MI with GPIIb/IIIa inhibitors compared with placebo or controls (10.8 of events vs. 1 1.8% of events; OR 0.91, 95% CI 0.84 - 0.94, P = 0.015). There was an unexpected and signif cant interaction between sex and treatment,with a benef t in men (OR 0.81, 95% CI 0.75 - 0.89)but not in women (OR 1.15, 95% CI 1.01 - 1.30,P = 0.0001 for heterogeneity). However, once patients were stratif ed according to troponin concentration,there was no difference in treatment response. Major bleeding complications were increased in women(3 of events vs. 1.4% of events; OR 2.2, 95% CI 1.6- 2.9) compared with men (2.1 of events vs. 1.4%of events; OR 1.6, 95% CI 1.3- 2), with no evidence of heterogeneity (P = 0.10) [ 41 ]. Can Rapid Risk Stratif cation of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) further explored bleeding and specif cally addressed differences between women and men. The adjusted OR for major bleeding in those treated with GPIIb/IIIa inhibitors (versus those not treated) was 2.78 (95% CI 2.34- 3.31) for women and 1.98 (95% CI 1.61 - 2.42)for men. Some explanations for the increased bleeding risk include women ’ s creatinine clearance averaged 20 points less than men ’ s and women were more likely to receive excess GPIIb/IIIa doses than men (46.4 vs. 17.2%; P < 0.0001, adjusted OR 3.81,95% CI 3.39 - 4.37)[ 42 ] .
Sex Considerations for the Choice of Antiplatelet Medication
After an ACS, women experience worse short-term and long-term outcomes than men. This may be secondary to dif ferences in comorbidities, higher bleeding rates, increased time to presentation, and differences in management. The higher bleeding rates are likely secondary to smaller blood vessels in females, higher vascular reactivity, and inappropriate dosing on the basis of body mass and renal function [ 10]. After an ACS, women are less likely to be prescribed DAPT [ 43]. Data from the Greek Antiplatelet Registry (GRAPE) revealed that women were more frequently dischar ged with clopidogrel therapy rather than with treatment with novel P2Y12inhibitors. Among women discharged with clopidogrel therapy or without treatment with a P2Y12inhibitor, 7.1% had previous stroke, 41.8%were 75 years or older, 16.3% weighed less than 60 kg, 24.5% had impaired renal function, and 4.3%reported a prior major bleeding episode. These high-risk features for bleeding can explain the difference in treatment choice for women in the study.The females in the registry who received the novel P2Y12inhibitors did not experience better ischemic outcomes but did experience higher bleeding rates compared with the clopidogrel cohort [ 44]. In a meta-analysis of seven trials with 24,494 women and 63,346 men, potent P2Y12inhibitors signif cantly reduced the risk of major adverse cardiovascular events by 14% in women (HR 0.86, 95% CI 0.78 -0.94) and by 15% in men (HR 0.85, 95% CI 0.8 - 0.9, P = 0.93 for interaction). The risk of MI was reduced by 13% in women (HR 0.87, 95%CI 0.78 - 0.96) and 16% in men (HR 0.84, 95%CI 0.77 - 0.91, P = 0.65 for interaction). The potent P2Y12inhibitors increased the risk of major bleeding in women (HR 1.28, 95% CI 0.87 - 1.88) and men (HR 1.52, 95% CI 1.12 - 2.07, P = 0.62 for interaction). These data provide further evidence that the eff cacy and safety of the potent P2Y12inhibitors are comparable between men and women [ 45].
Because of the increased bleeding risk in women,bleeding-reduction strategies are becoming an important area of focus in antiplatelet therapy research. Study of Access Site for Enhancing PCI for Women (SAFE-PCI for Women) was the f rst randomized trial comparing interventional strategies in women, comparing radial versus femoral access. There was no signif cant difference in the primary eff cacy end point (Bleeding Academic Research Consortium [BARC] type 2, 3, or 5 bleeding or vascular complications requiring intervention)between radial and femoral access among women undergoing PCI (1.2% for radial access vs. 2.9%for femoral access; OR 0.39, 95% CI 0.12 - 1.27).However, among women undergoing cardiac catheterization or PCI, radial access signif cantly reduced bleeding and vascular complications (0.6 vs. 1.7%;OR 0.32, 95% CI 0.12- 0.9) [ 46].
Other bleeding-reduction strategies include de-escalation of antiplatelet therapy, aspirin-free regimens, and shortening DAPT duration [ 47 - 49 ].There are limited data assessing the clinical impact of de-escalation of antiplatelet therapy based on the results of genetic testing; however, several randomized trials, including the use of rapid genetic testing, are in progress [ 50]. Nevertheless,there are data on the use of platelet function testing (PFT) to guide de-escalation [ 51]. The Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) trial investigated the safety and eff cacy of early de-escalation of antiplatelet treatment from prasugrel to clopidogrel guided by PFT [ 52]. Patients were enrolled if they had biomarker-positive ACS with successful PCI and a planned duration of DAPT of 12 months.Patients were randomly assigned to receive either standard treatment with prasugrel for 12 months(control group) or a step-down regimen (prasugrel for 1 week followed by clopidogrel for 1 week and PFT-guided maintenance therapy with clopidogrel or prasugrel from day 14 after hospital dischar ge;guided de-escalation group). The primary end point (net clinical benef t; cardiovascular death,MI, stroke, or BARC bleeding type 2 or higher)occurred in 7% of participants in the guided deescalation group and in 9% of participants in the control group (HR 0.81, 95% CI 0.62 - 1.06,P = 0.0004). This trial revealed that guided de-escalation of antiplatelet treatment was noninferior to standard treatment with prasugrel at 1 year after PCI with respect to net clinical benef t. Subgroup analysis revealed a similar net clinical ef fect between men (HR 0.78, 95% CI 0.57 - 1.06) and women(HR 0.92, 95% CI 0.53 - 1.62, P = 0.60) [ 52 ]. There are several ongoing trials to test whether other bleeding-reduction strategies can reduce bleeding while preserving eff cacy. Of these, the Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention (TWILIGHT) trial has female sex as one of the study entry criteria, with the goal of enriching the study with this typically underrepresented cohort [ 53].
Conclusion
Despite the progress in the f eld of antiplatelet therapy over the past years, there are still some knowledge gaps with regard to the eff cacy and safety of these therapies in women. This is lar gely due to the underrepresentation of women in clinical trials. Most of these trials were not powered to detect sex differences; however, on the basis of the available evidence, women benef t to a similar extent as men from antiplatelet therapy. However, studies also show that women experience more bleeding.These observations underscore the need to def ne antiplatelet treatment regimens with a more favorable ischemic and bleeding benef t prof le in female patients with CAD. This is further underscored by the ever-emerging prevalence of CAD among females.
Conflict of Interest
Dominick J. Angiolillo declares that he has received consulting fees or honoraria from Amgen, Aralez,AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck,PLx Pharma, Pf zer, Sanof , and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical. D.J.A. also declares that his institution has received research grants from Amgen,AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead,Janssen, Matsutani Chemical Industry Co., Merck,Novartis, Osprey Medical, and Renal Guard Solutions. Khadeeja Esmail declares that she has no conf icts of interest.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Challenges in Cardiovascular Risk Prediction and Stratification in Women
- Nonobstructive Coronary Artery Disease in Women: Risk Factors and Noninvasive Diagnostic Assessment
- Novel Imaging Approaches for the Diagnosis of Stable Ischemic Heart Disease in Women
- Psychosocial Stress, the Unpredictability Schema, and Cardiovascular Disease in Women
- Heart Disease in Pregnancy: A Special Look at Peripartum Cardiomyopathy
- Heart Failure with Preserved Ejection Fraction:Time to Revisit the Stiff Heart