Effect of Magnesium Atom on CL-20
——A DFT Treatment
2018-01-08Lemirker
Lemi Türker
(Department of Chemistry Üniversiteler, Middle East Technical University, Ankara Turkey 06231)
Effect of Magnesium Atom on CL-20
——A DFT Treatment
Lemi Türker
(Department of Chemistry Üniversiteler, Middle East Technical University, Ankara Turkey 06231)
The effect of magnesium atom on 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtizane (HNIW, CL-20) explosive is considered within the constraints of density functional theory at the level of B3LYP/6-31++G(d,p). The Mg atom transfers some electron population to CL-20 and one of the nitro groups linked to 6-membered piperazine ring system (base) is expelled in the prenitrite form. The total Mulliken charges on the NO2group reveals that the respective nitramine bond in CL-20 is the most susceptible one to impact. The calculated IR and UV-VIS spectra are investigated. The effect of Mg atom on the molecular orbital energies, especially the HOMO and LUMO has been discussed. Narrowing of the interfrontier molecular orbital energy gap (Δε) in the composite system occurs. Therefore, the composite system is more susceptible to impact compared to CL-20.
CL-20; hexanitro hexaazaisowurtizane; explosives; magnesium; density functional theory
Introduction
2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtizane which is a cage-like compound, (commonly called CL-20 or HNIW) was synthesized in 1987 by Nelsen[1-3]. CL-20 is a novel, high-density (2.04g/cm3) cyclic nitramine which can be used as an energetic component in explosive formulations and propellants. Its standard molar enthalpy of formation is of the order of about 419kJ/mol[3-5]. It has several polymorphs[4]. The epsilon-form is the one having the highest density and greatest thermal stability among its polymorphs[5-6]. Its gas phase dissociation yields high concentration of NO2and a few ring fragments with nitro groups (the ring fragments of [CxHyNz]+have been detected[7-8]. Cocrystal formation of CL-20 with HMX has been reported[9]. Some polymer based explosive (PBX) formulations having CL-20 have been established[10]. Thermally induced damage in CL-20 was reported by Tian et al.[11-12]and also by Zeman and coworkers[13]. Its shock sensitivity in a polyisobutylene matrix was investigated[14-15]. Temperature-dependent shock initiation of CL-20-based high explosives was investigated by Pi et al.[16]. A detailed quantum chemical analysis for the instability of CL-20 exposed to structural variations was reported[17].
Aluminum is widely used in explosive compositions for high performance and high blast including CL-20[18]. Magnesium is an important ingredient of high-energy mixtures containing an oxidant and a reductant. An oxidant contained in mixture facilitates its combustion without access to air oxygen, which means that the desired special effect is possible. Magnesium containing compositions have found a broad range of applications in show and military pyrotechnics, in rocket propellants and explosive mixtures[19].
Composites of porous metal (nickel, aluminum and magnesium) and nano-scale aluminum powder and CL-20 have been synthesized with UV (ultraviolet) ink method, respectively[20]and the structure, morphology, infrared characteristic, thermal decomposition of composites were characterized. Chan and Turner patented various thermobaric explosive formulations with metastable mechanical alloys which include nano-sized Al-Mg, Al-Mg-H, B-Mg, Al-B,Ti-B, incorporate high energy explosive material including, hexanitrohexaaza-isowurtzitane (CL-20)[21].
In the present study, the interaction of Mg atom and CL-20 explosive is considered quantum chemically within the limitations of density functional theory (DFT).
1 Method of calculation
In the present study, after initial structure optimizations by using MM2 method, semi-empirical PM3 self-consistent fields molecular orbital (SCF MO) method[22-23]at the restricted level[24-25], then STO, RHF and density functional theory (DFT) (B3LYP/6-31G(d,p), B3LYP/6-31++G(d,p) levels)[26-27]type quantum chemical calculations were performed consecutively for the optimization of structures. The exchange term of B3LYP consisted of hybrid Hartree-Fock and local spin density (LSD) exchange functions with Becke′s gradient correlation to LSD exchange[27-28]. The correlation term of B3LYP consists of Vosko, Wilk, Nusair (VWN3) local correlation functional[29]and Lee, Yang, Parr (LYP) correlation functional[30].Vibrational analyses were done (using the same basis set employed in the corresponding structure optimizations) for each set of calculations. For each structure the normal mode analysis yielded no imaginary frequencies for the 3N-6 vibrational degrees of freedom, where N is the number of atoms in the system. This indicates that the structure of each molecule corresponds to at least a local minimum on the potential energy surface. Furthermore, all the bond lengths were thoroughly searched in order to know if any bond cleavage occurred during the structure optimization process. Structure optimizations and the vibrational analysis computations were performed by using the Spartan 06 package program[31].
2 Results and discussion
Molecules and atoms engender potential fields around them originating from their protons, electrons and distribution of them in the molecular/atomic structure. When those species are in the vicinity of each other certain perturbations of electrostatic potential field occur. The consequence of it results some polarization effects and at the extreme cases ionization processes even bond dissociation(s) may occur.
Stability of an explosive material to various stimuli is an important phenomenon to be considered from various points of view, including its synthesis, storage, safety etc. One of the stimuli is electrostatic discharge which is a serious problem in general in the chemical industry and especially important related to explosive materials.
Magnesium atom having 1s22s22p63s2electronic configuration in the ground state is an active element. Mg, B and Al like elements are common ingredients of some composite energetic materials[32]. They contribute the heat evolved while they are oxidized. However, most of the explosives like CL-20 contain various numbers of nitro group(s) which is/are rather strong oxidizing moiety in general. On the other hand, those elements, especially Mg is an easily oxidizable metal. Therefore, some interaction of Mg with NO2having molecules are expected which may affect the ballistic properties as well as the shelf-life of those composite type explosives. On the other hand, it is to be mentioned that the oxidation power of NO2group depends on its location in a molecule where it is attached. CL-20 molecule possesses six nitramine type NO2groups. Therefore, any conjugative effects present and involving the NO2group may highly influence that oxidative power.
Figure 1 shows the optimized structures of CL-20 and its Mg composite, CL-20+Mg.
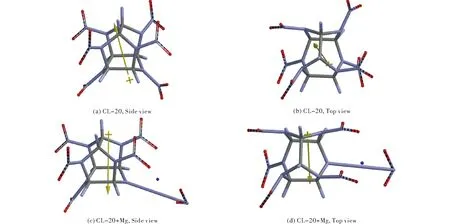
Fig.1 Optimized structures for CL-20 and CL-20+Mg
As seen from Figure l, the effect of Mg atom nearby CL-20 is very dramatic and causes the rupture of one of the nitramine bonds by expelling the NO2group off the structure. In the present study Mg atom initially located at various sides of the molecule, always yielded the optimized structure shown in Figure 1. The bond lengths/distance(s) in CL-20 and the Mg composite of it indicate that of the six nitramine bonds present in the structure, only the one associated with the 6-membered ring (piperazine base ring) undergoes bond cleavage (see Figure 1 and the Figure 2). In CL-20 the nitramine bonds of the piperazine ring are 1.41 Å, whereas in the composite system the intact nitramine bond is 1.40 Å. The other one is broken and the respective nitrogen to nitrogen distance is 4.16 Å. Also it is to be noted that the direction of dipole moment of CL-20 is almost reversed by the effect of Mg atom (see Figure 1).

Fig.2 Bond lengths/distances for CL-20 and CL-20+Mg (hydrogens are not shown)
The ESP charges (see Figure 3) are obtained by the program based on a numerical method that generates charges that reproduce the electrostatic potential field from the entire wavefunction[31]. The results indicate the Mg atom acquires some positive charge (1.442 esu) which is indicative of some electron transfer from the metal to CL-20 molecule. Indeed the charge of amino nitrogen of CL-20, next to the expelled NO2group changes from -0.018 esu (CL-20) to -1.017 esu (CL-20+Mg system).
Figure 4 shows the calculated IR spectra (time dependent DFT) of CL-20 and its magnesium composite. In the CL-20 spectrum, the peaks at 1691-1648 cm-1are due to N-O stretchings of various nitro groups. The C-H stretchings of the 6-membered base ring occur at 1421-1415cm-1. The peaks at 1380-1300cm-1belong to various bendings and stretchings. In the spectrum of CL-20 composite, N-O stretchings occur at 1683-1570cm-1. The region in between 1424-1409cm-1includes C-H stretchings of the 6-memebered ring system. Those C-H bonds bend at 1378-1369cm-1. The nitramine N-NO2stretchings occur at 1358cm-1overlapped with various bendings. The dispelled NO2stretchings take place at 1347cm-1.
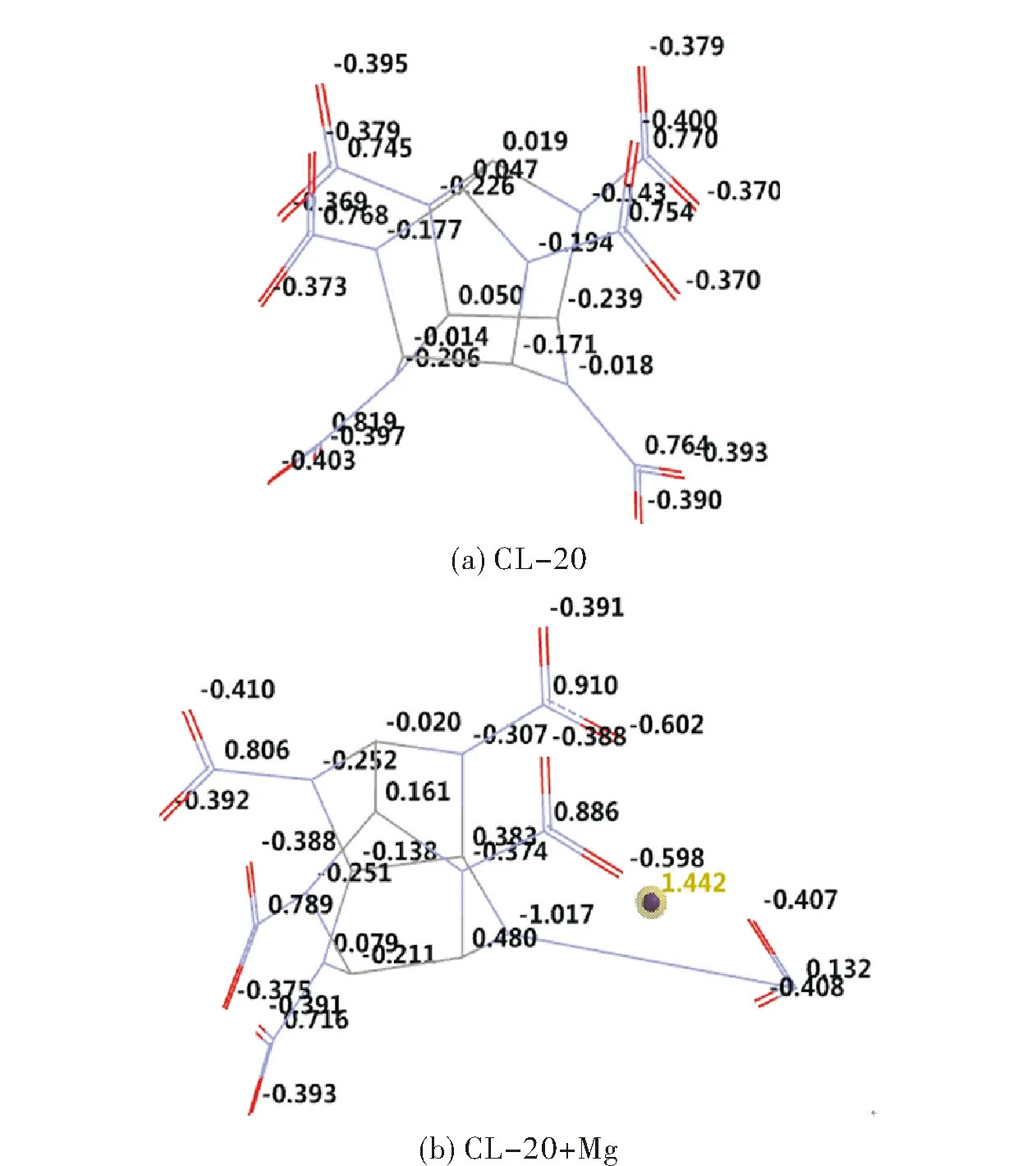
Fig.3 Electrostatic charges (ESP ) in CL-20 and CL-20+Mg (hydrogens not shown)
On the other hand, Zhang et al[33]. proposed a method of correlation to predict the impact sensitivities of nitro compounds based on nitro group charges (Mulliken). The more negative charge the nitro group possesses it is less likely to split from the backbone (R-NO2). In systems conjugated with the nitro group, some electron population can be transferred to NO2moiety via mesomerism and consequently the bond order between the NO2and its neighbor atom in the backbone increases. Meanwhile the bond length is expected to shorten. However, that relationship is not so explicitly occurring all the time.

Fig.4 IR spectra of CL-20 and CL-20+Mg
Figure 5 shows the numbering of atoms and Mulliken charges on CL-20 structure. In Table 1, the Mulliken charges on atoms of the nitro groups and the total charge of the nitro groups are depicted. The nitrogen labeled N6 is embedded in the 6-membered piperazine ring and the nitro group linked to it has the highest positive total charge. Consequently it is the most susceptible NO2group to be affected by an impact. In the present study, it is the most susceptible NO2group to the effect of Mg atom. Note that, although N5 and N6 atoms are embedded into the same ring (piperazine ring) the Mulliken charges on them as well as on the nitro groups linked to them are different. This unsymmetrical outcome should be due to the variations of conformational differences of the nitro groups present (see Figures 1 and 4).

Fig.5 Numbering of atoms and Mulliken charges in CL-20 (hydrogens not shown)
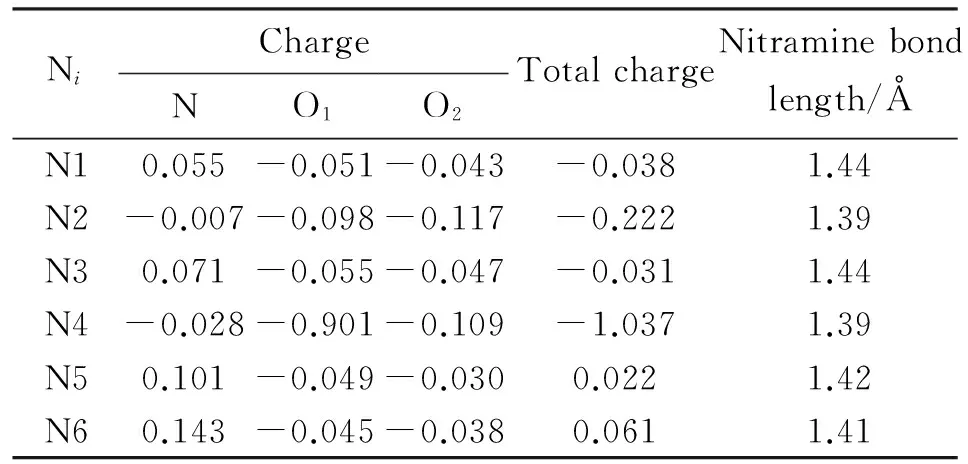
NiChargeTotalchargeNitraminebondlength/ÅNO1O2N10.055-0.051-0.043-0.0381.44N2-0.007-0.098-0.117-0.2221.39N30.071-0.055-0.047-0.0311.44N4-0.028-0.901-0.109-1.0371.39N50.101-0.049-0.0300.0221.42N60.143-0.045-0.0380.0611.41
Figure 6 displays some of the molecular orbitals of CL-20 and CL-20+Mg systems. Obvious from the figure, the presence of Mg atom highly perturbs the molecular orbital energy spectrum of CL-20. Table 2 includes the HOMO and LUMO energies and the interfrontier energy gaps (Δε) for these systems within the constraints of density functional theory. As seen in the table Mg atom raises up the HOMO but lowers the LUMO energy level and the consequence of it is narrowing of the interfrontier molecular orbital energy gap (Δε). Note that almost degenerate LUMO and next LUMO of CL-20 is widely separated in the composite system. It is known that the impact sensitivity of explosives increases as the HOMO-LUMO energy gap decreases[34]. Therefore, the Mg composite of CL-20 system should be more susceptible to an impact stimulus.
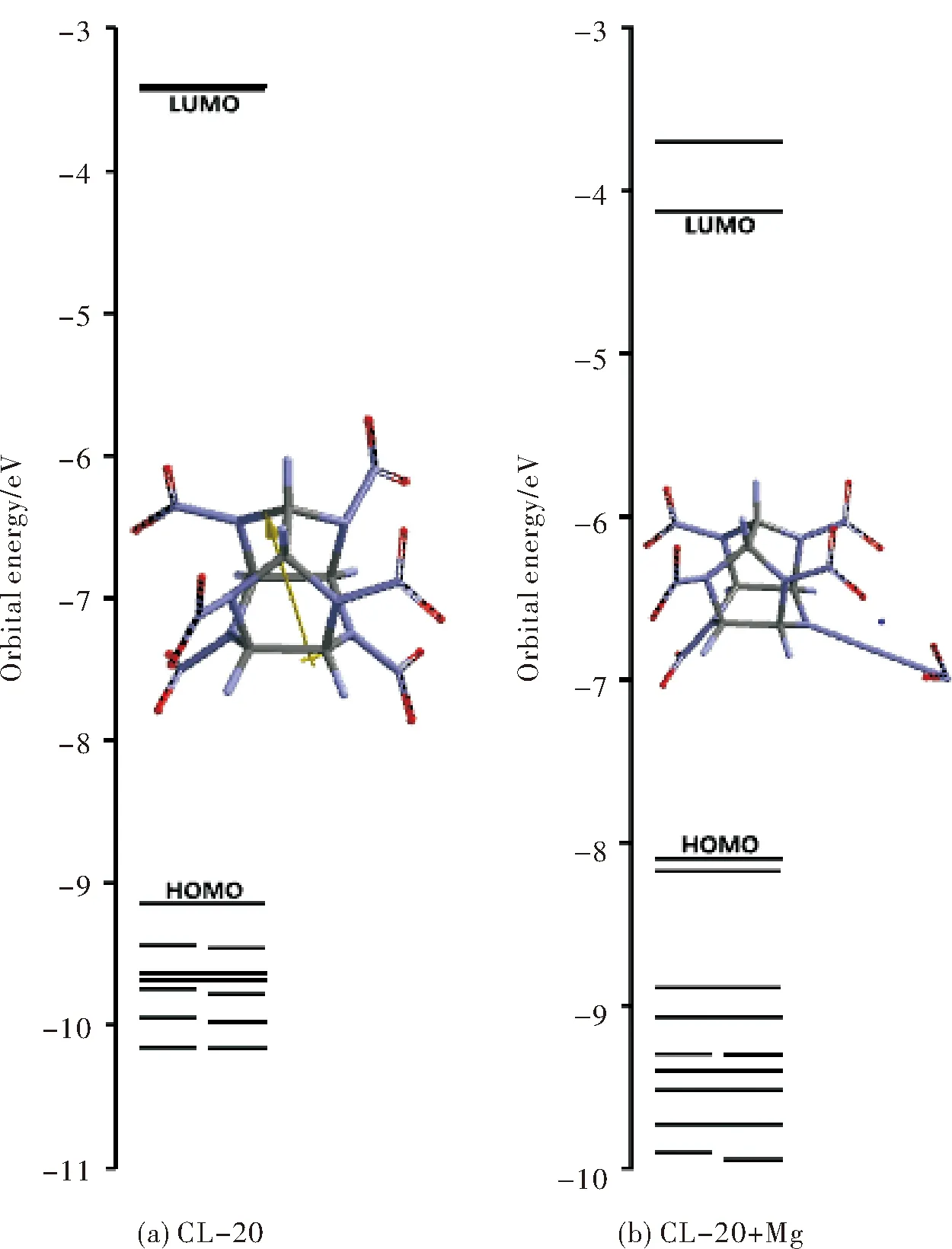
Fig.6 Some of the molecular orbital energies of CL-20 and CL-20+Mg

CompoundHOMO/(kJ·mol-1)LUMO/(kJ·mol-1)Δε/(kJ·mol-1)CL-20 -882.74-330.89551.85CL-20+Mg-781.50-397.77383.73
Note:Energies in kJ/mol.
Figure 7 shows the UV-VIS spectra (Time dependent DFT) of the systems of present concern (within the constraints of DFT). Since, the interfrontier molecular orbital energy gap of the composite system is narrower than CL-20, some bathochromic effect is observed in the spectrum of the composite, compared to CL-20 spectrum.

Fig.7 UV-VIS spectra of CL-20 and CL-20+Mg
Figure 8 shows the HOMO and LUMO pattern of CL-20 and its Mg composite. As seen in the figure, the effect of Mg atom on CL-20 is highly dramatic. Most of the atoms of CL-20 contributing to the HOMO and LUMO become ineffective in the composite system. The NO2group expelled has appreciable contribution to over all HOMO but nothing to LUMO of the composite system.


Fig.8 The HOMO and LUMO patterns of CL-20 and CL-20+Mg
Figure 9 shows the electrostatic potential maps of the systems considered. As seen in the figure, around NO2group dispelled from CL-20 some negative potential exists as revealed by charge calculations. In both systems core of the cage has some positive potential field development.
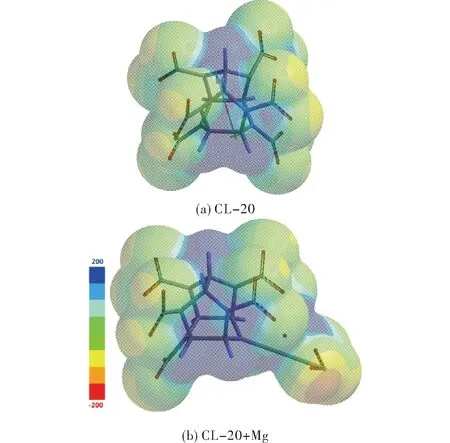
Fig.9 Electrostatic potential maps for CL-20 and CL-20+Mg
3 Conclusions
(1) Of the six nitramine bonds present in CL-20 only the one embedded in to 6-membered piperazine ring is expelled.
(2) The total electrostatic charge (ESP) of expelled NO2moiety is negative (-0.683 esu) and it is in prenitrite form.
(3) The Mg atom transfers some electron population to CL-20 system thereby acquires some positive charge.
(4) Mg atom raises up the HOMO but lowers the LUMO energy level of CL-20 and the consequence of it is narrowing of the interfrontier molecular orbital energy gap (Δε) in the composite system. Therefore, the composite system is more susceptible to impact.
[1] Nielsen A T. Caged polynitramine compound: US,5693794 [P]. 1997.
[2] Nielsen A T, Chafin A P, Christian S L, Moore D W, Nadler M P, Nissan R A, Vanderah D J, Gilardi R D, George C F,Flippen-Anderson J L. Synthesis of polyazapolycyclic caged polynitramines [J]. Tetrahedron, 1998, 54 : 11793-11812.
[3] Bayat Y, Mokhtari J, Farhadian N , Bayat M. Heteropolyacids: an efficient catalyst for synthesis of CL-20 [J]. Journal of Energetic Materials, 2012, 30 (2): 124-134.
[4] Politzer P, Murray J S. Energetic Materials, Part 1, Decomposition, Crystal and Molecular Properties [M]. Amsterdam: Elsevier, 2003.
[5] Foltz M, Coon C L, Garcia F, Nichols A L. III. The thermal stability of the polymorphs of hexanitrohexaazaisowurtzitane. Part I [J].Propellants, Explosives, Pyrotechnics,1994, 19: 133-145.
[6] Jiang X, Guo X, Ren H, Jiao Q. Preparation and characterization of desensitized ε-HNIW in solvent-antisolvent recrystallizations[J].CEJEM,2012,9:219-236.
[7] Doyle R J Jr. The gas-phase dissociation of a new polyazapolycyclic nitramine: hexanitrohexaazaisowurtzitane [J]. Org Mass Spectrom,1991,26:723-726.
[8] Patil D G, Brill T B. Thermal decomposition of energetic materials 53. Kinetics and mechanism of thermolysis of hexanitroazaisowurtizitane [J]. Combust Flame,1991,87:145-151.
[9] Bolton O, Simke L R, Pagoria P F, Matzger A J. High power explosive with good sensitivity: A 2:1 cocrystal of CL-20:HMX [J]. Cryst Growth Des, 2012, 12: 4311-4314.
[10] Nair U R, Asthana S N, Rao A S, and Gandhe B R. Advances in high energy materials [J]. Defence Science Journal, 2010,60(2):137-151,
[11] Tian Q, Yan G, Sun G, Huang C, Xie L, Chen B, Huang M, Li H, Liu Y , Wang J. Thermally induced damage in hexanitrohexaazaisowurtzitane [J]. CEJEM, 2013, 10:359-369.
[12] Wang D, Gao B, Yang G, Nie F, Huang H. Preparation of CL-20 explosive nanoparticles and their thermal decomposition property [J]. Journal of Nanomaterials, 2016, 20: 5462097/1- 5462097-7.
[13] Zeman S, Yan Q L , Vlacek M. Recent advances in the study of the initiation of energetic materials using characteristics of their thermal decomposition. Part I. Cyclic nitramines [J].CEJEM,2014,11:173-189.
[14] Pelikan V, Zeman S, Yan Q L, Erben M, Elbeih A, Akstein Z. Concerning the shock sensitivity of cyclic nitramines incorporated into a polyisobutylene matrix [J]. CEJEM,2014,11:219-235.
[15] Molt R W J, Bartlett R J, Watson T J, Bazanté A P. Conformers of CL-20 explosive and ab initio refinement using perturbation theory: implications to detonation mechanisms [J]. J Phys Chem A,2012,116(49):12129-12135.
[16] Pi Z, Chen L, Wu J. Temperature-dependent shock initiation of CL-20-based high explosives [J]. CEJEM, 2017, 14(2):361-374.
[17] Türker L. Instability of CL-20 exposed to the effects of α-particle [J]. Indian Journal of Chemistry A, 2015, 54: 858-866.
[18] Nicolich S M, Capellos C, Balas W A, Akestar J D, Hatch R L. High-blast explosive compositions containing particulate metal: US8168016 B1, US 10/907,599 [P].2012.
[19] Türker L. Thermobaric and enhanced blast explosives (TBX and EBX) [J]. Defence Technology,2016,12(6):423-445.
[20] Li Z H, Ren H, Jiao Q J, Dong J. Fabrication and characterization in composites of nanoenergetic materials and porous metal with uvink method [J]. Integrated Ferroelectrics, 2014,152:73-80.
[21] Chan M L, Turner A D. High energy blast explosives for confined spaces: US,20070113939 A1 (US 11/482,302) [P]. 2007.
[22] Stewart J J P. Optimization of parameters for semiempirical methods I. method [J]. J Comput Chem, 1989, 10:209-220.
[23] Stewart J J P. Optimization of parameters for semi empirical methods II. application [J]. J Comput Chem, 1989,10:221-264.
[24] Leach A R. Molecular Modeling [M]. Essex : Longman, 1997.
[25] Fletcher P. Practical Methods of Optimization [M]. New York :Wiley, 1990.
[26] Kohn W, Sham L J. Self-consistent equations including exchange and correlation effects [J]. Phys Rev, 1965,140 A1:1133-1138.
[27] Parr R G , Yang W. Density Functional Theory of Atoms and Molecules [M]. London: Oxford University Press, 1989.
[28] Becke A D. Density-functional exchange-energy approximation with correct asymptotic behavior [J]. Phys Rev A, 1988, 38:3098-3100.
[29] Vosko S H, Vilk L, Nusair M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis [J]. Can J Phys,1980,58:1200-1211.
[30] Lee C, Yang W, Parr R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density [J]. Phys Phys Rev,1988, 37B : 785-789.
[31] Spartan 06 Program[CP/DK].Wavefunction Inc, Irvine, CA.
[32] Klapötke T M.Chemistry of High-energy Materials [M]. Berlin:De Gruyter,2011.
[33] Zhang C, Shu Y, Huang Y, Zhao X, Dong H. Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds [J]. J Phys Chem B, 2005, 109: 8978-8982.
[34] Ovens E J. Relationship between impact induced reactivity of trinitroaromatic molecules and their molecular structure [J]. J Mol Struct (Theochem), 1984, 121: 213-220.
10.14077/j.issn.1007-7812.2017.06.003
TJ55;TQ560 Document Code:A Article ID:1007-7812(2017)06-0017-06
date:2017-09-17; Revised date:2017-11-10
Biography:Lemi Türker(1950-),male,Prof.,Dr.,research field:organic chemistry.E-mail: lturker@metu.edu.tr
