运动通过AMPK信号通路改善高脂诱导的Ⅱ型糖尿病小鼠心肌糖脂代谢
2018-02-05李国平
田 阁, 徐 昕, 李国平
运动通过AMPK信号通路改善高脂诱导的Ⅱ型糖尿病小鼠心肌糖脂代谢
田 阁1, 徐 昕2, 李国平3
1. 北京体育大学研究生院, 北京 100084; 2. 上海体育学院运动科学学院, 上海 200438; 3. 国家体育总局运动医学研究所, 北京 100061
目的:糖尿病是一种常见的慢性疾病,心肌功能受损是糖尿病患者心衰和死亡的重要原因之一。观察8周有氧联合抗阻运动对糖尿病小鼠心肌形态结构的影响,探讨AMPK信号通路在其中可能的作用机制,以期为防治糖尿病患者心肌受损提供新的靶向。方法:采用16周高脂饮食建立糖尿病模型,建模成功后将小鼠分为糖尿病安静组(DS组)和糖尿病运动组(DE组);同龄C57小鼠为空白对照组(CS组)。糖尿病组采用高脂喂养,DE组进行8周有氧联合抗阻运动干预。采用腹腔注射葡萄糖耐量实验(IPGTT)检测小鼠糖耐量,天狼腥红染色测定心肌纤维化,麦胚凝集素(WGA)染色检测心肌横截面积,Western blot法检测心肌AMPKα、磷酸化的AMPKαThr172(p-AMPKα)、沉默信息调节因子2同源蛋白1(SIRT1)、过氧化物酶体增殖物激活受体γ共激活因子-1α(PGC-1α)和过氧化物酶体增殖物激活受体α(PPARα)蛋白表达。结果:1)DS组小鼠体重和糖耐量均显著高于CS组(<0.01),DE组小鼠体重和AUC均显著低于DS组(<0.01);2)与CS组相比,DS组小鼠心肌血管周围纤维化比例显著增加(<0.05),重量和心脏重量/胫骨长度显著增加(<0.01),心肌横截面积有所增加但无显著性差异(>0.05);与DS组相比,DE组心肌血管周围纤维化比例显著降低(<0.05);3)与CS组相比,DS组小鼠心肌p-AMPKα非常显著降低(<0.01),SIRT1和PGC-1α显著降低(<0.05),PPARα显著升高(<0.05);与DS组相比,DE组小鼠心肌p-AMPKα、SIRT1和PGC-1α非常显著升高(<0.01),PPARα非常显著降低(<0.01)。结论:长期运动可以激活AMPK及下游通路,改善心肌糖脂代谢,从而减轻糖尿病后心肌重构。
运动;腺苷酸活化蛋白激酶;糖尿病;心肌;糖脂代谢
糖尿病是一种常见的慢性疾病,预计到2030年,世界糖尿病患者将达到3.66亿,约占世界人口的4%,成为重大的公共卫生问题[32]。糖尿病患者多存在心肌肥大和心脏舒张功能受损等症状,是糖尿病患者心衰和死亡的重要原因之一[19]。糖尿病患者心肌功能受损后的主要治疗方式包括药物治疗、基因处理和运动干预。药物治疗费用较高,同时可能存在副作用[30]。基因处理目前仍在研发中,临床应用尚需时间。运动干预可能是相对经济有效的治疗手段[18]。
腺苷酸活化蛋白激酶(5’-adenosinemono phosphate ctivated protein kinase, AMPK)作为能量感受器,在改善糖尿病能量代谢中起到非常重要的作用[15]。近年来研究发现,激活AMPK还可以改善胰岛素抵抗[14],减轻心肌肥大和心肌纤维化,改善心力衰竭[17]。同时,AMPK与运动关系比较密切。有氧运动联合抗阻运动是目前临床上推荐的糖尿病运动处方[8, 20],但其是否能够通过AMPK改善糖尿病心肌受损尚不明确。因此,本研究采用高脂饲养建立Ⅱ型糖尿病模型,观察8周有氧运动联合抗阻运动干预对心肌糖脂代谢的影响,探讨AMPK及其下游通路在糖尿病心肌受损运动康复中的作用。
1 研究对象与方法
1.1 研究对象
8周龄SPF级雄性C57小鼠36只,于第二军医大学动物实验中心购买,于上海体育学院实验动物中心饲养训练。小鼠自由进食饮水,4只/笼。环境温度20℃~24℃,相对湿度40%~60%,通风良好,昼夜12 h/12 h循环照明。所有操作均严格遵守上海体育学院道德伦理委员会的要求。
1.2 实验造模及分组
小鼠随机分为空白对照组(CS组,12只)和糖尿病组(24只)。CS组以普通饲料喂养,糖尿病组以高脂饲料喂养(脂肪:60% kcal、碳水化合物:20% kcal、蛋白质:20% kcal,OpenSource Diets No.D12492)。第15、16周末分别对糖尿病组小鼠进行随机血糖测试,连续2次随机血糖>11.1 mmol/L认为糖尿病建模成功[7]。23只小鼠造模成功,随机分为糖尿病安静组(DS组,12只)和糖尿病运动组(DE组,11只)。CS组和DS组无运动干预,DE组从第17周开始进行有氧联合抗阻干预8周。
1.3 运动方案
运动实验开始前,进行3 d运动预适应;每周一、三、五有氧运动干预:20 m/min,60 min[9];每周二、四、六抗阻运动干预:90°爬梯运动,速度不限且不负重,4次/组,共4组[16,22]。
1.4 腹腔注射葡萄糖耐量实验(Intraperitoneal glucose tolerance test,IPGTT)
24周后,对小鼠进行腹腔注射葡萄糖耐量实验。小鼠禁食12 h,腹腔注射葡萄糖溶液(2 g/kg),并分别在腹腔注射后0 min、30 min、60 min、90 min、120 min时进行剪尾(约0.5 cm)取血。采用血糖测试仪(强生公司,稳豪倍易型)和配套试纸条测定血糖值,并绘制血糖曲线,计算血糖曲线下面积(Area under the curve,AUC)[34]。
1.5 组织取材及处理
运动组小鼠末次运动后24 h取材,取材前禁食12 h,腹腔注射10%水合氯醛(2 mL/kg)麻醉,取心脏,将左心室上1/3置于多聚甲醛固定制作石蜡切片、下2/3置于-80℃,分别用于染色和Western blot。
1.5.1 天狼腥红染色
将石蜡切片脱蜡后置于0.1%苦味酸天狼猩红溶液染色 1 h,0.5%醋酸洗涤3×10 min,梯度脱水至二甲苯,封片。在显微镜下观察、拍照,采用Image Pro Plus 6.0计算心肌间质纤维化比例和血管周围纤维化比例。
1.5.2 麦胚凝集素(Wheat germ agglutinin,WGA)染色
将石蜡切片脱蜡后进行抗原修复,置于0.3%甲醇10 min,蒸馏水洗涤后置于0.01 mol/L柠檬酸盐缓冲液,98℃水浴40 min;PBS洗涤10 min后滴加WGA-Alexa Fluor®488储存液(1 mg/mL,Invitrogen公司),避光室温孵育2 h,PBS缓冲液避光漂洗3×5 min,封片。采用Image Pro Plus 6.0计算心肌细胞大小。
1.5.3 蛋白免疫印迹
1)总蛋白提取:将30 mg心肌组织置于30 μLRIPA裂解液(内含蛋白酶抑制剂PMFS)中,混合均匀后置于冰上匀浆;匀浆后将匀浆液置于离心机中16 000 rpm、4℃离心10 min;取上清,置于-20℃冻存备用。采用BCA法测定蛋白浓度,按照BCA 试剂盒(Thermo Scientific)说明书进行蛋白浓度测定。煮沸样本,100℃,5 min。2)Western blot:将样本分别加入凝胶孔中,每孔20μg样本。采用恒压电泳,浓缩胶:90 V恒压,30 min;分离胶120 V恒压,60 min。采用湿式转膜法将蛋白转至PVDF膜,100 V,恒压,1.5 h;5%脱脂牛奶室温封闭,2 h;分别采用p-AMPKα、AMPKα、PGC-1α和PPARα的一抗(1︰2 000,Abcam公司)4℃冰箱孵育过夜;TBST洗膜6×5 min;采用对应二抗(1︰3 000,Merck Millipore公司)室温孵育1 h;TBST洗膜6×5 min;将ECL发光液以A液和B液1︰1的比例混匀后滴于膜上,暗室曝光呈像。3)计算结果:采用Image J计算蛋白条带积分灰度值,Western blot结果以目的蛋白与内参蛋白的积分灰度值的比值表示。
1.6 统计学分析
采用SPSS 19.0统计分析数据,数据采用均值±标准差(±)表示。组间比较采用ANOVA单因素方差分析,以<0.05表示有显著性差异。
2 结果
2.1 各组小鼠的体重和糖耐量
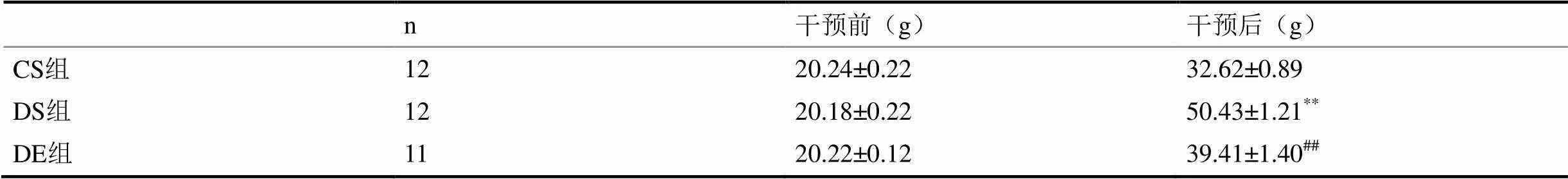
表1 各组小鼠的体重
注:*<0.05,**<0.01 vs CS组;#<0.05,##<0.01vs DS组;下同。
表1显示,实验前3组小鼠体重无显著性差异(>0.05);24周后,DS组小鼠体重显著高于CS组(<0.01),DE组小鼠体重显著低于DS组(<0.01)。图1显示,DS组小鼠腹腔注射葡萄糖后0 min、30 min、60 min、90 min、120 min血液葡萄糖水平及IPGTT血糖曲线下面积(AUC)非常显著高于CS组(<0.01);与DS组相比,DE组小鼠腹腔注射葡萄糖后30 min、60 min、90 min、120 min血液葡萄糖水平及IPGTT血糖曲线下面积(AUC)非常显著降低(<0.01)。
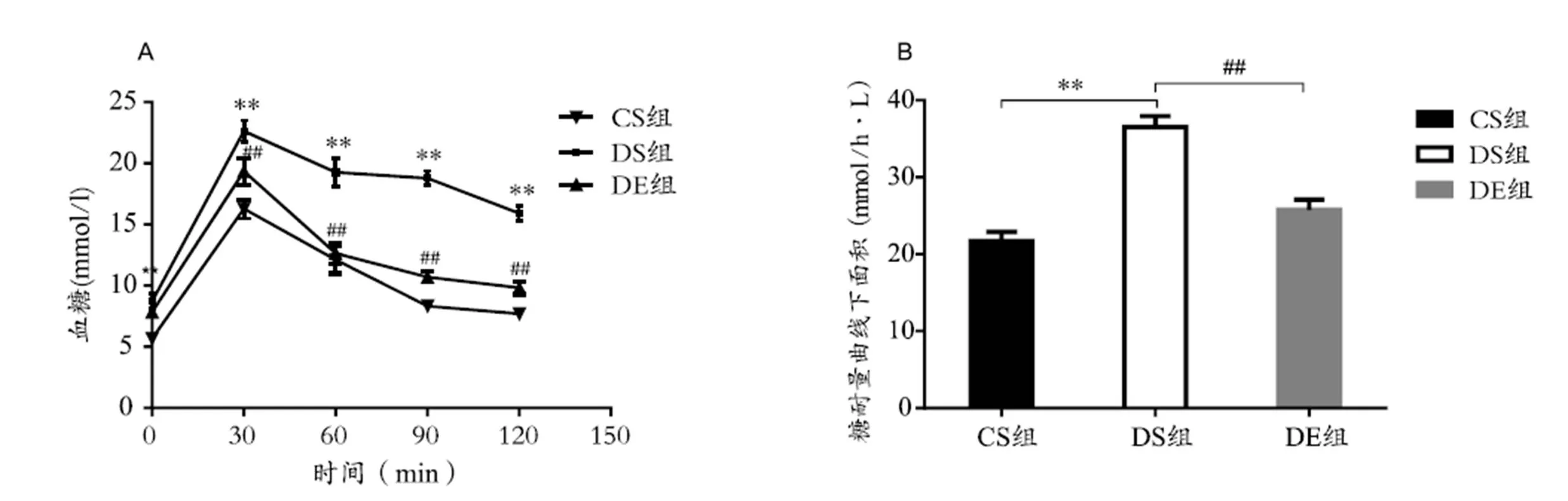
图1 各组小鼠葡萄糖耐量实验结果
Figure 1. Plasma Glucose Levels and AUC in IPGTT in Each Group
2.2 各组小鼠的心肌重量和形态学指标
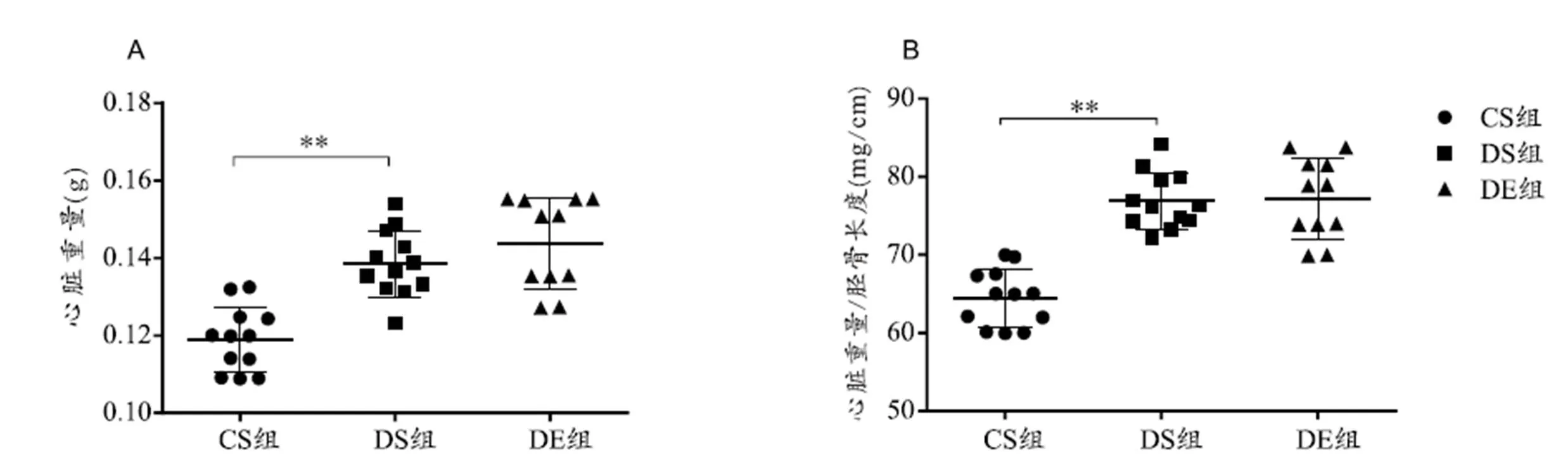
图2 各组小鼠心脏重量
Figure 2. Results of Heart Weight and Heart Weiht/Gibia Length

图3 小鼠心肌天狼腥红染色和WGA染色结果
Figure 3. Results of Sitius Red Staining and WGA Staining in Mice Myocardium
图2、图3结果显示,与CS组相比,DS组小鼠心脏重量和心脏重量/胫骨长度非常显著增加(<0.01),心肌间质纤维化比例无显著性差异(>0.05),血管周围纤维化比例升高(<0.05),心肌细胞横截面积显著增加,但无显著性差异(>0.05);与DS组相比,DE组小鼠心肌血管周围纤维化显著减低(<0.05),心肌细胞横截面积减小,但无显著性差异(>0.05),其他指标均无显著性差异(>0.05)。
2.3 各组小鼠心肌组织AMPKα及相关蛋白的表达
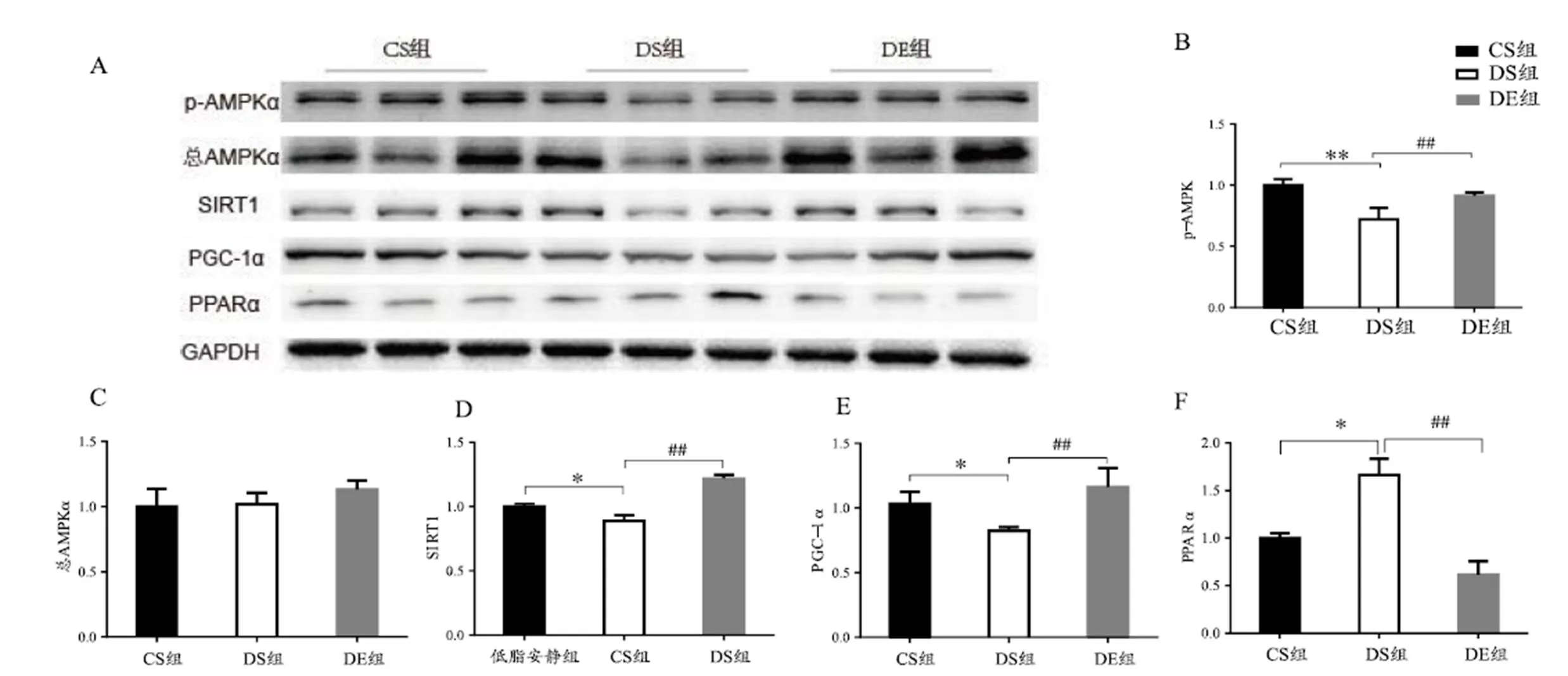
图4 心肌组织各蛋白表达水平
Figure 4. Expression of Relative Protein in Mice Myocardium
图4显示,与CS组相比,DS组小鼠心肌p-AMPKα非常显著降低(<0.01),SIRT1和PGC-1α显著降低(<0.05),PPARα显著升高(<0.05);与DS组相比,DE组小鼠心肌p-AMPKα、SIRT1和PGC-1α非常显著升高(<0.01),PPARα非常显著降低(<0.01)。
3 分析讨论
本研究发现,8周有氧联合抗阻运动可以明显改善高脂饮食诱导的Ⅱ型糖尿病小鼠糖耐量受损和心肌血管周围纤维化;同时,高脂饮食导致Ⅱ型糖尿病小鼠心肌p-AMPKα显著降低,沉默信息调节因子2同源蛋白1(Silent matingtype information regulation homolog1,SIRT1)和过氧化物酶体增殖物激活受体γ共激活因子-1 alpha (Peroxisome proliferator-activated receptor γ coactivator-1,PGC-1α)显著降低,过氧化物酶体增殖剂激活受体α(Peroxisome proliferators-activated receptor alpha,PPARα)显著升高,而运动可以激活AMPKα,增加SIRT1和 PGC-1α表达,抑制PPARα表达。可见,长期有氧联合抗阻运动可以通过激活AMPK及其下游通路改善Ⅱ型糖尿病小鼠心肌糖脂代谢紊乱,减轻心肌重塑。
3.1 Ⅱ型糖尿病小鼠模型建立
糖尿病患者中Ⅱ型糖尿病约占90%。而在饮食诱导的糖尿病模型中,动物心脏受损进程与人体较为相似[13],因此,本研究采用高脂饮食建立Ⅱ型糖尿病模型。Ⅱ型糖尿病是由脂肪组织、骨骼肌和肝脏胰岛素抵抗并伴随胰岛素分泌缺陷导致的,中心性肥胖是其基本特征,糖耐量受损是其重要诊断标准之一[1, 2]。本研究发现,24周后,DS组小鼠体重和AUC显著高于CS组,说明DS组小鼠出现肥胖和糖耐量受损,进一步证明糖尿病造模成功。同时,本研究发现,高脂喂养后,小鼠心脏重量和心脏重量/胫骨长度以及心肌血管周围纤维化比例显著增加,糖尿病小鼠心肌出现能量代谢紊乱,以及血管周围纤维化和心肌肥大,此结果与以往大多数研究相一致。Zeng等(2015)和Ouwens等(2005)发现,高脂饮食后小鼠和大鼠心脏重量和心脏重量/胫骨长度或者心脏重量/体重显著增加,出现病理性心肌肥厚。Cannon等(2016)发现,16周高脂喂养后,小鼠出现心肌纤维化和心肌肥大,心脏舒缩功能受损。但Patel等(2016)采用高脂喂养小鼠24周后发现小鼠未出现心肌肥厚,可能与其高脂饲料中脂肪含量较低(45% kcal)有关。
3.2 运动对心肌形态学的影响
连续泵血使心脏成为机体对能量需求较高的器官之一,其ATP大部分由线粒体氧化磷酸化产生,恒定流量的底物输送到线粒体是满足心脏高能量需求的关键。心脏中葡萄糖和脂肪酸是主要的代谢底物,正常心脏的一个重要特征是具有在不同生理和饮食条件下确保代谢灵活性和适当ATP生产率的能力。糖尿病心脏主要依赖于脂肪酸氧化,并伴随着葡萄糖氧化的减少[29]。缺乏代谢灵活性已被认为是心力衰竭,包括糖尿病心肌病,发展的根本,但其机制尚不清楚。糖尿病心脏中,游离脂肪酸储存增加会引起心脏脂毒性,导致心肌细胞凋亡和心肌纤维化[3]。同时,血糖增高引发间质糖基化,可能会导致心肌纤维化和心室顺应性降低[25]。因此,改善糖脂代谢可能是防治糖尿病及其并发症的关键。
前瞻性研究发现,运动可以减少Ⅱ型糖尿病和心血管疾病风险,降低全因死亡率[18,31,32]。Duvivier等(2017)发现,运动可以降低Ⅱ型糖尿病患者血糖水平,增加胰岛素敏感性,改善机体胰岛素抵抗。Xu等(2015)人发现,采用主动脉弓缩窄术诱导大鼠心衰前进行4 W运动预适应干预,可以明显减轻小鼠心肌病理性肥大和心脏收缩功能受损。本研究发现,DE组小鼠心脏重量、心脏重量/胫骨长度和心肌血管纤维化比例非常显著降低,心肌细胞横截面积出现一定程度减小。可见,8周有氧联合抗阻运动可以改善高脂诱导的糖尿病小鼠心肌重塑。
3.3 运动对糖尿病小鼠心肌AMPK及其下游通路的影响
AMPK是一个由功能亚基(α)和调节亚基(β,γ)构成的异源三聚体蛋白[21],通过醛缩酶或感受胞浆内AMP/ATP比值变化,广泛参与机体能量代谢调节[38]。AMPK在调节心肌能量代谢中起重要作用,Chang等(2013)发现,小檗碱调节胰岛素抵抗大鼠心肌细胞葡萄糖代谢、减轻胰岛素抵抗具有AMPK依赖性。以往研究表明,运动是激活体内AMPK的最主要生理应激[23]。运动可以通过激活脂肪组织、肝脏[27]、胰腺和骨骼肌[24]等组织中的AMPK改善胰岛素抵抗,提示,运动激活AMPK也可能是改善糖尿病心肌代谢紊乱的关键。
Canto等(2009)发现,AMPK通过增加NAD/NADH比率或烟酰胺磷酸核糖转移酶(Nicotinamide phosphoribosyltransferase,NAMPT)激活SIRT1及其下游转录因子PGC-1α和FOXO家族成员等。Xu等(2010)采用高脂喂养SIRT1基因敲除杂合子小鼠后发现,SIRT1缺乏后肝脏脂肪酸氧化相关基因PGC-1α、肉毒碱棕榈酰基转移酶(Carnitinepalmitoyl transferase1A,CPT1A)和细胞色素-C(Cytochrome C,cytoc)表达减少,炎症因子NF-κB表达增加,加重了高脂诱导的肝脏能量代谢紊乱和肝脂肪变性。Doan等(2015)采用细胞培养,发现AMPK/SIRT1/PGC-1α通路在没食子酸(Gallic acid)调节肥胖小鼠肩胛间棕色脂肪组织脂肪代谢,改善周身糖耐量受损中起重要作用。另外,PPARα是AMPK下游重要的脂质调节因子,Finck等(2002)发现,PPARα基因过表达小鼠心肌脂肪酸氧化率增加,葡萄糖摄取和利用减少,同时出现心肌病理性肥厚等与糖尿病心脏类似的表征。本研究发现,糖尿病小鼠心肌p-AMPKα显著降低,SIRT1和PGC-1α显著降低,PPARα显著升高,而运动可以激活AMPKα,增加SIRT1和PGC-1α表达,抑制PPARα表达。可见,AMPK/SIRT1/PGC-1α和AMPK/ PPARα也可能是运动改善糖尿病心肌中代谢紊乱的重要通路。
4 结论
8周有氧联合抗阻运动可能是通过激活AMPK/SIRT1/ PGC-1α和AMPK/PPARα通路,促进心肌脂质代谢,减轻糖耐量受损,从而改善高脂诱导的Ⅱ型糖尿病小鼠心肌重塑。
[1] 王正珍. ACSM运动测试与运动处方指南[J]. 北京:人民卫生出版社, 2015: 274-275.
[2] AMERICAN DIABETES ASSOCIATION. Classification and dia-gnosis of diabetes[J]. Diabetes Care, 2017, 40(Suppl 1): S11-S24.
[3] BUGGER H, ABEL E D. Molecular mechanisms of diabetic card-iomyopathy[J]. Diabetologia, 2014, 57(4): 660-671.
[4] CANNON M V, SILLJE H H, SIJBESMA J W,. LXRalpha improves myocardial glucose tolerance and reduces cardiac hyper-trophy in a mouse model of obesity-induced type 2 diabetes[J]. Diabetologia, 2016, 59(3): 634-643.
[5] CANTO C, GERHART-HINES Z, FEIGE J N,. AMPK regul-ates energy expenditure by modulating NAD+ metabolism and SIRT1 activity[J]. Nat, 2009, 458(7241): 1056-1060.
[6] CHANG W, ZHANG M, LI J,. Berberine improves insulin resistance in cardiomyocytes via activation of 5'-adenosine monophosphate-activated protein kinase[J]. Metabolism, 2013, 62 (8): 1159-1167.
[7] CHEN F, XIONG H, WANG J,. Antidiabetic effect of total flavonoids from Sanguis draxonis in type 2 diabetic rats[J]. J Ethnopharmacol, 2013, 149(3): 729-736.
[8] CHURCH T S, BLAIR S N, COCREHAM S,. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial[J]. JAMA, 2010, 304(20): 2253-2262.
[9] DE ANGELIS K, WICHI R B, JESUS W R,. Exercise training changes autonomic cardiovascular balance in mice[J]. J Appl Physiol (1985), 2004, 96(6): 2174-2178.
[10] DOAN K V, KO C M, KINYUA A W,. Gallic acid regulates body weight and glucose homeostasis through AMPK activation [J]. Endocrinol, 2015, 156(1): 157-168.
[11] DUVIVIER B M, SCHAPER N C, HESSELINK M K,. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes[J]. Diabetologia, 2017, 60(3): 490-498.
[12] FINCK B N, LEHMAN J J, LEONE T C,. The cardiac phen-otype induced by PPARα overexpression mimics that caused by diabetes mellitus[J]. J Clin Invest, 2002, 109(1): 121-130.
[13] FUENTES-ANTRAS J, PICATOSTE B, GOMEZ-HERNANDEZ A,. Updating experimental models of diabetic cardiomyo pathy [J]. J Diabetes Res, 2015, 2015: 656795.
[14] GAUTAM S, ISHRAT N, YADAV P,. 4-Hydroxyisoleucine attenuates the inflammation-mediated insulin resistance by the activation of AMPK and suppression of SOCS-3 coimmunopre cipitation with both the IR-beta subunit as well as IRS-1[J]. Mol Cell Biochem, 2016, 414(1-2): 95-104.
[15] HU M, YE P, LIAO H,. Metformin protects H9C2 cardiomyocytes from high-glucose and hypoxia/reoxygenation injury via inhibition of reactive oxygen species generation and inflammatory responses: Role of AMPK and JNK[J]. J Diabetes Res, 2016, 2016: 2961954.
[16] KIM H J, SO B, CHOI M,. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans[J]. Exp Gerontol, 2015, 70: 11-17.
[17] LEE J E, YI C O, JEON B T,. alpha-Lipoic acid attenuates cardiac fibrosis in Otsuka Long-Evans Tokushima Fatty rats[J]. Cardiovasc Diabetol, 2012, 11: 111.
[18] LI G, ZHANG P, WANG J,. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study[J]. Lancet Diabetes Endocrinol, 2014, 2(6): 474-480.
[19] LIU F, SONG R, FENG Y,. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of per-oxisome proliferation-activated receptor alpha[J]. Circulation, 2015, 131(9): 795-804.
[20] MAIORANA A, O'DRISCOLL G, GOODMAN C,. Combin-ed aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes[J]. Diabetes Res Clin Pract, 2002, 56(2): 115-123.
[21] MINOKOSHI Y, ALQUIER T, FURUKAWA N,. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus[J]. Nat, 2004, 428(6982): 569-574.
[22] MOLANOURI SHAMSI M, MAHDAVI M, QUINN L S,. Effect of resistance exercise training on expression of Hsp70 and inflammatory cytokines in skeletal muscle and adipose tissue of STZ-induced diabetic rats[J]. Cell Stress Chaperon, 2016, 21(5): 783-791.
[23] MUSI N, HIRSHMAN M F, ARAD M,. Functional role of AMP-activated protein kinase in the heart during exercise[J]. FEBS Lett, 2005, 579(10): 2045-2050.
[24] NIU Y, WANG T, LIU S,. Exercise-induced GLUT4 transcription via inactivation of HDAC4/5 in mouse skeletal muscle in an AMPKalpha2-dependent manner[J]. Biochim Biophys Acta, 2017, 1863(9): 2372-2381.
[25] NUNODA S, GENDA A, SUGIHARA N,. Quantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitus[J]. Heart Vessels, 1985, 1(1): 43-47.
[26] OUWENS D M, BOER C, FODOR M,. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats[J]. Diabetologia, 2005, 48(6): 1229-1237.
[27] PARK H, KAUSHIK V K, CONSTANT S,. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise[J]. J Biol Chem, 2002, 277(36): 32571-32577.
[28] PATEL V B, MORI J, MCLEAN B A,. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity[J]. Diabetes, 2016, 65(1): 85-95.
[29] PETERSON L R, HERRERO P, SCHECHTMAN K B,. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women[J]. Circulation, 2004, 109(18): 2191-2196.
[30] QIN F, SIWIK D A, LUPTAK I,. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice[J]. Circulation, 2012, 125(14): 1757-1764, S1751-1756.
[31] Surgeon General's report on physical activity and health. From the centers for disease control and prevention[J]. JAMA, 1996, 276 (7):522.
[33] WILD S, ROGLIC G, GREEN A,. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030[J]. Diabetes Care, 2004, 27(5): 1047-1053.
[34] WOLEVER T M. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values[J]. Br J Nutr, 2004, 91(2): 295-301.
[35] XU F, GAO Z, ZHANG J,. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: a role of lipid mobilization and inflammation[J]. Endocrinol, 2010, 151(6): 2504-2514.
[36] XU T, TANG H, ZHANG B,. Exercise preconditioning atten-uates pressure overload-induced pathological cardiac hypertrophy [J]. Int J Clin Exp Pathol, 2015, 8(1): 530-540.
[37] ZENG H, VAKA V R, HE X,. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss[J]. J Cell Mol Med, 2015, 19(8): 1847-1856.
[38] ZHANG C S, HAWLEY S A, ZONG Y,. Fructose-1,6-bisph-osphate and aldolase mediate glucose sensing by AMPK[J]. Nat, 2017, 548(7665): 112-116.
Exercise Improving Cardiac Glycolipid Metabolism in High Fat Diet Induced Type 2 Diabetic Mice through AMPK Pathway
TIAN Ge1, XU Xin2, LI Guo-ping3
1.Beijing Sport University, Beijing 100084, China; 2. Shanghai University of Sport, Shanghai 200438, China; 3. National ResearchInstitute of Sport Medicine, Beijing 100061, China.
Objective: Diabetes mellitus is an emerging global threat to human health, and impaired myocardial function in diabetic patients is the major pathogenic factor leading to heart failure or sudden death. To test the hypothesis that exercise may protect the heart from metabolic disturbance in type 2 diabetes heart via AMPK, we studied the expression of AMPK and its downstream proteins in high-fat diet (HFD)-induced type 2 diabetic mice with eight weeks aerobic combined with resistance exercise intervention. Methods: The diabetic mice model were induced by HFD for sixteen weeks. After that, diabetes mice were divided into diabetic sedentary group (DS) and diabetic exercise group (DE) randomly. DE group was intervened with eight weeks aerobic combined with aerobic resistance exercise. The intraperitoneal glucose tolerance test (IPGTT) was used to detect glucose tolerance, Sirius red staining was applied to detect fibrosis and wheat germ agglutinin (WGA) staining was used to evaluate myocyte size in heart tissue. Western blot was used to measure the protein level of AMPK pathway. Results: Compared with controls, HFD increased body weight, blood glucose levels in IPGTT in diabetic mice(<0.01), heart weight (<0.05), heart weight / tibia length (<0.01) and perivascular fibrosis (<0.05) . Eight weeks combined exercise program could alleviate compromised glucose tolerance (<0.01) and decrease perivascular fibrosis (<0.01). More importantly, we identified that exercise might alleviate glycolipid metabolic disturbance in HFD-induced diabetic heart via activating AMPK (<0.01) and its downstream protein, involving upregulation of SIRT1(<0.01), PGC-1a (<0.01) and downregulation of PPARa (<0.01). Conclusion: The results suggest that eight weeks aerobic combined with resistance exercise could alleviate glycolipid metabolic disturbance and myocardial remodeling in HFD-induced diabetic heart AMPK and its downstream. These data indicated that long-term aerobic combined with resistance exercise might exert a cardiac protective effect against diabetes mellitus and associated cardiomyopathy.
G804.5
A
1000-677X(2018)01-0049-06
10.16469/j.css.201801007
2017-10-12;
2017-12-26
国家自然科学基金资助项目(81370197)。
田阁,女,在读博士研究生,主要研究方向为运动损伤与康复, E-mail: tiangebsu@163.com;徐昕,男,教授,主要研究方向为运动与心脏, E-mail: xxu2000@outlook.com;李国平,男,教授,主要研究方向为运动医学, E-mail: Ligp@263.net。
