Ballistic Testing and Thermal Behavior of Cast Double-base Propellant Containing BuNENA
2018-01-08AhmedMaradenPetrStojanRobertMatyaJanZigmund
Ahmed Maraden, Petr Stojan,Robert Matyaš,Jan Zigmund
(1. Institute of Energetic Materials, Faculty of Chemical Technology, University of Pardubice, CZ;2. OZM Research, s.r.o., Blížňovice 32, 538 62 Hroch v T nec, CZ ; 3.Explosia a.s.,Semtín 107, 530 02 Pardubice, Czech Republic)
Ballistic Testing and Thermal Behavior of Cast Double-base Propellant Containing BuNENA
Ahmed Maraden1, Petr Stojan2,Robert Matyaš1,Jan Zigmund3
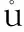
Preparation of cast double-propellant grains depends on the ability of nitrocellulose powder to swell and coalesce into a coherent mass when treated with a suitable solvent. The cast double-base process has been developed into a highly versatile technique for manufacturing solid rocket charges. Propellants manufactured by this process provide a wide range of energies and burning rates. Successful preparation of cast double-base propellant grains has been performed using compatible casting liquid with the casting powder. BuNENA was used as an energetic plasticizer for manufacturing of casting powder. Burning rate measurements have been performed using closed bomb SV-2 to investigate the burning behavior along a wide range of operating pressure. Plateau burning had been detected in pressure range (50-70)×105Pa for the composition included BuNENA. DTA and TGA thermal analysis were conducted to evaluate the thermal behavior of the prepared cast double-base propellants. Results from DTA were used to calculate the apparent activation energy.
cast double-base propellant; burning rate; thermal analysis; BuNENA plasticizer
Introduction
Cast double-base(CDB) propellant is designed to meet the desired ballistic requirements[1]. The casting process is a technique for forming the solid propellant grains based on nitrocellulose as the polymeric binder and nitroglycerin or other high energy liquids as plasticizers. Many factors can affect the performance of CDB propellants such as the composition, manufacturing process, loading density, addition of burning rate modifier and the propellant grain surface area[2]. The use of energetic additives, mainly binder and plasticizers containing groups such as nitro, nitrato, fluorodinitro, etc. is considered to be one of the practical ways to improve the energy level, physical integrity and other technical performances of solid propellants[3-5]. The n-butyl nitroxyethylnitramine (BuNENA) have excellent plasticizing characteristics[6-11]which impart good physicochemical properties to the propellant.
The manufacturing process consists of two essential steps:(1) production of casting powder consisting of nitrocellulose, plasticizers, and any solid ingredients. (2) Casting and curing: casting powder is loaded into a mold; the interstices between the granules are filled with a casting solvent containig plasticizer. On heating to moderate temperatures, interdiffusion of polymer and plasticizer occurs and knits the two-component system into a single monolithic grain. The homogeneity of ingredients to each other′s is important to determine if the resulting propellant is of uniform composition. The ratio of liquid to solid is also important in preparing CDB grains. The high surface area per unit mass of nitrocellulose in the casting powder may cause in rapid absorption of the casting liquid by the casting powder. The composition of the casting liquid can be varied widely depending on the overall ballistic properties desired in the cured propellant grain. The aim of this work is to acheive an appropriate homogeneous composition including an energetic plasticizer, BuNENA, to produce a CDB propellant grain which has a good ballistic and thermal properties and plateau burning-effect.
1 Experimental
1.1 Preparation of CDB propellant
The first step in preparation of CDB propellant is to produce casting powder. The manufacturing process may be divided conveniently into four steps: mixing, granulating, drying and then finalizing the product. Nitrocellulose (w(N)=13.1%) was introduced into the process in a lumpy condition. All other solid ingredients are added and the resulting premixed serves as a feed for the mixer, a heavy duty, horizontal, sigma-blade Brabender mixer. The optimum capacity for the mixer is 200 grams of dough. The mixer is jacketed to provide circulation of hot or cold water during various stages in the mixing operation maintaining mixing temperature of 20℃. Table 1 shows the formulations of casting powder used to manufacture of cast double base grains. A solution of volatile solvents (mytalal, ethanol and acetone; 40∶40∶20 by volume) is poured to the premixed solid ingredients and gelatinizing nitrocellulose for 90minutes at 20r/min. The shaping or granulating operation proceeds by loading the dough into a cylinder and subjecting it to pressure of 10bars for extrusion. Extrusion rates are normally controlled by orifices in the hydraulic supply line and the velocity of piston head (73mm/min). After extrusion, the strands of diameter 1mm are cut using standard small arms. Precise control ofL/Dis complex since it depends on shrinkage characteristics during drying. Thus, the cutting operation must be conducted with full awareness of dimensional changes to be expected in the extrusion operation preceding it and the drying operation following it.

Table 1 Formulations of the prepared nitrocellulose based casting powder
A considerable amount of volatile solvent is removed by evaporation during the latter stages of mixing. The remainder must be removed in the drying operation. Therefore, it goes through a water dry operation, after complete drying, the average grain length is 0.81mm and average diameter is 0.79mm, several operations are required such as glazing, which reduces the electrostatic charge and promotes easy flow. A small amount (0.05%) of graphite is added to the propellant granules and distributed over the surface of the granules. Finally, screening takes place to remove clusters, dust, and foreign material.
The second stage for manufacturing of cast double base is casting process. The rate and uniformity with which casting powder granules are plasticized by casting solvent are critical in determining the proper cure conditions and the properties of the final propellant. In this work, the process of "in situ" casting, also called granular casting, has been used. The first basic step is to fill a mold or the motor case with casting powder. Ideally, the technique selected to fill the mold is one which results in maximum reproducible loading density. Simple dumping and pouring yields low packing density. In well-loaded units, the packing density is such that 68%(vomule fraction) of the container is occupied by casting powder. Simple dumping might lead to only 57%. In combination with the above techniques, vibration has also been used to achieve maximum bulk density in mold. The powder bed is usually vibrated by externally mounted, pneumatic vibrators. After the mold is filled with casting powder, it is subjected to a vacuum. The casting step consists of filling the interstitial space in the powder bed with casting solvent. A mixture of nitroglycerin with triacetein (7∶3)(mass ratio) was used as energetic plasticizer with triacetein to facilitate the diffusion of nitroglycerin into nitrocellulose. The ratio of casting liquid to casting powder in the mold was 1.1∶1.0 by mass. The casting solvent may be introduced to the bed from the top of the mold or from the bottom. The curing process is carried out during two distinct periods: an ambient rest period in which the unit is simply stored one or more days at 25℃ and a second period in a cure bay at 50-60℃ for a longer period. After several days to 2 weeks at the elevated cure temperature, the propellant has been converted to a macroscopically homogeneous mass by mutual diffusion of nitrocellulose and plasticizers. The propellant charge is then permitted to cool to room temperature.
1.2 Burning rate measurement
In general the burning rates obtained by different techniques are not the same; even using identical specimens and the same technique at different facilities, the measured burning rates are different due to a variety of details not fully controllable or controlled. Several closed vessel configurations are currently available to obtain the burning rate of the propellant from experimental pressure records in time. The SV-2 closed bomb is used for measuring the dependency of the burning rate of solid rocket propellants on the operating pressure. A single shot is sufficient for plotting burning rates in the whole pressure range.
1.3 Thermal analysis
Various data are available on the thermal behaviour and kinetics of decomposition of nitrocellulose compounds[12-22]. A DTA 550 Ex apparatus was used for differential thermal analysis[23]. The measurements were carried out at atmospheric pressure, with the tested sample in direct contact with the air. The sample (5 mg) was placed in a test tube made of Simax glass, 5 mm in diameter and 50 mm long. The reference standard was 0.05g aluminium oxide. A linear heating rate of 5, 10, 15 and 20℃/min was used. Results were treated with the Kissinger model to evaluate activation energies for double-base propellant compositions[24-27].
DSC experiments were carried out on a model DSC190S instrument at atmospheric pressure and laboratory temperature of 20℃. The conditions of DSC were reference standard was an empty crucible, sample mass about 2.00mg, heating rates 2, 5, 10, 15 and 20℃/min, a flow rate of N2gas of 45mL/min. Thermal analysis tests were performed for temperature range starts with lab temperature of 20℃ and ends with400 ℃.
Thermal decomposition experiments of the propellant samples were carried out under nitrogen atmosphere in a TGA instrument. In all experiments,2.0-3.0mg of sample was loaded in an open 90μL alumina pan and heated. Nitrogen at a flow rate of 50mL/min was used as the purge gas. TG runs were conducted at heating rates (β) 2, 5, 10 and 15℃/min and the collected data was used for further analysis.
2 Results and Discussion
2.1 Double base formulations
Three different cast double-base grains were prepared by introducing the casting liquid from the bottom of the casting powder bed. The curing time was about 15 days at elevated temperature of 60℃. The curing time for samples containig single-base casting powder is slightly longer than that samples containig RDX-based casting powders. The grains are left to reach lab temperature of 20℃. The appearance of the grains was good and showed that the casting liquid was completely diffused into casting powder forming homogenous double base grains. Cylindrical grains of 50mm height, 28mm diameter and 50g mass were prepared. The calculated density of the cylindrical grains was about 1.67g/cm3.
2.2 Ballistic testing using Stojan Vessel
Studying the burning dependency on formulations was carried out by three shots for each composition at lab temperature. The pressure profiles were reported. An advanced mathematical procedure based on the most modern computational and ballistic procedures was applied to calculate the burning rate (u) dependency on the pressure. Figure 1 shows the results obtained from firing of one CDB propellant grain for each composition.
As predicted, the burning rate is increasing with pressure. The behavior of burning rate is studied for pressure range (15-65)×105Pa. For compositions A-01 and A-02, the burning rate increased linearly with pressure and did not show a plateau burning region. Rate of increase in burning rate for composition A-01, which has BuNENA as an energetic plasticizer, is higher than that for A-02. Composition A-04 showed relatively higher burning rate regime compared with other two compositions. It also showed independency of burning rate on pressure for pressure range (50-70)×105Pa offering a reasonable plateau burning region.

Fig.1 Burning rate dependancy on operating pressure using SV-2
2.3 Thermal analysis
Figure 2 shows DTA curves for the prepared cast double base at heating rate 5℃/min. Exothermic peaks corresponding to the decomposition of compositions showed a slight temperature difference. Maximum peak temperatures for each composition at different heating rates are given in Table 2. A-01 decomposition curve showed three distinct peaks for BuNENA, nitrocellulose and RDX at 170℃, 192℃ and 230℃ respectively.
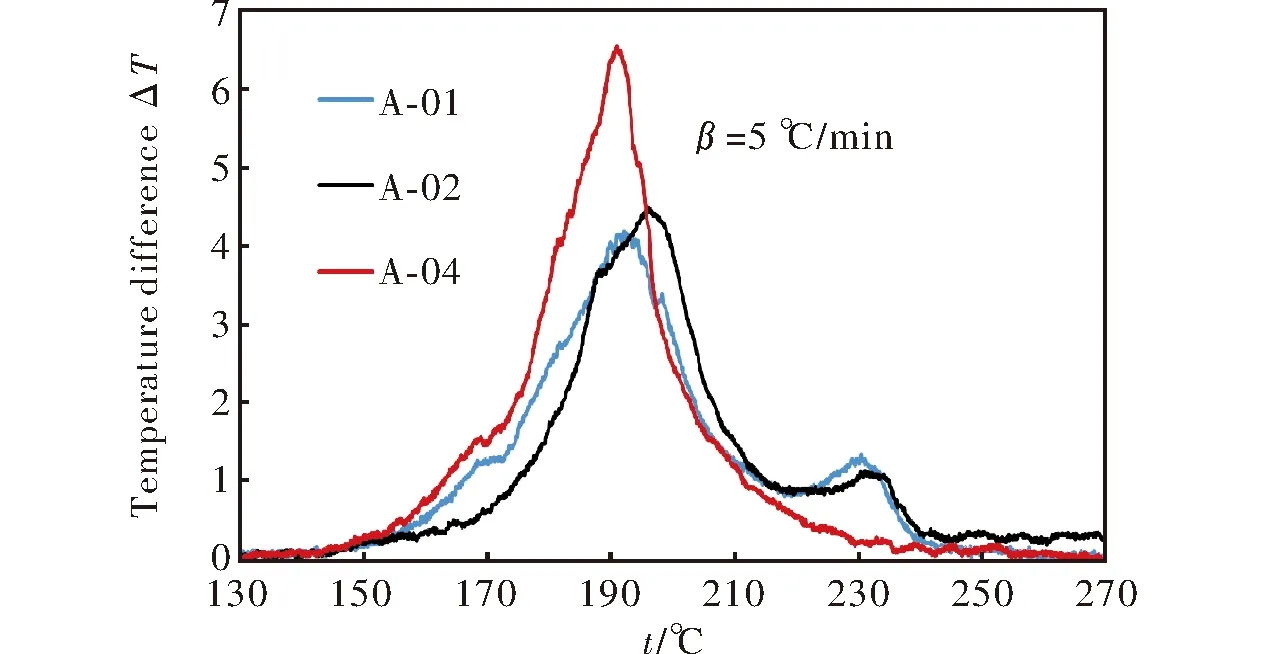
Fig.2 DTA curves for samples at heating rate of 5℃/min
A-02 showed only two decomposition peaks according to decomposition of nitrocellulose at 196℃ and decomposition of RDX at 232℃. A-04 also showed two decomposition peaks due to BuNENA decomposition at 169℃ and nitrocellulose decomposition at 194℃.

Table 2 Maximum peak temperatures at different heating rates
DTA data are treated for quantitative evaluation of activation energies by using the model proposed by Kissinger which takes form[28-30].
(1)
Where,Φis the heating rate (℃/min),Eais the activation energy (kJ/mol),Ris the gas constant,Ais the frequency factor for thermal decomposition andTis the exothermic peak temperature (K).
Figure 3 shows the graphical representation for DTA data from table 2 treated by Kissinger model.

Fig.3 Plots of Kissinger to evaluate the activation energy Ea
The plot of the ln (ΦT-2) against 1/Twas straight lines, which indicated that the mechanism of thermal decomposition of powders is first order[31-32]. The preliminary estimation for activation energies can be done from the slopes of the regression lines. The slope of the lines is equal to -Ea/R. Therefore, the activation energy (Ea) was obtained from the slope of the graph. Values of the activation energy and logarithm of frequency factor for samples are tabulated in Table 3.

Table 3 Calculated activation energy using Kissinger model
The value ofEacan be determined by an isoconversional method without assuming the kinetic model function[33-34]. A single-step rate equation cannot generally be adequate for a
multi-step mechanism. However, it can provide an adequate kinetic representation of a multi-step process that has a single rate-limiting step. The occurrence of a multi-step process does not immediately invalidate the application of the isoconversional principle, although the latter holds strictly for a single-step process. The principle continues to work as a reasonable approximation because isoconversional methods describe the process kinetics by using multiple single step kinetic equations[35]. In case of a mechanism of two consecutive reactions when the first reaction is significantly slower than the second, the first process would determine the overall kinetics that would obey a single-step, whereas the mechanism involves two steps.
On the other hand, the activation energies were studied using differential scanning calorimetry (DSC) and (Ea) was calculated by Friedman method[12-14]. In this method the degree of conversion can be computed on the basis of the DSC curves. The activation energy can be determined by Friedman method without a precise knowledge of the reaction mechanism from plots of the logarithm of the heat flux versus the inverse of the temperature. The regression correlation coefficientsR2are all greater than 0.98. Figure 4 shows DSC curves for propellant compositions at heating rate 5℃/min. The calculated activation energies and the dependency of the activation energy on conversion rate are illustrated in Table 4.
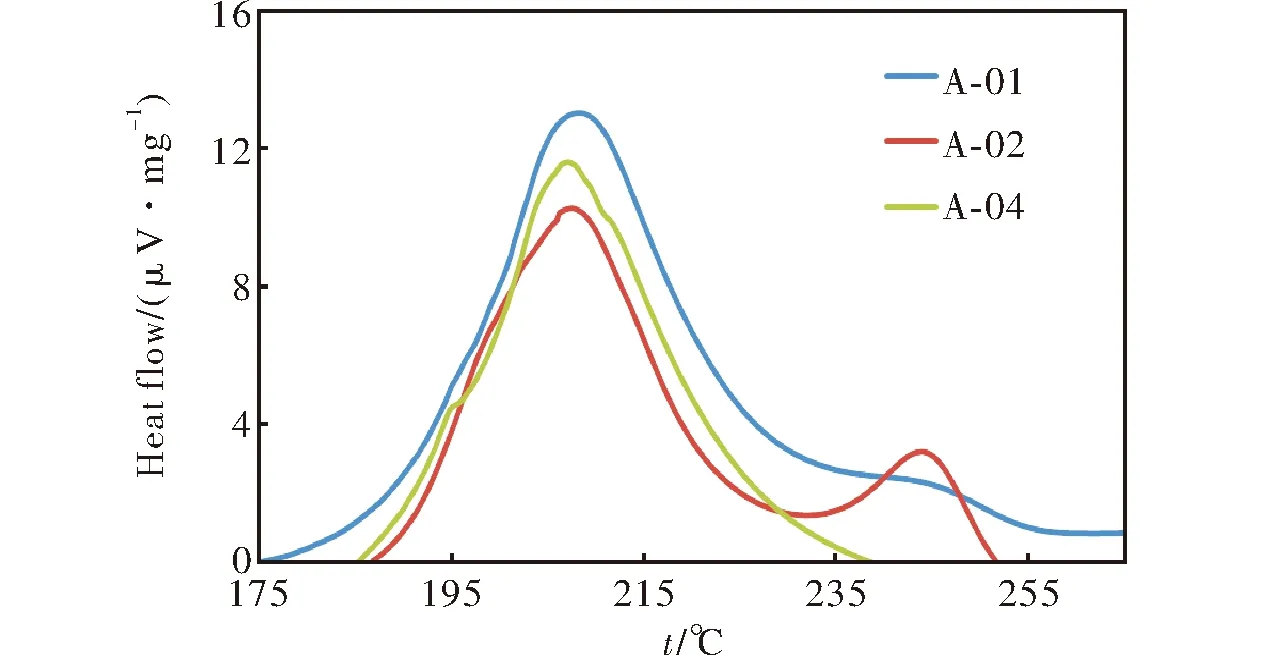
Fig.4 DSC curves for samples at heating rate of 5℃/min
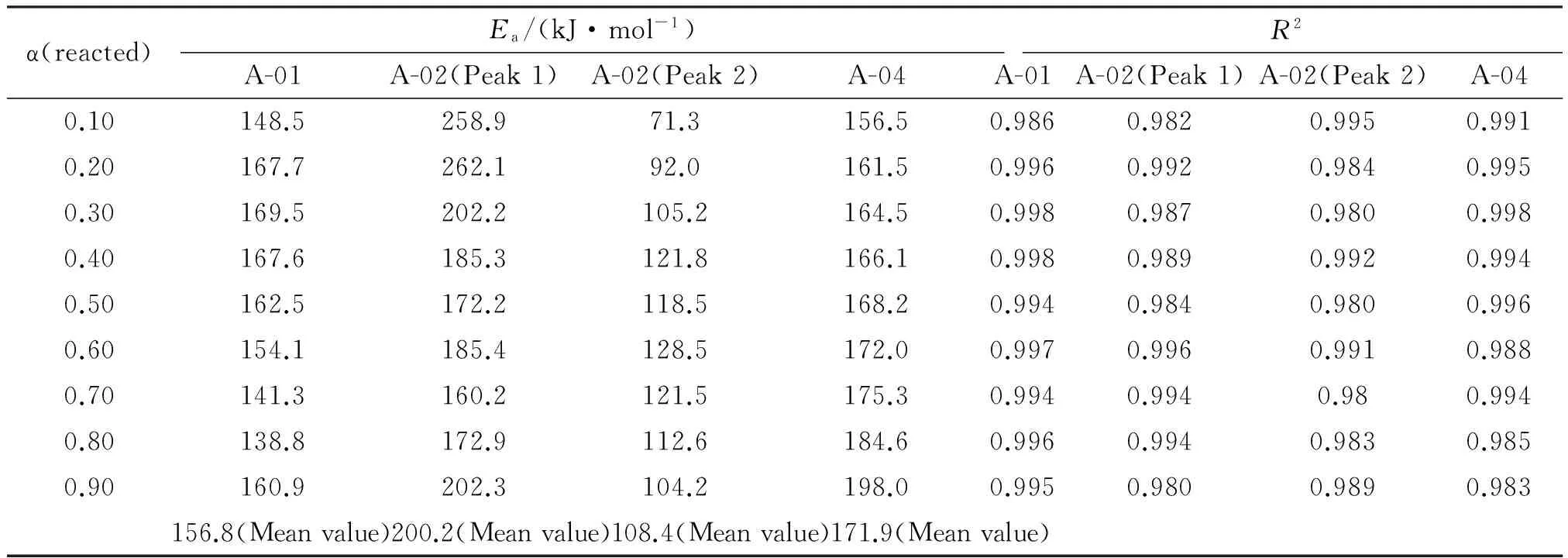
α(reacted)Ea/(kJ·mol-1)R2A-01A-02(Peak1)A-02(Peak2)A-04A-01A-02(Peak1)A-02(Peak2)A-040.10148.5258.971.3156.50.9860.9820.9950.9910.20167.7262.192.0161.50.9960.9920.9840.9950.30169.5202.2105.2164.50.9980.9870.9800.9980.40167.6185.3121.8166.10.9980.9890.9920.9940.50162.5172.2118.5168.20.9940.9840.9800.9960.60154.1185.4128.5172.00.9970.9960.9910.9880.70141.3160.2121.5175.30.9940.9940.980.9940.80138.8172.9112.6184.60.9960.9940.9830.9850.90160.9202.3104.2198.00.9950.9800.9890.983156.8(Meanvalue)200.2(Meanvalue)108.4(Meanvalue)171.9(Meanvalue)
Thermal decomposition characteristics of double-base propellants are investigated by thermo gravimetric analysis (TGA). Figure 5 shows the TG curves of the decomposition processes at heating rate of 2℃/min. The percentage mass loss and residual mass percent of each sample between 50 to 400℃ have been evaluated and properly presented in Table 5.

Fig.5 TG curves of the decomposition processes at heating rate of 2℃/min
TG-DTG curves confirmed the DTA and DSC curves and showed same thermal decomposition stages. A-01 formulation decomposed in 3 steps related to decomposition of BuNENA, nitrocellulose and RDX respectively. A-02 and A-04 decomposed in only 2 steps related to decomposition of nitrocellulose and RDX for A-02 while peaks are related to BuNENA and nitrocellulose for A-04. The highest mass loss happened during the nitrocellulose decomposition steps. Nitrocellulose starts decomposition at earlier temperature about 130℃ and continues to decompose till 190℃.
3 Conclusions
(1)Cast double-base propellant grains containing BuNENA energetic plasticizer were successfully built up. The harmonicity between the casting powder and the casting liquid has been achieved by using the proper and compatible ingredients. The selection of the percentage between the casting powder and the casting liquid is very important to get a totally cured, coherent and solid cast double base grain after the diffusion of casting liquid into casting powder taking into consideration the optimum conditions for curing process.
(2)The prepared cast double-base propellant A-04 has a plateau burning region at pressure range (50-70)×105Pa,while the other two compositions A-01 and A-02 have a linear dependency of burning rate on operating pressure. A-04 composition which contains 29% BuNENA in its casting powder has the highest burning rate regime compared with A-01 and A-02 which contain less amount of BuNENA and greater amount of RDX.
(3)Thermal analysis showed that the decomposition of nitrocellulose is the rate determining step with the highest peak parameters in the decomposition process. Kissinger model has been applied for determining the activation energies for the prepared cast double base formulations.
[1] Sutton G P.Rocket Propulsion Elements[M]. Sixth Edition.New York:Wiley Interscience Publication,1992.
[2] Kubuta N.Propellants and Explosives: Thermochemical Aspects of Combustion[M].New York:Wiley-VCH Publication,2002.
[3] Schack C J, Flanagan J E. Alkyl, Azido, Nitro Ethers and Method of Preparation:US,4522756[P].1985-06-11.
[4] Witucki E F, Flanagan J E. Azido Esters:US,4419286[P].1983-12-06.
[5] Ou Y, Chen B, Yan H, Jia H,Li J and Dong S. Development of energetic additives for propellants in China[J]. Journal of Propulsion and Power,1995:11:838.
[6] Cartwright R V. Volatility of NENA and other energetic plasticizer determined by thermogravimetric analysis[J]. Propellants, Explosives, Pyrotechnics,1995:20:51.
[7] Howard W M. Triaminoguanidine Nitrate-containing Propellants:US,4381958[P].1983-04-03.
[8] Fang L A, Hua S Q, Xin L and Ling V G. Preliminary study of BuNENA gun propellants[C]∥27th International Annual Conference of ICT. Karlsruhe:ICT,1996:51/1.
[9] Johnson R A,Mulley J J. Stability and performance characteristics of NENA materials and formulations[C]∥Joint International Symposium on Energetic Materials Technology. New Orleans:[s.n.],1992:116.
[10] Silver P A,Stanley N F. BuNENA gun propellants[J]. JANNAF Propulsion Meeting,1990,2:515.
[11] Avrami L and Hutchinson R. The sensitivity to impact and friction in energetic materials technology of inorganic acides[M].New York:Plenum Press, 1977:111.
[12] Song X D,Zhao F Q,Liu Z R,Pan Q,Luo Y. Thermal decomposition mechanism, non-isothermal reaction kinetics of bismuth citrate and its catalytic effect on combustion of double-base propellant[J]. Chem J Chin Univ,2006,27 (1):125-128.
[13] Hu R Z, Gao S L,Zhao F Q,Shi Q Z,Zhang T L,Zhang J J. Thermal Analysis Kinetics[M]. 2nd ed..Beijing:Science Press, 2008.
[14] Yi J H,Zhao F Q,Xu S Y,Gao H X,Hu R Z,Hao H X,Pei Q ,Gao Y. Nonisothermal thermal decomposition reaction kinetics of double-base propellant catalyzed with lanthanum citrate[J]. Acta Phys. Chim. Sin, 2007, 23 (9), 1316-1320.
[15] Gizycki J F. The thermal decomposition of nitrocellulose[J].Chem. Ztg,1950,74:649-651.
[16] Smith R D. Pyrolysis of dissolved nitrocellulose[J].Nature,1952,170:844-845.
[17] Makashir P S,Mahajan R R,Agrawal J P. Studies on kinetics and mechanism of initial thermal decomposition of nitrocellulose[J]. J. Therm. Anal. Cal,1995,45:501.
[18] Binke N, Rong L, Zhengquan Y,Yuan W,Pu Y.Rongzu H,Qingsen Y. Studies on the kinetics of the first order autocatalytic decomposition reaction of highly nitrated nitrocellulose[J]. J. Therm. Anal. Cal,1999,58:403.
[19] Binke N, Rong L,Xianqi C,Yuan W, Rongzu H, Qingsen Y.Study on the melting process of nitrocellulose by thermal analysis method[J]. J. Therm. Anal. Cal,1999,58:249.
[20] Paulik F, Paulik J,Arnold M. TG and TGT investigations of the decomposition of nitrocellulose under quasi-isothermal conditions[J]. J. Therm. Anal. Cal,1977,12:383.
[21] Herder G,de Klerk W P C. Measurement of the relaxation transitions of nitrocellulose based gunpowder[J]. J. Therm. Anal. Cal,2006,85:169.
[22] Rong L, Binke N,Yuan W,Zhengquan Y,Rongzu H. Estimation of the critical temperature of thermal explosion for the highly nitrated nitrocellulose using non-isothermal DSC[J]. J. Therm. Anal. Cal,1999,58:369.
[23] Krupka M. Devices and equipment for testing of energetic materials, in: J. Vágenknecht (Ed.)[C]∥Proc. 4th International Seminar NTREM.Pardubice:Univ. Pardubice,2001:222.
[24] Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957, 29:1702-1709.
[25] Ksiazczak A, Radomski A, Zielenkiewicz T. Nitrocellulose porosity thermoporometry[J]. J. Therm. Anal. Cal,2003,74:559.
[26] Ettre K, Varadi P. Pyrolysis-gas chromatographic technique, effect of temperature on thermal degradation of polymers[J]. Anal. Chem,1963,35:68-73.
[27] Huwei L, Ruonong F.Studies on thermal decomposition of nitrocellulose by pyrolysis-gas chromatography[J]. J. Anal. Pyrolysis,1988,14:163-167.
[28] Kissinger H E. Variation of peak temperature with heating rate in differential thermal analysis[J].Journal of Research of the National Bureau of Standards,1956,57:217-221.
[29] Sunitha M,Reghunadhan Nair C P,Krishnan K,Ninan K N. Kinetics of alder-ene reaction of tris(2-allylphenoxy)triphenoxycyclotriphosphazene and bismaleimides; a DSC study, thermochim[J]. Acta,2001,374:159-169.
[30] Bohn M A. Kinetic modelling of the concentrations of the stabilizer DPA and some of its consecutive products as function of time and temperature[J]. J Therm Anal Calorim,2001,65:103e20.
[31] Pourmortazavi S, Hosseini S, Rahimi-Nasrabadi M, Hajimirsadeghi S,Momenian H. Effect of nitrate content on thermal decomposition of nitrocellulose[J]. Journal of Hazardous Materials, 2009,162:1141-1144.
[32] Sovizi M, Hajimirsadeghi S,Naderizadeh B. Effect of particle size on thermal decomposition of nitrocellulose[J]. Journal of Hazardous Materials, 2009,168:1134-1139.
[33] Friedman H L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry-application to a phenolic resin[J]. J. Polym. Sci,1964, 6 (Pt. C): 183-195.
[34] Ozawa T. Applicability of Friedman plot[J]. J. Therm. Anal,1986, 31: 547- 551.
[35] Vyazovkin S,Burnham A K,Criado J M,Pérez-Maqueda L A,Popescu C N. Sbirrazzuoli ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data thermochim[J]. Acta,2011, 520:1-19.
10.14077/j.issn.1007-7812.2017.06.004
TJ55;V512 Document Code:A Article ID:1007-7812(2017)06-0023-06
date:2017-09-18; Revised date:2017-10-23
Biography:Ahmed Maraden(1982-),male,MSc.Master of Science,research field:solid rocket propellant.E-mail:ahmedmaraden@yahoo.com
