The effects of aerobic exercise training on oxidant-antioxidant balance, neurotrophic factor levels, and blood-brain barrier function in obese and non-obese men
2018-01-08HeeTeRohWiYoungSo
Hee-Te Roh, Wi-Young So
aDepartment of physica; Educatiom. College of Arts and Physical Education, Dong-A University, Busan 604-714, Republic of Korea
bSpiorts and Health Care Major, Cpllege of Humanities and Arts, Korea National University of Transportation, Chungju-si 380-702, Republic of Korea
The resulting oxidative stress induces DNA denaturation and apoptosis,12causing cardiovascular disease,diabetes,cancer,and neurodegenerative diseases.13,14In particular,the brain contains significan amounts of unsaturated fatty acid and circulating oxygen but has decreased antioxidant enzyme activity compared with other organs,increasing the risk for the development of neurodegenerative diseases through the apoptosis of vulnerable neurons.15,16Moreover,the brain’s blood vessels comprise a blood–brain barrier(BBB)composed of tight junctions,pericytes,astrocyte end-feet,and basal lamina.The BBB protects the brain from sudden changes in blood components by selectively blocking toxic substances that threaten normal brain function.17However,excessive oxidative stress can damage the BBB18,19and result in various neurologic diseases.20
Benedict et al.21reported that increased peripheral blood levels of neuron-specifi enolase(NSE)and S100β,circulating brain-specifi proteins,may be indicative of neuronal damage,impaired BBB function,or both.Moreover,obesity regulates neuronal survival,plasticity,and neurotransmitter release and is related to brain-derived neurotrophic factor(BDNF)expression,which can prevent cognitive dysfunction and neurodegenerative diseases.22Gardiner et al.23suggested that an increase in oxidative stress can be linked with the downregulation of this neurotrophic factor.
On the other hand,regular exercise training is an effective treatment for obesity that reduces oxidative stress caused by obesity or diseases such as metabolic syndrome24and induces an increase in neurotrophic factors.25However,previous human studies have been limited to the measurement of only BDNF,nerve grow th factor(NGF),and glial cell line-derived neurotrophic factor(GDNF).
As noted earlier,regular exercise training can alleviate oxidative stress in obese subjects and can affect neurotrophic factor levels,which promote brain cell growth and support the BBB protecting the brain.However,no previous study has determined the relationship between regular exercise training,oxidative stress,BDNF levels,and BBB damage.Thus,this study aimed to investigate the effects of regular exercise training on the oxidant–antioxidant balance,neurotrophic factor levels,and BBB function in obese subjects.
2.Methods
2.1.Subjects
Subjects included in this study did not participate in regular exercise and understood the purpose of this study.We included 2 groups:10 healthy non-obese males with a body mass index(BM I)<25 kg/m2and 10 obese males with a BM I≥25 kg/m2based on the World Health Organization/International Association for the Study of Obesity/International Obesity Task Force definitio of obesity.26The subjects did not take any medication,and no dietary modification were made during the study.The subjects were informed about data collection and purposes of the study,and all subjects agreed to participate,signing a written informed consent statement.The protocol of this study was approved by the National Research Foundation of Korea(NRF-2013S1A5B5A07049580),and the physical characteristics of the subjects are shown in Table 1.
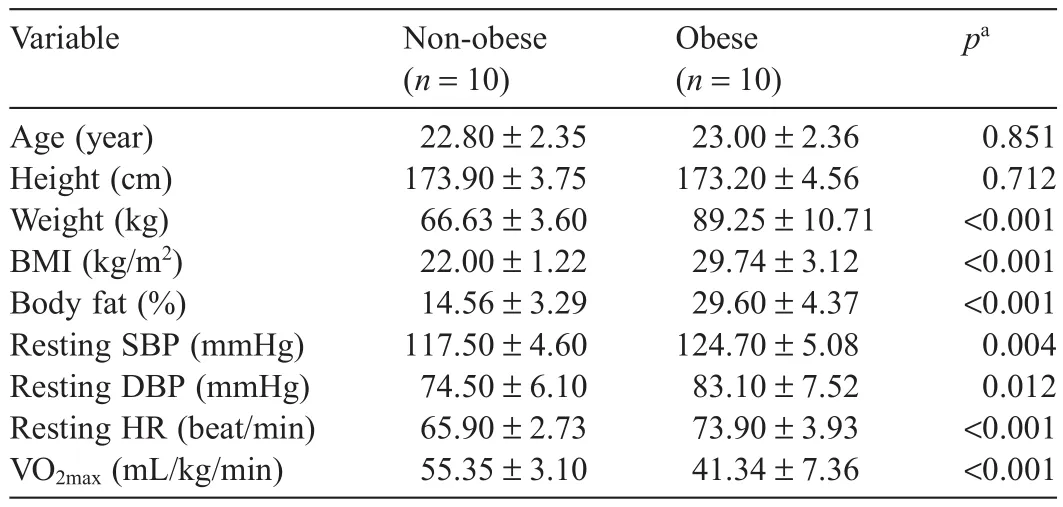
Table1 Physical characteristics of the subjects included in this study(mean±SD).
2.2.Anthropometric measurements
Anthropometric measurements including height,weight,BM I,percentage of fat,resting blood pressure(BP),resting heart rate(HR),and maximum oxygen uptake(VO2max)were obtained.Height was measured using semiautomatic height measurement equipment(HD;STDK,Tokyo,Japan),and weight and body composition were measured using a bioelectrical impedance analysis body composition analyzer(Inbody220;Biospace,Seoul,Korea).Resting BP in the brachial artery was obtained by a nurse with a mercury sphygmomanometer(Trim line;PyMaH,Somerville,NJ,USA)after subjects had relaxed in a comfortably seated position for at least 10 min.The HR was measured with a wireless HR analyzer(Polar A5;Polar,Kempele,Finland).VO2maxwas measured on the treadmill(Q65;Quinton,Seattle,WA,USA)at1.7mph and a 10%grade using the Bruce protocol with an increase of 0.8–0.9 mph and 2%grade every 3m in.27Breath-by-breath analysis was applied using a gas analyzer(Metamax 3B;Cortex,Leipzig,Germany)and a wireless HR analyzer(Polar A5).Repeat measurements of weight,body composition,resting BP,resting HR,and VO2maxwere conducted after 8 weeks of training to record changes in obesity and cardiovascular parameters.
2.3.Exercise training method
Running exercise was performed on a treadmill 3 times weekly for 8 weeks in accordance with a previously described training method28and exercise prescription guidelines for obese subjects.29Exercise intensity was set at 70%HR reserve using the Karvonen formula,in which the resting HR(HRrest)and maximum HR(HRmax)are measured during the VO2maxtest.30Exercise intensity during training was controlled ata±5%error range of the target HR using the wireless HR analyzer(Polar A5).Exercise duration was 60min,including 10 min warm-up(stretching)and cool-down periods(stretching)and 40m in of treadmill exercise.
2.4.Blood collection and analysis methods
In all,10mL of blood was collected from the antecubital vein with a 22-gauge needle and serum separator tubes before and after 8 weeks of training.The blood separation was performed by centrifugation at 3000 rpm for 15m in,and serum was kept at −80°C until the analysis of serum oxidant–antioxidant status(ROS and SOD),neurotrophic factors(BDNF,NGF,and GDNF),and BBB function-related factors(S100βand NSE)was performed.
2.5.Blood oxidant–antioxidant marker analysis methods
The analysis of serum ROS was conducted using the OxiSelect In Vitro ROS/RNS Assay Kit(#STA-347;Cell Biolabs,San Diego,CA,USA).In this assay 2′,7′-dichlorodihydrofluorescei is converted to 2′,7′-dichlorodihydrofluorescei diacetate by ROS.Fluorescence was measured at480 nm and 530 nm using a fluorescenc plate reader(LS 55 Lum inescence Spectrometer;PerkinElmer,Waltham,MA,USA).The analysis of serum SOD activity was performed using a colorimetric assay with the Superoxide Dismutase Assay Kit(#CM 706002;IBL International,Hamburg,Germany)at 450 nm with a microplate reader(GENios;TECAN,Salzburg,Austria).
2.6.Blood neurotrophic factor analysis methods
Serum BDNF,NGF,and GDNF levels were measured using sandwich enzyme-linked immunosorbent assays(ELISAs).For BDNF,we used the Human BDNF ELISA Kit(#DBD00;R&D Systems,Minneapolis,MN,USA);for NGF we used the NGF sandwich ELISA Kit(#CYT304;ChemiKine,Temecula,CA,USA),and for GDNF we used the GDNF Human ELISA Kit(#ab100525;Abcam,Cambridge,MA,USA).Fluorescence was measured at 450 nm with a microplate reader(Emax;Molecular Devices,Sunnyvale,CA,USA).
2.7.BBB function-related marker analysis methods
Serum S100β levels were measured with a S100β (Human)ELISA Kit(#KA0037;Abnova,Taiwan,China),and NSE levels were measured with a Human NSE ELISA Kit(#M-0050;A lpha Diagnostic International,San Antonio,TX,USA).Florescence was measured at450 nm with a microplate reader(Emax)with ELISA.
2.8.Statistical analysis
The data from this study are expressed as mean±SD using SPSS/PC+Version 21.0 for Windows(IBM,Armonk,NY,USA).Two-way repeated analysis of variance(ANOVA)was conducted to examine the differences in each dependent variable and group before and after exercise training.An independent t test was conducted to examine the differences between the obese and non-obese groups prior to training.Statistical significanc(α)was set at0.05.
3.Results
3.1.Changes in body composition and cardiovascular parameters
Changes in body composition(weight,BM I,and percentage of fat)and cardiovascular parameters(BP,HR,and VO2max)in the non-obese and obese groups before and after aerobic exercise training are shown in Table 2.The two-way repeated ANOVA for body composition and VO2maxshowed an interaction effect in weight(F(1,18)=16.474,p=0.001),BM I(F(1,18)=18.384,p<0.001),percentage of fat(F(1,18)=18.384,p<0.001),and VO2max(F(1,18)=12.292,p=0.003),which showed significan differences.There were no significan differences in resting systolic blood pressure(F(1,18)=0.485,p=0.495),diastolic blood pressure (F(1,18)=1.427,p=0.248),and HR(F(1,18)=1.458,p=0.243).The post hoc test results revealed that the non-obese group did not show significan differences in these parameters before and after training,but the obese group showed significant y lower weight,BM I,and percentage of fat(all p<0.05)and a significant y higher VO2max(p<0.05)after training.Moreover,the obese group showed a significant y higher weight,BM I,and percentage of fat and a significant y lower VO2maxbefore and after training compared with the non-obese group(all p<0.05).
3.2.Changes in blood oxidant–antioxidant balance
Changes in the oxidant–antioxidant balance in the non-obese and obese groups before and after aerobic exercise training areshown in Table 3.The two-way repeated ANOVA revealed significan differences in blood ROS levels and SOD activity between the 2 groups before and after training (F(1,18)=10.209,p=0.005;F(1,18)=9.502,p=0.006,respectively).The post hoc test results showed that the obese group had significant y higher ROS levels and lower SOD activity at baseline compared with the non-obese group(both p<0.05).In addition,the non-obese group showed no significan differences in these values before and after training,whereas the obese group showed significant y decreased ROS levels and increased SOD activity after training(both p<0.05).
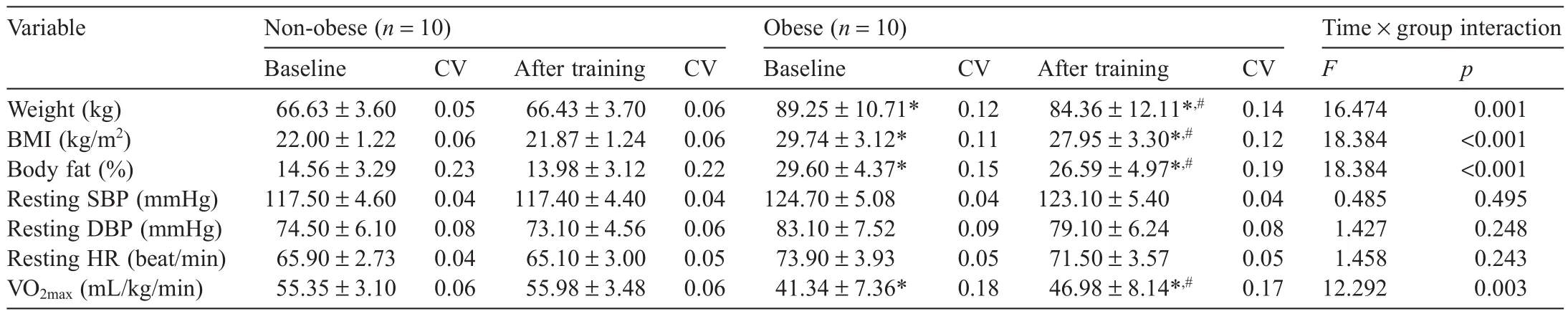
Table2 Changes in body composition and cardiovascular assessments before and after training(mean±SD).
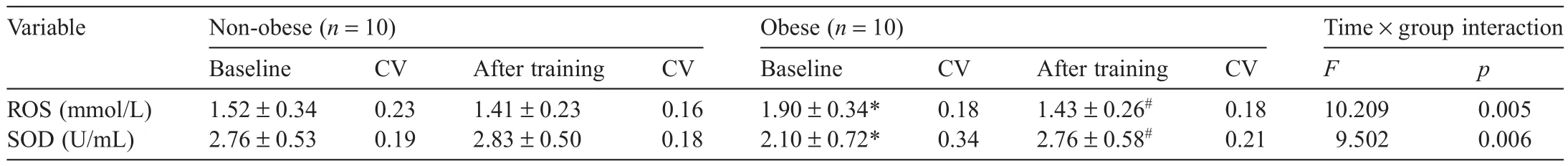
Table3 Changes in blood oxidant–antioxidant status before and after training(mean ± SD).
3.3.Changes in blood neurotrophic factor levels
Changes in blood neurotrophic factor levels in the non-obese and obese groups before and after aerobic exercise training are shown in Table4.The two-way repeated ANOVA showed interaction effects for blood BDNF before and after training,indicating a significan difference(F(1,18)=7.665,p=0.013).There were no significan differences in blood NGF and GDNF levels(F(1,18)=1.802,p=0.196;F(1,18)=1.854,p=0.190,respectively).According to the posthoc test results,the obese group showed a significant y lower BDNF level at baseline compared with the non-obese group(p<0.05).In addition,the non-obese group showed no significan difference in blood neurotrophic factor levels before and after training,whereas the obese group showed a significant y higher BDNF level after training(p<0.05).
3.4.Changes in serum BBB function markers
Changes in serum BBB function markers in the non-obese and obese groups before and after aerobic exercise training are shown in Table 5.The two-way ANOVA showed interaction effects for blood S100β and NSE levels before and after training,indicating significan differences(F(1,18)=9.202,p=0.007;F(1,18)=9.271,p=0.007,respectively).According to the posthoc test results,the obese group showed significant y higher S100β levels at baseline compared with the non-obese group(p<0.05).Furthermore,the non-obese group showed no significan differences before or after training,whereas the obese group showed significant y lower S100β levels and NSE levels after training(both p<0.05).
4.Discussion
Regular exercise,along with dietary control,is effective in the prevention of obesity.31In our study,body composition parameters including weight,BM I,and percentage of fat were significant y reduced in the obese group after training,indicating a positive effect of training on obesity reduction.In addition,the obese group showed a significant y increased VO2max,whereas the non-obese group showed no significan change inVO2maxwith training.We believe this occurred because the VO2maxof the non-obese group was in the upper10th percentile before training(VO2max>51.4mL/kg/m in),32and therefore a greater exercise effort was required to promote cardiorespiratory fitnes in the non-obese group.
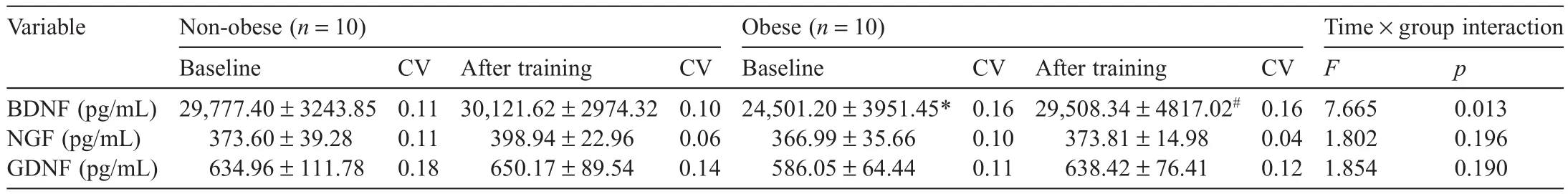
Table4 Changes in blood neurotrophic factor levels before and after training(mean±SD).
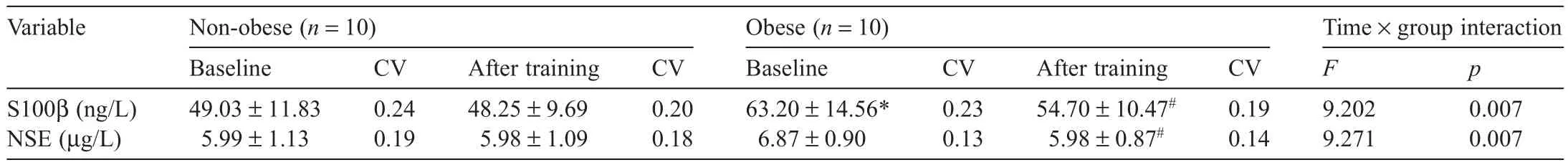
Table5 Changes in blood blood-brain barrier function markers before and after training(mean±SD).
Oxidative stress occurs when pro-oxidants are predominant compared with antioxidants,33and it has been reported that obesity is associated with chronically increased oxidative stress.24In this study,the obese group had a significant y higher ROS level and significant y lower SOD activity at baseline compared with the non-obese group.This result supports previous studies reporting that obese subjects show a higher prooxidant/antioxidant ratio compared with that of normal-weight subjects34and implies that obesity can increase oxidative stress.On the other hand,ROS levels decreased and SOD activity levels increased significant y in the obese group after training.It is assumed that exercise training improved the antioxidant balance in the obese group as well as significant y decreasing weight and BM I.Vincent and Taylor5reported that obesity induces an imbalance in pro-oxidant–antioxidant imbalance by depleting enzymatic antioxidants such as SOD,but that exercise training increases antioxidant status,and weight loss ameliorates increased oxidative stress in obese subjects.In addition,according to a large epidemiologic study,obesity is highly associated with BM I and oxidative stress,supporting the results of this study.35Moreover,high blood glucose levels may be associated with obesity and increased oxidative stress,but glucose levels were not assessed in this study.This is a limitation of our study,and future studies should examine the association between glucose levels,exercise training,and oxidative stress.
Recent studies have reported that obesity can increase the body’s oxidative stress.High oxidative stress reduces neurotrophic factor levels,has a negative impact on brain function,and is related to the occurrence of neurodegenerative diseases.36,37In this study,we analyzed serum BDNF,NGF,and GDNF levels to examine the effects of obesity and exercise training on neurotrophic factor levels.The obese group showed significant y lower serum BDNF levels at baseline compared with those of the non-obese group,but BDNF levels in the obese group increased significant y after exercise training.This result supports those of previous studies,which reported that obese and overweight subjects showed significant y lower serum BDNF levels compared with normal-weight subjects38,39and that aerobic exercise training significant y increased serum BDNF levels in obese subjects.39,40The changes in serum ROS levelsand SOD activity in this study indicate that the reduced oxidative stress and improved antioxidant ability with exercise training can significant y affect BDNF levels.Increased oxidative stress levels can downregulate neurotrophic factors,23and BDNF has shown a negative correlation with oxidative stress and a positive correlation with antioxidant activity.41,42Moreover,recent studies have shown improved antioxidant activity with regular exercise and that the use of various antioxidants can promote the expression of neurotrophic factors,43-45supporting the results of this study.On the other hand,there was a significan difference in BDNF levels between the obese and non-obese groups at baseline.The obese group showed significant increases in BDNF after training,but no increase was observed in NGF and GDNF levels.These results support those of a previous study reporting that exercise training significant y increased hippocampal BDNF mRNA expression but not NGF and GDNF mRNA expression in rats.46NGF,GDNF,and BDNF levels play an important role in the survival,maintenance,and regeneration of a specifi neuronal population.47BDNF also plays an important role in the central and peripheral molecular processes of energy metabolism and homeostasis.45,48Additional studies are needed to determine the effects of calorie restriction on these neurotrophic factors.
The BBB is amulticellular vascular structure that separates the central nervous system from the peripheral blood circulation.The BBB actively regulates influ and efflu at the blood–brain interface.49A disruption of the BBB may play a role in the etiology of various cerebrovascular and nervous diseases such as is chemic stroke,epilepsy,amyotrophic lateral sclerosis,and neuromyelitis optica.49Thus,the maintenance of BBB function is important for long-term brain health.In this study,we measured serum S100β and NSE levels to examine the effects of obesity and exercise training on BBB function.The obese group showed significant y higher serum S100βlevels at baseline(63.20±14.56 ng/L)compared with those of the non-obese group(49.03±11.83 ng/L).This correlation between obesity and serum S100β levels was similar to that reported by Steiner et al.,50who showed that overweight subjects with a BMI of 25–29.9 kg/m2showed significant y higher serum S100β levels(about 60 ng/L)compared with control subjects(about 50 ng/L)with a BMI of 25 kg/m2.Also,S100β and NSE levels were significant y reduced in the obese group after exercise training.These results show that obesity exacerbates BBB dysfunction by increasing oxidative stress,but exercise training reduces ROS levels and increases SOD activity.Microglia activation,the upregulation of proinflammato y cytokines,and an increase in oxidative stress largely account for the obesityinduced BBB disruption.18However,antioxidants such as catalase and SOD have been shown to attenuate the BBB hyperpermeability resulting from hyperglycemia.51Schulpis et al.52reported a negative correlation between serum S100β levels and total antioxidant status(r=−0.64).Moreover,Al-Jarrah and Jamous53previously reported that treadmill exercise training decreased S100β and NSE expression in a Parkinson’s disease mouse model,supporting the results of this study.In addition,even though serum S100βand NSE levels are blood biomarkers for BBB disruption and a BBB permeability increase,21,54future studies analyzing circulating tight junction proteins and matrix met alloproteinases may more specifical y reflec BBB function because S100β is expressed in various peripheral tissues,including skelet al muscle,and NSE reflect brain damage.55,56
5.Conclusion
In conclusion,obesity can reduce serum neurotrophic factor levels and induce BBB dysfunction.Increased oxidative stress caused by obesity is largely responsible for this phenomenon.On the other hand,regular aerobic exercise can improve the oxidant–antioxidant imbalance resulting from obesity,increase neurotrophic factor levels,and limit BBB dysfunction.However,future studies should investigate calorie restriction because neurotrophic factors,such as BDNF,are also involved in energy metabolism,45,48and serum S100β levels show a high correlation with changes in weight.50
Acknowledgment
This work was supported by the Dong-A University research fund.
Authors’contributions
HTR participated in study design,subject recruitment,data collection,data processing,and data analysis and drafted the manuscript;WYS conceived of the study,participated in its design and coordination,and helped draft the manuscript.Both authors have read and approved the fina version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
1.Ji LL.Antioxidants and oxidative stress in exercise.Proc Soc Exp Biol Med 1999;222:283–92.
2.Powers SK,Talbert EE,Adhihetty PJ.Reactive oxygen and nitrogen species as intracellular signals in skelet al muscle.J Physiol 2011;589:2129–38.
3.Nikolaidis MG,Jamurtas AZ,Paschalis V,Fatouros IG,Koutedakis Y,Kouretas D.The effect of muscle-damaging exercise on blood and skelet al muscle oxidative stress:magnitude and time-course considerations.Sports Med 2008;38:579–606.
4.Bengesser SA,Lackner N,Birner A,Fellendorf FT,Platzer M,Mitteregger A,et al.Peripheral markers of oxidative stress and antioxidative defense in euthymia of bipolar disorder—gender and obesity effects.J Affect Disord 2015;172:367–74.
5.Vincent HK,Taylor AG.Biomarkers and potential mechanisms of obesityinduced oxidant stress in humans.Int J Obes(Lond)2006;30:400–18.
6.Steinberg HO,Baron AD.Vascular function,insulin resistance and fatty acids.Diabetologia 2002;45:623–34.
7.Son SM,Whalin MK,Harrison DG,Griendling KK.Oxidative stress and diabetic vascular complications.Curr Diab Rep 2004;4:247–52.
8.Le Lay S,Simard G,Martinez MC,Andriantsitohaina R.Oxidative stress and metabolic pathologies:from an adipocentric point of view.Oxid Med Cell Longev 2014;2014:doi:10.1155/2014/908539
9.Konukog˘lu D,Serin O,Ercan M,Turhan MS.Plasma homocysteine levels in obese and non-obese subjects with or without hypertension;its relationship with oxidative stress and copper.Clin Biochem 2003;36:405–8.
10.Ozata M,Mergen M,Oktenli C,Aydin A,Sanisoglu SY,Bolu E,et al.Increased oxidative stress and hypozincemia in male obesity.Clin Biochem 2002;35:627–31.
11.Olusi SO.Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans.Int J Obes Relat Metab Disord 2002;26:1159–64.
12.Higuchi Y.Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress.Biochem Pharmacol 2003;66:1527–35.
13.Khan AA,Rahmani AH,Aldebasi YH,Aly SM.Biochemical and pathological studies on peroxidases—an updated review.Glob J Health Sci 2014;6:87–98.
14.Stadtman ER,Berlett BS.Reactive oxygen-mediated protein oxidation in aging and disease.Drug Metab Rev 1998;30:225–43.
15.Knott AB,Perkins G,Schwarzenbacher R,Bossy-Wetzel E.Mitochondrial fragmentation in neurodegeneration.Nat Rev Neurosci 2008;9:505–18.
16.Reynolds A,Laurie C,Mosley RL,Gendelman HE.Oxidative stress and the pathogenesis of neurodegenerative disorders.Int Rev Neurobiol 2007;82:297–325.
17.Ballabh P,Braun A,Nedergaard M.The blood-brain barrier:an overview:structure,regulation,and clinical implications.Neurobiol Dis 2004;16:1–13.
18.Tucsek Z,Toth P,Sosnowska D,Gautam T,Mitschelen M,Koller A,et al.Obesity in aging exacerbates blood-brain barrier disruption,neuroinflammation and oxidative stress in the mouse hippocampus:effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease.J Gerontol A Biol Sci Med Sci 2014;69:1212–26.
19.Vieira AA,Michels M,Florentino D,Nascimento DZ,Rezin GT,Leffa DD,et al.Obesity promotes oxidative stress and exacerbates sepsis-induced brain damage.Curr Neurovasc Res 2015;12:147–54.
20.Karamanos Y,Gosselet F,Dehouck MP,CecchelliR.Blood-brain barrier proteomics:towards the understanding of neurodegenerative diseases.Arch Med Res 2014;45:730–7.
21.Benedict C,Cedernaes J,Giedraitis V,Nilsson EK,Hogenkamp PS,Vågesjö E,et al.Acute sleep deprivation increases serum levels of neuron-specifi enolase(NSE)and S100 calcium binding protein B(S-100B)in healthy young men.Sleep 2014;37:195–8.
22.Huang CJ,Mari DC,Whitehurst M,Slusher A,Wilson A,Shibata Y.Brain-derived neurotrophic factor expression ex vivo in obesity.Physiol Behav 2014;123:76–9.
23.Gardiner J,Barton D,Overall R,Marc J.Neurotrophic support and oxidative stress:converging effects in the normal and diseased nervous system.Neuroscientist 2009;15:47–61.
24.Vincent HK,Innes KE,Vincent KR.Oxidative stress and potential interventions to reduce oxidativestress in overweight and obesity.Diabetes Obes Metab 2007;9:813–39.
25.Mattson MP,Wan R.Beneficia effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems.J Nutr Biochem 2005;16:129–37.
26.World Health Organization/International Association for the Study of Obesity/International Obesity Task Force.The Asia-Pacifi perspective:redefinin obesity and its treatment.Sydney:Health Communications Australia Pty Ltd.;2000.
27.Bruce RA,Blackmon JR,Jones JW,Strait G.Exercising testing in adult normal subjects and cardiac patients.Pediatrics 1963;32:742–56.
28.Paik IY,Jin CH,Jin HE,Kim YI,Cho SY,Roh HT,et al.Effects of the NADPH oxidase p22phox C242T polymorphism on endurance exercise performance and oxidative DNA damage in response to aerobic exercise training.Mol Cells 2009;27:557–62.
29.American College of Sports Medicine.ACSM’s guidelines for exercise testing and prescription.Philadelphia,PA:Lippincott Williams&Wilkins;2006.
30.Karvonen MJ,Kentala E,Mustala O.The effects of training on heart rate;a longitudinal study.Ann Med Exp Biol Fenn 1957;35:307–15.
31.Snow V,Barry P,Fitterman N,Qaseem A,Weiss K,Clinical Effica y Assessment Subcommittee of the American College of Physicians.Pharmacologic and surgical management of obesity in primary care:a clinical practice guideline from the American College of Physicians.Ann Intern Med 2005;142:525–31.
32.American College of Sports Medicine.ACSM’s health-related physical fitnes assessment manual.3rd ed.Baltimore,MD:Lippincott Williams&Wilkins;2009.
33.Fisher-Wellman K,Bell HK,Bloomer RJ.Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders.Oxid Med Cell Longev 2009;2:43–51.
34.Razavi A,Baghshani MR,Ardabili HM,Andalibi MS,Rahsepar AA,Moohebati M,et al.Obese subjects have significant y higher serum prooxidantantioxidant balance values compared to normal-weight subjects.Clin Lab 2013;59:257–61.
35.Keaney Jr JF,Larson MG,Vasan RS,Wilson PW,Lipinska I,Corey D,et al.Obesity and systemic oxidative stress:clinical correlates of oxidative stress in the Framingham Study.Arterioscler Thromb Vasc Biol 2003;23:434–9.
36.Kishi T,Hirooka Y,Nagayama T,Isegawa K,Katsuki M,Takesue K,et al.Calorie restriction improves cognitive decline via up-regulation of brainderived neurotrophic factor:tropomyosin-related kinase B in hippocampus of obesity-induced hypertensive rats.Int Heart J 2015;56:110–5.
37.Liu Y,Fu X,Lan N,Li S,Zhang J,Wang S,et al.Luteolin protects against high fat diet-induced cognitive deficit in obesity mice.Behav Brain Res 2014;267:178–88.
38.El-Gharbawy AH,Adler-Wailes DC,Mirch MC,Theim KR,Ranzenhofer L,Tanofsky-Kraff M,et al.Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents.J Clin Endocrinol Metab 2006;91:3548–52.
39.Lee SS,Yoo JH,Kang S,Woo JH,Shin KO,Kim KB,et al.The effects of 12 weeks regular aerobic exercise on brain-derived neurotrophic factor and inflammato y factors in juvenile obesity and type 2 diabetes mellitus.J Phys Ther Sci2014;26:1199–204.
40.Araya AV,Orellana X,Godoy D,Soto L,Fiedler J.Effect of exercise on circulating levels of brain-derived neurotrophic factor(BDNF)in overweight and obese subjects.Horm Metab Res 2013;45:541–4.
41.Eraldemir FC,Ozsoy D,Bek S,Kir H,Dervisoglu E.The relationship between brain-derived neurotrophic factor levels,oxidative and nitrosative stress and depressive symptoms:a study on peritoneal dialysis.Ren Fail 2015;37:722–6.
42.Jain S,Banerjee BD,Ahmed RS,Arora VK,Mediratta PK.Possible role of oxidative stress and brain derived neurotrophic factor in triazophos induced cognitive impairment in rats.Neurochem Res 2013;38:2136–47.
43.Andrade JP,Assunção M.Protective effects of chronic green tea consumption on age-related neurodegeneration.Curr Pharm Des 2012;18:4–14.
44.Meeusen R.Exercise,nutrition and the brain.Sports Med 2014;44(Suppl.1):S47-56.
45.Ozawa Y,Sasaki M,Takahashi N,Kamoshita M,Miyake S,Tsubota K.Neuroprotective effects of lutein in the retina.Curr Pharm Des 2012;18:51–6.
46.Jiang P,Dang RL,Li HD,Zhang LH,Zhu WY,Xue Y,et al.The impacts of swimming exercise on hippocampal expression of neurotrophic factors in rats exposed to chronic unpredictable mild stress.Evid Based Complement Alternat Med 2014:729827.doi:10.1155/2014/729827
47.Allen SJ,Watson JJ,Shoemark DK,Barua NU,Patel NK.GDNF,NGF and BDNF as therapeutic options for neurodegeneration.Pharmacol Ther 2013;138:155–75.
48.Wisse BE,Schwartz MW.The skinny on neurotrophins.Nat Neurosci 2003;6:655–6.
49.Obermeier B,Daneman R,Ransohoff RM.Development,maintenance and disruption of the blood-brain barrier.Nat Med 2013;19:1584–96.
50.Steiner J,Schiltz K,Walter M,Wunderlich MT,Keilhoff G,Brisch R,et al.S100B serum levels are closely correlated with body mass index:an important caveat in neuropsychiatric research.Psychoneuroendocrinology 2010;35:321–4.
51.Allen CL,Bayraktutan U.Antioxidants attenuate hyperglycaemiamediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability.Diabetes Obes Metab 2009;11:480–90.
52.Schulpis KH,Moukas M,Parthimos T,Tsakiris T,Parthimos N,Tsakiris S.The effect of alpha-Tocopherol supplementation on training-induced elevation of S100B protein in sera of basketball players.Clin Biochem 2007;40:900–6.
53.A l-Jarrah MD,Jamous M.Effect of endurance exercise training on the expression of GFAP,S100B,and NSE in the striatum of chronic/progressive mouse model of Parkinson’s disease.Neurorehabilitation 2011;28:359–63.
54.Kazmierski R,Michalak S,Wencel-Warot A,Nowinski WL.Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients.Neurology 2012;79:1677–85.
55.Heizmann CW,Fritz G,Schäfer BW.S100 proteins:structure,functions and pathology.Front Biosci 2002;7:d1356–68.
56.Kapural M,Krizanac-Bengez L,Barnett G,Perl J,Masaryk T,Apollo D,et al.Serum S-100β as a possible marker of blood-brain barrier disruption.Brain Res 2002;940:102–4.
杂志排行
Journal of Sport and Health Science的其它文章
- Footfall patterns of a runner with an Achilles tendon rupture
- Three-dimensional impact kinetics with foot-strike manipulations during running
- Shock attenuation,spatio-temporal and physiological parameter comparisons between land treadmill and water treadmill running
- Tribulus terrestris extracts alleviate muscle damage and promote anaerobic performance of trained male boxers and its mechanisms:Roles of androgen,IGF-1,and IGF binding protein-3
- Heart rate variability to assess ventilatory thresholds in professional basketball players
- The influenc of different exercise intensities on kicking accuracy and velocity in soccer players
