铬杂氮团簇的生成及光解
2018-01-08李陶琦丁可伟许洪光卜建华郑卫军葛忠学
李陶琦,丁可伟,2,许洪光,卜建华,2,肖 啸,郑卫军,葛忠学,2
(1.西安近代化学研究所, 陕西 西安 710065;2.氟氮化工资源高效开发与利用国家重点实验室,陕西 西安 710065;3.北京分子科学国家实验室,中国科学院化学研究所,北京 100190)
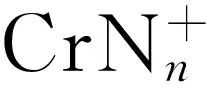
李陶琦1,丁可伟1,2,许洪光3,卜建华1,2,肖 啸1,郑卫军3,葛忠学1,2
(1.西安近代化学研究所, 陕西 西安 710065;2.氟氮化工资源高效开发与利用国家重点实验室,陕西 西安 710065;3.北京分子科学国家实验室,中国科学院化学研究所,北京 100190)
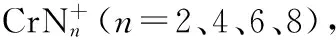
氮团簇;铬杂氮团簇;激光溅射;光解;高能量密度材料
引 言
氮团簇是近年来发展起来的新型绿色高能量密度材料候选物[1],但是其稳定性差,难以制备。由于金属极易和全氮基团通过配位、静电等作用形成金属杂氮团簇,在一定程度上能提高全氮基团的稳定性,因此金属杂氮团簇以其丰富多样的结构和相对较好的稳定性吸引了国内外研究人员的关注。Haiges R等[2-6]合成了M(N3)2(M=Ti、V、Nb、Ta、Mo、W);Filippou A C 等[7]合成了Ge(N3)2;Knapp C等[8]合成了Bi(N3)2;Klapötke T M等[9]合成了Se(N3)2;Villinger A等[10]合成了Te(N3)2,并用核磁进行了表征,用红外光谱或拉曼光谱进行了检测和理论计算验证;Gagliardi L等[11]通过理论计算研究了四叠氮化物形态的金属杂氮团簇M(N3)4(M=Ti、Zr、Hf、Th);Li Q S等[12]用密度泛函理论研究了三叠氮化物形态的金属杂氮团簇M(N3)3(M=Sc、Y、La、Al、Ga、In、Tl)和四叠氮化物形态的M(N3)4(M=Ti、Zr、Hf、Ge、Sn、Pb)的结构及稳定性。
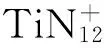
在目前已经开展的金属杂氮团簇研究中,有关ⅥB族金属和氮原子形成团簇的研究报道很少。由于铬具有特殊的原子外层电子构型3d54s1, 本研究以金属铬和氮化物BN为原料,采用激光溅射法[25-31],探索了铬杂氮团簇的生成,并对高丰度团簇进行了光解研究,探讨了团簇的组成。
1 实 验
1.1 仪器和试剂
飞行时间质谱仪[31],自制;Nd:YAG Surelite II-10激光器,美国Continuum 公司。
铬金属粉末(纯度99.5%)、氮化硼(纯度99.6%),上海麦克林生物试剂有限责任公司;氮气,纯度99.99%,西安卫光气体有限公司。
1.2 激光溅射及光解实验
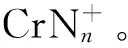
2 结果与讨论
2.1 铬杂氮团簇的生成与光解

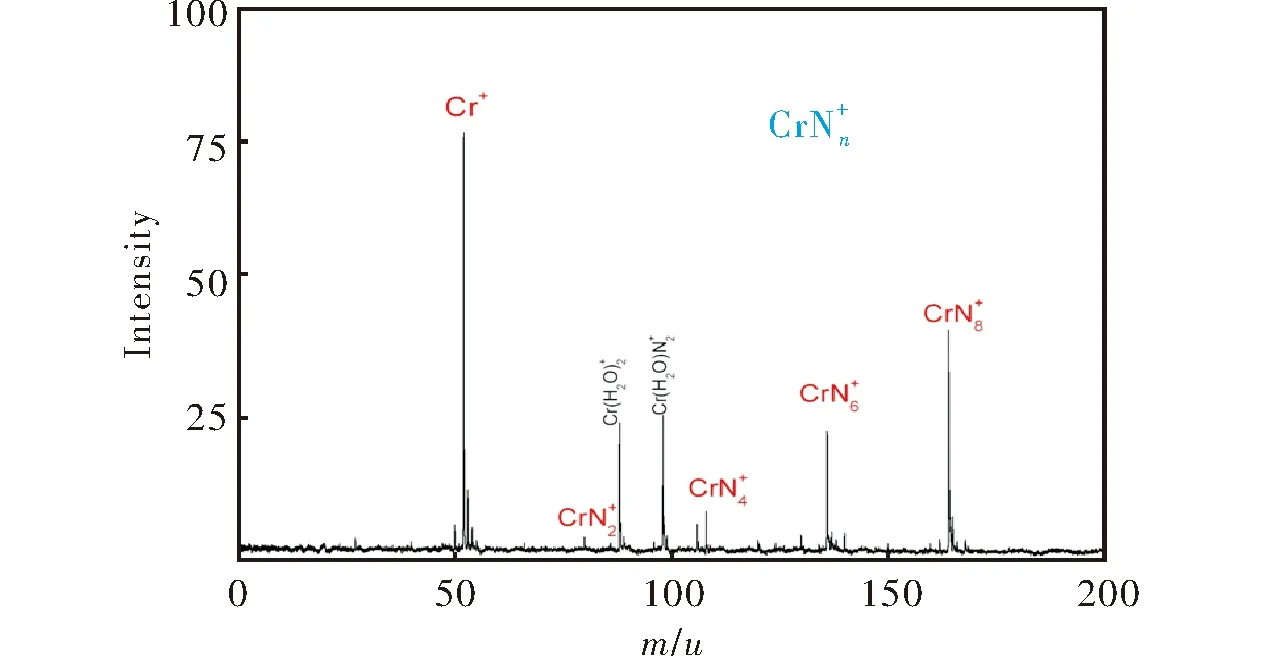
图1 激光轰击Cr/BN样品产生铬杂氮团簇的质谱图Fig.1 MS spectra of Cr-N clusters generated by laser ablation of Cr/BN sample

图团簇和团簇在266nm激光波长下的光解质谱图Fig.2 MS spectra of clusters Cr and Cr photodissociation by laser at a wavelength of 266nm

2.2 铬杂氮团簇的组成
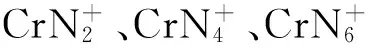
3 结 论
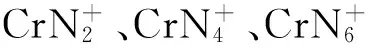
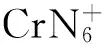
[1] 张光全,董海山. 氮簇合物——潜在的高能密度材料候选物[J]. 含能材料, 2004,12(增刊):105.
ZHANG Guang-quan,DONG Hai-shan. Nitrogen clusters——potential candidates as high-enerng dencity materials[J]. Chinese Journal of Energetic Materials,2004, 12(Supplement):105.
[2] Haiges R,Boatz J A,Schneider S,et al.The binary group 4 azides [Ti(N3)4], [P(C6H5)4][Ti(N3)5]and [P(C6H5)4]2[Ti(N3)6] and on linear Ti-N-NN coordination[J]. Angewandte Chemie International Edition,2004,43:3148-3152.
[3] Haiges R,Boatz J A,SchroerT,et al. Experimental evidence for linear metal-azido coordination: the binary group 5 azides [Nb(N3)5], [Ta(N3)5],[Nb(N3)6]-,and [Ta(N3)6]-,and 1∶1 acetonitrile adducts [Nb(N3)5(CH3CN)] and [Ta(N3)5(CH3CN)] [J]. Angewandte Chemie International Edition,2006, 45:4830-4835.
[6] Haiges R,Boatz J A,Bau R,et al. The first binary group 6 azides, Mo(N3)6, W(N3)6,[Mo(N3)7]-,and [W(N3)7]-,and the [NW(N3)4]-and [NMo(N3)4]-ions[J]. Angewandte Chemie International Edition,2005,44:1860-1865.
[7] Filippou A C,Portius P,Schnakenburg G. The hexaazidosilicate(IV) ion: synthesis, properties,and molecular structure[J]. Journal of the American Chemical Society,2002,124:12396-12397.
[8] Knapp C,Passmore J. On the way to “solid nitrogen” at normal temperature and pressure-binary azides of heavier group 15 and 16 elements[J]. Angewandte Chemie International Edition, 2004,43:4834-4836.
[11] Gagliardi L,Pyykkö P I. Predicted group 4 tetra-azides M(N-3)(4) (M = Ti-Hf,Th):the first examples of linear M-NNN coordination[J]. Chemisrty,2003,42(9):3074-3078.
[12] Li Q S,Duan H X. Density functional theoretical study of a series of binary azides M(N-3)(n)(n=3, 4)[J]. Journal of Physics Chemistry A,2005,109(40):9089-9094.
[13] Doeff M M ,Parker S F,Barrett P H,et al. Reactions of matrix-isolated iron atoms with dinitrogen[J]. Inorganic Chemistry,1984, 23(24):4108-4010.
[14] Chertihin G V,Andrews L,Neurock M. Reactions of laser-ablated iron atoms with nitrogen atoms and molecules. Matrix infrared spectra and density functional calculations of novel iron nitride molecules[J]. Journal of Physics Chemistry,1996,100(35):14609-14617.
[15] Chertihin G V,Andrews L,Bauschlicher C W. Reactions of laser-ablated scandium atoms with nitrogen: matrix infrared spectra and DFT calculations for scandium nitrides and the fixation of nitrogen by two scandium atoms[J]. Journal of the American Chemistry Soceity,1998,120(13):3205-3212.
[16] Citra A,Andrews L. Matrix infrared spectra of the osmium and ruthenium dinitride molecules. Evidence for direct insertion of osmium into the dinitrogen bond at cryogenic temperatures[J]. Journal of the American Chemistry Soceity,1999,121(49):11567-11568.
[17] Brock L R,Duncan M A. Photoionization spectroscopy of the IN-N-2 van-der-waals complex[J]. Journal of Chemistry Physics ,1995,102(24):9498-9505.
[18] Brock L R,Duncan M A. Threshold photoionization of Al-(N-2)(X) and Al-(CO2)(X) complexes-evidence for solvation-induced reactions[J]. Journal of Physics Chemistry,1995,99(45):16571-16575.
[19] Robbins D L,Brock L R,Pilgrim J S,et al. Electronic spectroscopy of the MG+-N-2 complex-evidence for photoinduced activation of N-2[J]. Journal of Chemistry Physics ,1995,102(4):1481-1492.
[20] Pullins S H,Reddic J E,France M R,et al. Photodissociation spectroscopy of the Ca+-N-2 complex[J]. Journal of Chemistry Physics ,1998,108(7):2725-2732.
[21] Pillai E D,Jaeger T D,Duncan M A. IR spectroscopy of Nb+(N2)ncomplexes:coordination,structures,and spin states[J]. Journal of the American Chemistry Soceity,2007,129:2297-2307.
[22] Pillai E D,Jaeger T D,Duncan M A. IR spectroscopy and density functional theory of small V+(N2)ncomplexes[J]. Journal of Physics Chemistry A,2005,109:3521-3526.
[23] Yang X,Gerasimov I,Dagdigian P. Electronic spectroscopy and excited state dynamics of the Al-N(2) complex[J]. Journal of Chemistry Physics,1998,239(1-3):207-221.



[26] 黄荣彬,刘朝阳,黄丰,等. 氮原子簇离子的质谱发现[J].化学通报,1995,68( 3):39.
HUANG Rong-bin,LIU ZHAO-yang,HUANG Feng,et al. Findation of N-cluster ions by mass spectrometry[J].Chemistry,1995,68(3):39.
[27] 唐紫超,石磊,黄荣彬,等. 碳氮二元簇离子的激光产生与碰撞诱导解离研究[J]. 化学学报,1997, 55(3),1191-1197.
TANG Zi-chao,SHI Lei,HUANG Rong-bin,et al. Collision induced dissociation of carbon-nitrogen cluster ions produced from laser bblation binary clusters[J].Acta Chimica Sinica,1997,55(3):1191-1197.
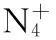
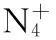
[29] 丁可伟,李陶琦,葛忠学,等. 多叠氮类化合物的合成研究进展[J].含能材料,2013,21(1):116-120.
DING Ke-wei,LI Tao-qi,GE Zhong-xue,et al. Review on synthesis of polyazides chines[J]. Journal of Energetic Materials,2013,21(1):116-120.
[30] 丁可伟,许洪光,李陶琦,等. TiN12团簇的制备研究[J]. 化学通报,2014,77(10):998.
DING Ke-wei,XU Hong-guang,LI Tao-qi,et al. Study on preparation of clusters TiN12[J]. Chemistry,2014,77(10): 998.
[31] Zhao Y C,Zhang Z G,Yuan J Y,et al. Modification of reflectron time-of-flight sass spectrometer for photodissociation of mass-selected cluster ionsy[J]. Chinese Journal of Chemistry Physics, 2009,22(6):655-662.
LI Tao-qi1,DING Ke-wei1,2,XU Hong-guang3,BU Jian-hua1,2,XIAO Xiao1,ZHENG Wei-jun3,GE Zhong-xue1,2
(1.Xi′an Modern Chemistry Research Institute,Xi′an 710065,China;2.State Key Laboratory of Fluorine & Nitrogen Chemicals,Xi′an 710065,China;3.Beijing National Laboratory for Molecular Science,Institute of Chemistry,Chinese Academy of Science,Beijing 100190,China)
nitrogen cluster;Cr-doped nitrogen clusters ; laser ablation;photodissociation;high energy density material
2017-04-07;
2017-07-07
装备发展部重大专项
李陶琦(1964-),男,副研究员,从事多氮化合物合成研究。E-mail:thankli64@163.com
郑卫军(1971-),男,从事分子动力学研究。E-mail:zhengwj@iccas.ac.cn
10.14077/j.issn.1007-7812.2017.06.009
TJ55;TQ203
A
1007-7812(2017)06-0055-04
