The PhoR/PhoP two-component system regulates fengycin production in Bacillus subtilis NCD-2 under low-phosphate conditions
2018-01-04GUOQinggangDONGLihongWANGPeipeiLIShezengZHAOWeisongLUXiuyunZHANGXiaoyunMAPing
GUO Qing-gang, DONG Li-hong, WANG Pei-pei, LI She-zeng, ZHAO Wei-song, LU Xiu-yun, ZHANG Xiao-yun, MA Ping
Institute of Plant Protection, Hebei Academy of Agriculture and Forestry Sciences/Integrated Pest Management Center of Hebei Province/Key Laboratory of IPM on Crops in Northern Region of North China, Ministry of Agriculture, Baoding 071000, P.R.China
RESEARCH ARTICLE
The PhoR/PhoP two-component system regulates fengycin production in Bacillus subtilis NCD-2 under low-phosphate conditions
GUO Qing-gang, DONG Li-hong, WANG Pei-pei, LI She-zeng, ZHAO Wei-song, LU Xiu-yun, ZHANG Xiao-yun, MA Ping
Institute of Plant Protection, Hebei Academy of Agriculture and Forestry Sciences/Integrated Pest Management Center of Hebei Province/Key Laboratory of IPM on Crops in Northern Region of North China, Ministry of Agriculture, Baoding 071000, P.R.China
Bacillus subtilis strain NCD-2 is an excellent biocontrol agent for plant soil-borne diseases, and the lipopeptide fengycin is one of the active antifungal compounds in strain NCD-2. The regulator PhoP and its sensor kinase PhoR compose a two-component system in B. subtilis. In this study, the phoR- and phoP-knockout mutants were constructed by in-frame deletion and the role of PhoR/PhoP on the production of fengycin was determined. Inactivation of phoR or phoP in B. subtilis decreased its inhibition ability against Botrytis cinerea growth in vitro compared to the strain NCD-2 wild type.The lipopeptides were extracted from strain NCD-2 wild type and its mutant strains by hydrochloric acid precipitate, and the lipopeptides from phoR-null mutant or phoP-null mutant almost lost the inhibition ability against B. cinerea growth compared to the lipopeptides from strain NCD-2 wild type. Fast protein liquid chromatography (FPLC) analysis of the lipopeptides showed that inactivation of phoR or phoP genes reduced the production of fengycin by strain NCD-2. The fengycin production abilities were compared for bacteria under low-phosphate medium (LPM) and high-phosphate medium (HPM), respectively.Results indicated that the regulation of fengycin production by the PhoR/PhoP two-component system occurred in LPM but not in HPM. Reverse transcriptional-PCR con firmed that the fengycin synthetase gene fenC was positively regulated by phoP when cultured in LPM. All of these characteristics could be partially restored by complementation of intact phoR or phoP gene in the mutant. These data indicated that the PhoR/PhoP two-component system greatly regulated fengycin production and antifungal ability in B. subtilis NCD-2 mainly under low-phosphate conditions.
Bacillus subtilis, two-component system, lipopeptides, transcriptional factor, PhoR/PhoP
1. Introduction
Bacillus subtilis strain NCD-2 was first isolated from the rhizosphere of cotton in Hebei Province, China, and is recognized as an excellent biocontrol agent for plant diseases(Li et al. 2005). This strain was used as the main active component of the bio-fungicide “109spore per gram Bacillus subtilis wettable powder”, which has been registered for managing plant soil-borne diseases such as cotton Verticillium wilt, ginseng Fusarium wilt, and ginseng root rot. The biological control capability of strain NCD-2 is mainly due to the production of antifungal compounds and its ef ficient colonization ability in plant rhizospheres (Guo et al. 2013). Strain NCD-2 can produce at least four lipopeptide antibiotics, including surfactin, fengycin, bacillaene,and subtilosin, as nucleotide sequences encoding those compounds have been identi fied in the genome sequence of strain NCD-2 (GenBank accession number kx646742 for surfactin, GQ906579 for fengycin, kx646741 for bacillaene and kx696450 for subtilosin, respectively). Among them,fengycin has been con firmed to play a major role in inhibiting growth of pathogenic fungi as well as managing plant diseases (Guo et al. 2014).
Fengycin is a cyclic lipopeptide containing ten amino acids and a 14–18 carbon fatty acid residue that is attached to the N terminus of the lipopeptide (Vanittanakom et al.1986). It is synthesized nonribosomally by an enzyme complex formed by fengycin synthetases in the order of FenC,FenD, FenE, FenA, and FenB (Wan et al. 2009). Fengycin shows strong antifungal activity, especially to filamentous fungi (Yanez-Mendizabal et al. 2012), and elicits induced systematic resistance in plants (Ongena et al. 2007). Due to its interesting biological properties, fengycin has been isolated from many B. subtilis strains and other closely related species presumed to have biocontrol capabilities(Tao et al. 2011; Rebib et al. 2012; Yanez-Mendizabal et al.2012). The physicochemical and biological properties of fengycin have been well characterized, but compared to other lipopeptides (e.g., surfactin and bacillomycin D), the regulatory mechanism of fengycin production is largely unknown (Jacques 2011; Wang et al. 2015).
The global regulators perceive a variety of external nutrition stress signals such as phosphate, nitrogen or carbohydrate starvation to activate downstream speci fic pathway regulators, thereby regulating the biosynthesis of secondary metabolites (Allenby et al. 2005; Martin and Liras 2010). Among these signals, phosphate has essential metabolic, structural, and regulatory roles during bacterial growth. B. subtilis responds to phosphate starvation stress by inducing the PhoP and SigB regulons. Genes in the PhoP regulon provide a speci fic response to phosphate starvation stress, while genes in the SigB regulon provide a general response to the resulting energy stress (Pragai and Harwood 2002). In B. subtilis group, the expression of genes, which is controlled by phosphate involved in the primary and secondary metabolites is mainly mediated by the PhoR/PhoP two-component system (Bisicchia et al. 2010). When the concentration of phosphate drops below a certain level, the sensor protein kinase PhoR self-phosphorylates at a conserved histidine residue,forming phosphorylated PhoP (PhoR~P). The phosphorylated kinase PhoR then changes the phosphorylated state of the response regulator PhoP by transferring the phosphoryl group to a conserved aspartate in the response regulator. The PhoP~P represses or activates expression of phosphate-regulated genes, termed the Pho regulon(Botella et al. 2011). Together, the products of the Pho regulon genes allow the cells to overcome the phosphate limitation in the environment (Cleveland et al. 2002). The induction or repression of Pho regulon genes is mediated by the binding of PhoP~P to Pho box sequences, which are four repeat TT(A/T/C)ACA-like sequences with a (5±2)-bp spacer on the coding strand between nucleotides 60 and 20 relative to the transcription start site (Liu and Hulett 1998). Based on genome-wide transcriptional analysis,42 genes were identi fied as potential Pho members in B. subtilis, including genes for plipastatin and surfactin synthetase. However, no assay was performed to detect the production of lipopeptides under a phoR or phoP-deficient background (Allenby et al. 2005).
Previously, we demonstrated that the inactivation of phoP in B. subtilis strain NCD-2 decreased its antifungal ability (Dong et al. 2014). In the present study, efforts were made to further reveal the role of the PhoR/PhoP two-component system in the antifungal ability and production of fengycin in strain NCD-2. In addition, we provide evidence that the expression of the fengycin synthetase gene in strain NCD-2 was affected by phoP deletion when cultured in low-phosphate medium (LPM), but not in high-phosphate medium (HPM). The results indicated the important role of the PhoR/PhoP two-component system in regulating fengycin biosynthesis.
2. Materials and methods
2.1. Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. B. subtilis strain NCD-2 (deposited as CGMCC No. 1019 in the China General Microbiological Culture Collection Center, CGMCC) and its derivative strains were maintained at –76°C in Luria-Bertani (LB) broth supplemented with 30% glycerol. B. subtilis strains were routinely grown in LB medium at 37°C. Escherichia coli DH5α was used for plasmid maintenance and grown in LB medium at 37°C. Botrytis cinerea (deposited as CGMCC No. 3.15253 in the CGMCC) was obtained from stocks in our lab and maintained on potato dextrose agar (PDA) and incubated at 25–28°C. When required, antibiotics were added to the media at the following concentrations: for E. coli, 100 μg mL–1of ampicillin (Amp) and 20 μg mL–1of tetracycline (Tet); for B. subtilis, 5 μg mL–1of erythromycinand 10 μg mL–1tetracycline.
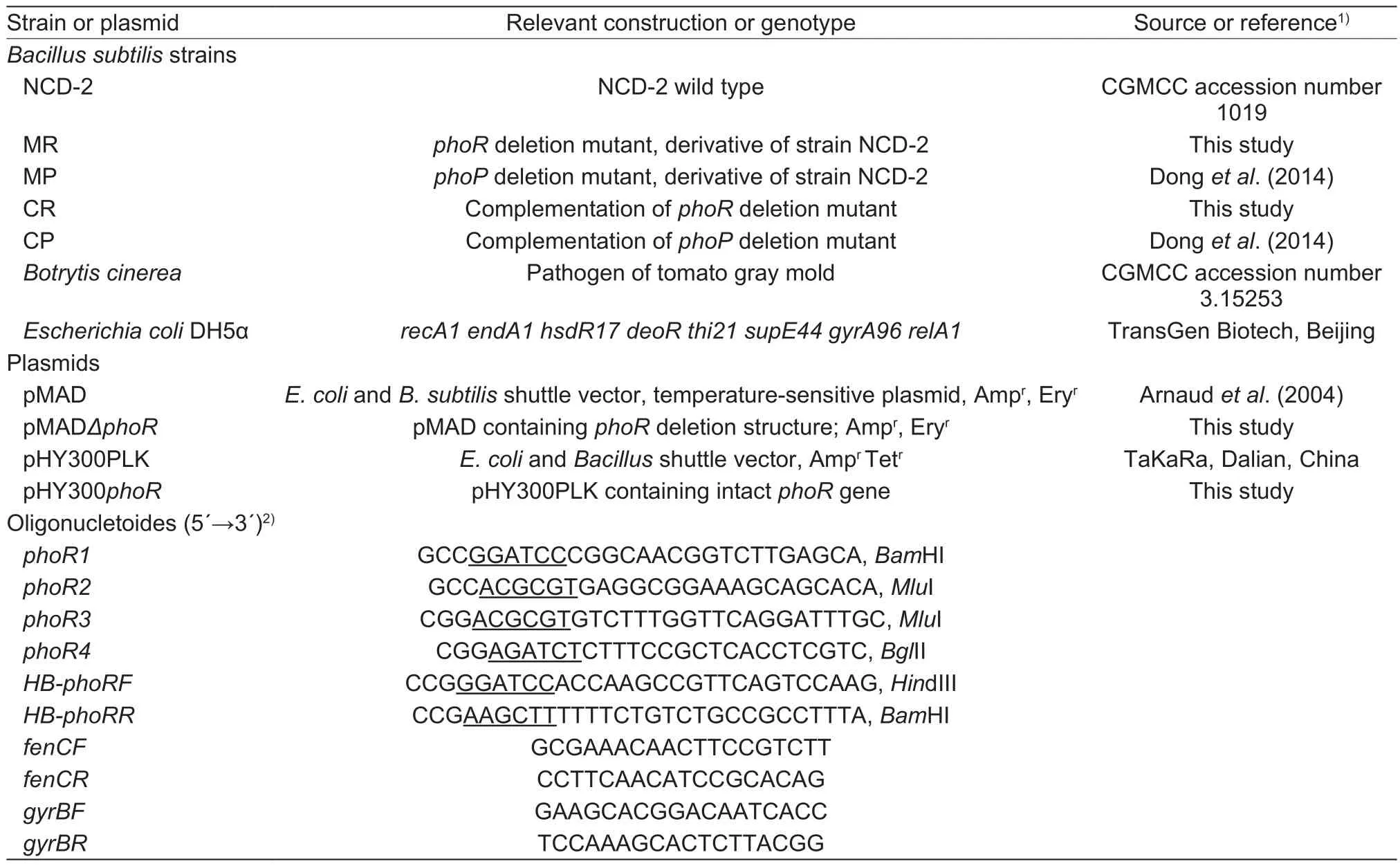
Table 1 Strains, plasmids and oligonucletoides in this study
2.2. DNA manipulation and sequencing
Routine DNA manipulations were done as described by Sambrook and Russell (2001). All restriction enzymes, as well as DNA polymerase and T4 DNA ligase were purchased from TaKaRa Biotech (Dalian) Co., Ltd., China and used according to the manufacturers’ recommendations. PCR ampli fications were carried out using LATaq polymerase.DNA from PCR products was puri fied by the MiniBEST DNA Fragment Puri fication Kit (TaKaRa). Plasmids were extracted with a MiniBEST Plasmid Puri fication Kit (TaKa-Ra). All constructs described below were con firmed by DNA sequencing performed by Sangon Biotech (Shanghai)Co., Ltd., China.
2.3. Gene knock-out and complementation
The phoP mutant and its complemented strain were constructed by Dong et al. (2014) and are referred to as MP and CP in this study, respectively. To create the phoR mutant with in-frame deletion of phoR, plasmid pMADΔphoR was constructed. The plasmid was generated using the temperature-sensitive vector pMAD (Arnaud et al. 2004).Two fragments flanking phoR were ampli fied by PCR.One fragment was created by primers phoR1 and phoR2(Table 1), which introduced the BamHI and MluI sites,respectively; the other fragment was ampli fied by primers phoR3 and phoR4 (Table 1), which introduced the MluI and BglII sites, respectively. After treatment with MluI restriction enzymes, the two fragments were ligated using T4 DNA ligase. The ligation mixture was used as a template for PCR ampli fication with primers phoR1 and phoR4, and the resulting fragment was digested with BamHI and BglII,and cloned into corresponding restriction sites of the pMAD vector, yielding pMADΔphoR. Then the plasmid was transformed into strain NCD-2 by the protoplast fusion method(Martin et al. 1981), and the in-frame deletion of phoR in the B. subtilis chromosome was performed according to the procedure described by Arnaud et al. (2004). The in-frame deletion was con firmed by PCR ampli fication and DNA sequencing, and the mutant was named MR in the present study (Table 1). For complementation of the mutant MR, a DNA fragment containing intact phoR (GenBank accession number EU165270) CDS and its promoter was ampli fied from genomic DNA of strain NCD-2 using primers HB-phoRF and phoRR which introduced the HindIII and BamHI sites, respectively (Table 1). After digestion by the relevant restriction enzymes, these PCR products were ligated into plasmid pHY300PLK, which can replicate and be expressed in both E. coli and B. subtilis (Guo et al. 2010), to produce the plasmid pHBphoR (Table 1). The complementing plasmid pHBphoR was then introduced into the mutant strain MR using standard electroporation. Colony selection was performed on LB medium plates supplemented with Tet, and the appropriate phenotypes were evaluated for the validated strain in the next experiments.
2.4. Antifungal activity assay in vitro
Antifungal activity of B. subtilis strains and their lipopeptide extracts were tested for their ability to inhibit the growth of B. cinerea using the dual-culture technique (Yoshida et al.2001). PDA plates were overlaid with soft PDA (0.8% agar)containing 2×105B. cinerea conidiospores mL–1. After solidifying, 2 μL of B. subtilis cell suspension (OD600=0.5)was dropped onto the dual-layer plates and blow dry at the laminar flow bench. Inhibition zones were observed after incubation for 5 days at 27°C. Lipopeptides produced by B. subtilis strains were extracted according to the method of Chitarra et al. (2003) with some modi fications. Strain NCD-2 and its derivative mutant strains were grown in Nutrient Broth (NB) medium for 3 days at 30°C with shaking at 180 r min–1; lipopeptides were extracted by hydrochloric acid precipitate and then dissolved with 50% methanol to a final concentration of 5 mg mL–1. The antifungal activities of lipopeptide extracts were tested against B. cinerea on double-layer PDA plates as described above, except the antagonistic strain was replaced by a 150-μL aliquot of the lipopeptide extract.
2.5. Detection of fengycin production by FPLC
The lipopeptides extracted from B. subtilis strain NCD-2 wild type and its derivative strains were filtered through a 0.2-μm-pore-size polytetra fluoroethylene membrane(JP020, Advantec, Tokyo), and the filtrates were analyzed by Fast protein liquid chromatography (FPLC) as described previously (Li et al. 2010). A 10-μL portion of the lipopeptide fraction was loaded into a SOURCETM5RPC ST 4.6/150 column (Uppsala, Sweden) and separated by FPLC with an AKTA Puri fier (GE Healthcare, USA). The lipopeptide was eluted at a flow rate of 1.0 mL min–1with two solvent gradients of 20% acetonitrile in 0.065% (v/v) tri fluoroacetic acid (TFA) (solvent A) and 80% (v/v) acetonitrile containing 0.065% TFA (solvent B). The gradient conditions were as follows: starting at 100% solvent A and 0% solvent B,solvent A was linearly decreased to 0% by increasing the amount of solvent B over 50 min and was kept at 0% for over 10 min. Peaks eluting from the column were detected by their absorbance at 215 nm by an ultraviolet detector. To con firm the production of fengycin in the lipopeptide extracts, the 10 μg mL–1fengycin standard (Sigma, USA) was also included in the FPLC analysis.
2.6. Effect of phosphate on fengycin production in B. subtilis strains
To test the effect of phosphate on fengycin production, the strain NCD-2 wild type and its derivative strains were grown in phosphate de fined medium and cultured at 30°C and 180 r min–1for 48 h. This medium includes 50 mmol L–1Tris,3.03 mmol L–1ammonium sulphate, 6.8 mmol L–1sodium citrate, 3.04 mmol L–1ferric chloride, 1 mmol L–1manganese sulphate, 3.5 mmol L–1magnesium sulphate, 0.01 mmol L–1zinc chloride, 50 mmol L–1KH2PO4, 0.5% glucose (w/v),0.05% casamino acids (w/v), and 10 mmol L–1L-arginine.The KH2PO4, glucose, casamino acids, and L-arginine were sterilized separately and added to the medium before it was used. For LPM, 0.42 μmol L–1phosphate was used, and for HPM, 10 μmol L–1phosphate was used. The population densities of bacteria were measured by counting bacterial colony forming units (CFUs) on LB agar plates. Lipopeptides were extracted from strain NCD-2 and its derivative strains cultured in LPM and HPM 48 h after inoculation. Lipopeptides were analyzed with FPLC as described above and the fengycin production ability was determined according to the ratio of fengycin peak areas to the density of bacteria culture (OD600).
2.7. Quantitative reverse transcription PCR (RT-qPCR)
B. subtilis strain NCD-2 wild type and its derivative strains were cultured in 50 mL LPM or HPM at 32°C with shaking at 180 r min–1, and 400 μL samples of bacterial cell suspensions were collected at the mid-log growth phase (6 h after inoculation). Total RNA was extracted with the RNAprep Pure Cell/Bacteria Kit (Tiangen Biotech, Beijing) and RNA was treated with DNase I and then puri fied with the RNA Clean Kit (Tiangen Biotech). The concentration of RNA was determined with a NanoDrop ND-2000 spectrophotometer(NanoDrop Technologies, Wilmington, DE) and adjusted to 100 ng μL–1. RNA (0.8 μg) was used as a template to produce cDNA with Primerscript Reverse Transcriptase(TaKaRa), following the manufacturers’ protocol in a total volume of 20 μL. Real-time PCR was performed using a reaction mixture that contained 2 μL of cDNA, 0.2 μmol L–1fenCF and 0.2 μmol L–1fenCR (Table 1) using Transe-Start Green qPCR SuperMix (TransGen Biotech, Beijing)in an ABI StepOneTMReal-Time PCR System (Applied Biosystems, California, Inc., USA). The cycling program included a 5-min initial pre-incubation at 95°C followed by 40 cycles of 95°C for 15 s, 60°C for 15 s, and 72°C for 15 s. A segment of gyrB between 1 042 and 1 208 nt was ampli fied with primers gyrBF and gyrBR (Table 1) and used as an internal control. Three technical replicates were carried out for each sample and two biological replicates were carried out to detect gene expression. The relative change in target gene expression was calculated using the formula 2–ΔΔCTas described by Ramachandran (2008).To ensure the absence of DNA contamination, the same procedure was also performed for gyrB primers and mRNA preparation in the absence of reverse transcriptase in all experiments; no PCR product was detected. All expression data were normalized to fenC expression in HPM in the strain NCD-2 wild type.
2.8. Statistical analysis
Data were analyzed by one-way analysis of variance using SAS for Windows ver. 9.0 (SAS Institute Inc., Cary, NC), differences were considered to be signi ficant at a 95% (or higher)con fidence level, and the column plots were built with software Origin 7.0 (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. The phoR/phoP mutants reduce antagonism to B. cinerea
To fully understand the role of the PhoR/PhoP two-component system in antagonism to B. cinerea, the wild-type B. subtilis strain NCD-2 and its mutant strains MR and MP were used in the antagonistic assay against B. cinerea(Fig. 1-A). Our results showed that the strain NCD-2 wild type remarkably inhibited the growth of B. cinerea.Both of the mutant strains MR and MP had a remarkable decrease in the inhibitory effect against B. cinerea growth compared to wild type. To understand the mechanism that caused the de ficiency of the mutant strains MR and MP in inhibiting B. cinerea, antimicrobial lipopeptides were extracted from strain NCD-2 wild type and its derivative strains and their inhibitory effects on B. cinerea were tested as described. As shown in Fig. 1-B, the inhibitory zones of lipopeptides extracted from the mutant strains MR and MP were remarkably smaller than that of strain NCD-2 wild type. The inhibitory abilities of both the mutant strains MR and MP could be restored to the level of strain NCD-2 wild type when complemented with the intact phoR (strain CR)or phoP (strain CP), respectively. These results indicated that the PhoR/PhoP two-component system was involved in regulating the production of antimicrobial secondary metabolites in strain NCD-2.
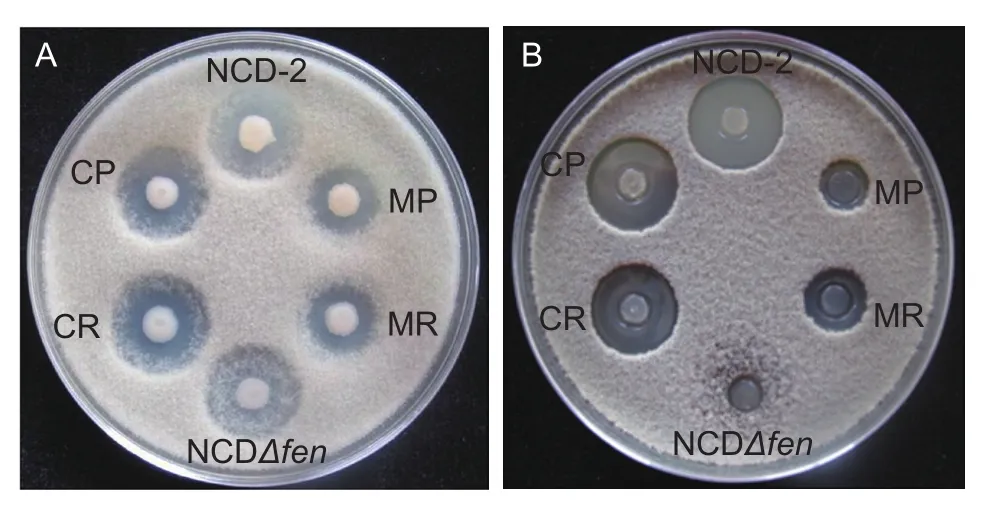
Fig. 1 Inhibitory capability of Bacillus subtilis strains, NCD-2,CP, CR, MP, MR, and NCDΔfen (A) and the lipopeptides extracted from B. subtilis strains (B) against the growth of Botrytis cinerea in vitro.
3.2. The phoR/phoP mutants reduce the production of fengycin in strain NCD-2
Our previous studies indicated that fengycin is one of the active antifungal compounds produced by strain NCD-2(Guo et al. 2014). In this study, the fengycin-de ficient mutant of strain NCD-2 was also included in the antifungal test. Results indicated that in the early co-culture stage,the fengycin-de ficient mutant also displayed a remarkable inhibitory capability against B. cinerea, but in the late co-culture stage, the fengycin-de ficient mutant was covered by hyphae of B. cinerea. However, the strain NCD-2 wild type still had remarkable inhibition of hyphal growth of B. cinerea in the late co-culture stage. In addition, lipopeptide extracts of the fengycin-de ficient mutant almost lost the inhibitory capability against B. cinerea growth,compared to that of the strain NCD-2 wild type (Fig. 1).To test the regulation of fengycin production by the PhoR/PhoP two-component system, lipopeptides were extracted from the NB cultures of the strain NCD-2 wild type and its derivatives, and the production of fengycin was tested with FPLC. The FPLC data showed that fengycin production by the mutant strains MR and MP was remarkably lower than that of the strain NCD-2 wild type (Fig. 2). The de ficiency in fengycin production of the mutant strains MR and MP could be complemented by an intact phoR (strain CR) or phoP (strain CP), respectively. This result indicated that the PhoR/PhoP two-component system is involved in regulating fengycin production in strain NCD-2.
3.3. Regulation by the PhoR/PhoP two-component system of fengycin production is in fluenced by phosphate concentration
The fengycin production capabilities were compared between strain NCD-2 wild type and its derivative strains cultured in LPM and HPM (Table 2). Results indicated that strain NCD-2 produced more fengycin when cultured in LPM than when cultured in HPM. Fengycin production capabilities were decreased in both MR and MP cultured in LPM, compared to that of strain NCD-2 wild type. However,when cultured in HPM, no signi ficant fengycin production differences were observed between strain NCD-2 wild type and strain MR. Therefore, the regulation of fengycin production by the PhoR/PhoP two-component system was executed mainly in the low-phosphate condition.
3.4. Effect of PhoP regulator on transcription of the fengycin synthetase complex
RT-qPCR assays were used to characterize the expression pro files of the first fengycin synthetase fenC gene under low-phosphate conditions. Since the regulator in thetwo-component system was responsible for regulating target gene expression, only the mutant strain MP and its complemented strain CP were chosen to investigate the effect of the PhoR/PhoP two-component system on fenC expression. As shown in Fig. 3, the expression of the fengycin synthetase fenC gene was higher when cultured in LPM than when cultured in HPM. Expression of fenC in LPM was reduced about 2.5 fold in the mutant strain MP compared to the wild type strain, but no signi ficant difference was observed for the expression of fenC in HPM. Therefore, the PhoR/PhoP two-component system regulated the expression of fenC only in the low phosphate condition.

Table 2 The effect of phosphate on the production of fengycin in Bacillus subtilis strain NCD-2 wild type and its derivative strains
4. Discussion
4.1. Compounds with antifungal activity regulated by the PhoR/PhoP two-component system
A previous study revealed that the lipopeptide fengycin played a major role in B. subtilis strain NCD-2 suppression of cotton damping-off disease caused by Rhizoctonia solani (Guo et al. 2014). In this study, we found that a fengycin-de ficient mutant showed similar antifungal ability to the strain NCD-2 wild type in the early co-culture stage,but the fengycin-de ficient mutant almost completely lost its antifungal ability in the late co-culture stage. Therefore,other compounds with antifungal activity must also be produced by strain NCD-2, in addition to fengycin. The lipopeptide extract from the fengycin-de ficient mutant almost completely lost its antifungal ability, therefore, we think that some unknown compounds with antifungal activity could not be extracted by the hydrochloric acid precipitation method used in this study. Lipopeptide extracts from the phoR-null mutant and the phoP-null mutant signi ficantly decreased the antifungal abilities compared to the strain NCD-2 wild type.Therefore, it was expected that the reduction of antifungal ability of the mutant strains was due to the decrease of the fengycin production in either the phoR-null mutant or the phoP-null mutant. The comparison of fengycin production in the lipopeptide extracts of strain NCD-2 wild type and its derivative strains revealed a signi ficant decrease in fengycin production by the phoR deletion or the phoP deletion.Therefore, our results indicated that fengycin biosynthesis in B. subtilis was positively regulated by the PhoR/PhoP two-component system.
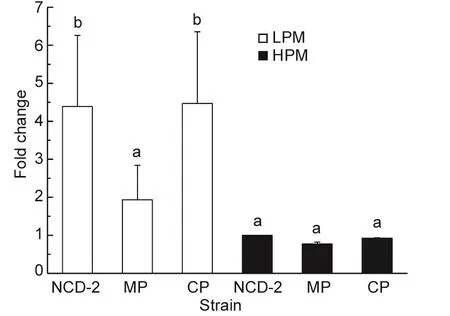
Fig. 3 Comparison of expression of the fenC gene in Bacillus subtilis NCD-2 wild type and its derivative strains cultured in Low-phosphate medium (LPM) and High-phosphate medium(HPM) medium using reverse transcriptional (RT)-PCR. The expression fold change was calculated using 2–ΔΔCT, and all expression data were normalized to fenC expression in HPM in the NCD-2 wild type strain. All values are the means of three replicates±SD. Columns of the same experiment with the different capital letters are signi ficantly different at the P=0.01 level analyzed by one-way analysis of variance.
4.2. Phosphate limition on the regulation of fengycin production by the PhoR/PhoP two-component system
In responding to phosphate starvation, B. subtilis could induce the PhoP and SigB regulons to resist phosphate stress. Based on genome-wide transcriptional analysis of phosphate starvation, Allenby et al. (2005) determined that expression of the fengycin biosynthase fenC (ppsA) gene could be induced in the sigB-null mutant in response to phosphate starvation. This result tentatively assigned fenC as a potential gene of the Pho regulon, but no evidence was obtained for the regulation of fengycin production by the PhoR/PhoP two-component system. In this study, fengycin production was first compared between the strain NCD-2 wild type and its derivative strains cultured in LPM and HPM.Results indicated that fengycin was mainly produced and regulated by phoR and phoP at low-phosphate condition.Reverse transcriptional (RT)-PCR results also con firmed a signi ficant decrease of fenC expression in the phoP mutant mainly in LPM, but not in HPM. Therefore, we concluded that PhoP regulated fengycin synthetase in the low-phosphate condition. We also noticed that there was only a 2.5-fold decrease in expression of fenC in the phoP mutant, and fengycin could still be detected even after deletion of phoP.It was suggested that the fengycin synthetase operon was transcriptionally activated only partially due to the induction of PhoP~P, and other signal pathways or regulators could also contribute to the transcription of fengycin synthetases in B. subtilis NCD-2. Wang et al. (2015) reported that DegQ also regulated fengycin production in B. subtilis strain NCD-2.In B. subtilis, DegU/DegS is a two-component system, in which DegS is a sensor histidine kinase and DegU is a response regulator. DegQ stimulates the phosphotransfer from DegS-P to DegU. Therefore, the DegU/DegS two-component system may also regulate fengycin production.However, the relationship between PhoR/PhoP and DegU/DegS has not been clari fied.
4.3. The possible mechanism of PhoR/PhoP two-component system regulation of fengycin production
N-Terminal fatty acids are key structural elements of the lipopeptide fengycin, because fatty acid moieties strongly in fluence the activity and properties of lipopeptides.Acetyl-CoA is an essential building block for synthesis of fatty acids (Kraas et al. 2010). B. subtilis uses glucose as a preferred source of carbon, and glucose is converted to acetyl-CoA via glycolysis and the pentose phosphate pathway. Under normal conditions, B. subtilis mainly use the glycolysis pathway to produce acetyl-CoA, but under phosphate limition, glucose is metabolized mainly by the pentose phosphate pathway; the pentose phosphate pathway is suggested as having a central role in generating anabolic precursors (Blencke et al. 2003). We employed a phenotype microarray analysis (Biolog Inc., CA) to screen for phenotypes associated with the NCD-2 wild type strain,the phoR-null mutant and the phoP-null mutant. Results revealed that the phoR-null mutant and the phoP-null mutant decreased the utilization for glucose-6-phosphate,glucosamine-6-phosphate, cytidine 5´-monophosphate, and the main intermediates in the pentose phosphate pathway.Therefore, we speculated that the PhoR/PhoP two component system regulates the pentose phosphate pathway in B. subtilis strain NCD-2.
The induction of Pho regulon genes was mediated by the binding of phosphorylated PhoP to Pho box sequences,which are four direct repeats of TT(A/T/C)ACA with a(5±2)-bp spacer, and are located on the coding strand of the promoter region (Liu and Hulett 1998). In strain NCD-2,the promoter sequences of the fengycin synthetase operon were obtained (Appendix A), but no PhoP-binding site was found. Therefore, we speculated that PhoP does not bind directly to the promoters of fengycin synthetase, and the PhoP regulatory effect on fengycin biosynthase might be exerted through signaling cascades involving intermediate regulatory genes or through interaction of PhoP with other regulators.
5. Conclusion
The PhoR/PhoP two-component system regulated the antifungal activity and fengycin production of B. subtilis strain NCD-2. Regulation of fengycin production and expression of the fengycin synthetase gene by the PhoR/PhoP two-component system mainly occurred under the low-phosphate condition.
Acknowledgements
This work was funded by the earmarked fund for the China Agriculture Research System (CARS-18-15), the National Natural Science Foundation of China (31272085,31572051), and the Special Fund for Agro-scienti fic Rensearch in the Public Interest, China (201503109).
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
Allenby N E E, O’Connor N, Pragai Z, Ward A C, Wipat A,Harwood C R. 2005. Genome-wide transcriptional analysis of the phosphate starvation stimulon of Bacillus subtilis.Journal of Bacteriology,187, 8063–8080.
Arnaud M, Chastanet A, Debarbouille M. 2004. New vector for ef ficient allelic replacement in naturally nontransformable,low-GC-content, Gram-positive bacteria. Applied and Environmental Microbiology,70, 6887–6891.
Bisicchia P, Lioliou E, Noone D, Salzberg L I, Botella E,Hubner S, Devine K M. 2010. Peptidoglycan metabolism is controlled by the WalRK (YycFG) and PhoPR twocomponent systems in phosphate-limited Bacillus subtilis cells. Molecular Microbiology,75, 972–989.
Blencke H, Homuth G, Ludwig H, Mader U, Hecker M, Stulke J. 2003. Transcriptional pro filing of gene expression in response to glucose in Bacillus subtilis: Regulation of the central metabolic pathways. Metabolic Engineering,5,133–149.
Botella E, Hubner S, Hokamp K, Hansen A, Bisicchia P, Noone D, Powell L, Salzberg L I, Devine K M. 2011. Cell envelope gene expression in phosphate-limited Bacillus subtilis cells.Microbiology,157, 2470–2484.
Chitarra G S, Breeuwer P, Nout M J R, Van Aelst A C, Rombouts F M, Tjakko A. 2003. An antifungal compound produced by Bacillus subtilis YM 10–20 inhibits germination of Penicillium roqueforti conidiospores. Journal of Applied Microbiology,94, 159–166.
Cleveland C C, Townsend A R, Schmidt S K. 2002. Phosphorus limitation of microbial processes in moist tropical forests:Evidence from short-term laboratory incubations and field studies. Ecosystems,5, 680–691.
Dong W, Li S, Lu X, Zhang X, Wang P, Ma P, Guo Q. 2014.Regulation of fengycin biosynthase by regulator PhoP in the Bacillus subtilis strain NCD-2. Acta Phytopathology Sinica,44, 180–187. (in Chinese)
Guo Q, Dong W, Li S, Lu X, Wang P, Zhang X, Wang Y, Ma P.2014. Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiological Research,169, 533–540.
Guo Q, Li S, Lu X, Li B, Ma P. 2010. PhoR/PhoP two component regulatory system affects biocontrol capability of Bacillus subtilis NCD-2. Genetics and Molecular Biology,33,333–340.
Guo Q, Wu Y, Li S, Lu X, Wang H, Ma P. 2013. Functional analysis of ywbB to the bio film formation and root colonization in Bacillus subtilis strain NCD-2. Acta Phytophylacica Sinica,40, 45–50. (in Chinese)
Jacques P. 2011. Surfactin and other lipopeptides from Bacillus spp. In: Biosurfactants. Springer, Berlin, Heidelberg. pp.57–91.
Kraas F I, Helmetag V, Wittmann M, Strieker M, Marahiel M A.2010. Functional dissection of surfactin synthetase initiation module reveals insights into the mechanism of lipoinitiation.Chemistry & Biology,17, 872–880.
Li B, Lu X, Guo Q, Qian C, Li S, Ma P. 2010. Isolation and identi fication of lipopeptides and volatile compounds produced by Bacillus subtilis strain BAB-1. Scientia Agricultura Sinica,43, 3547–3554. (in Chinese)
Li S, Lu X, Ma P, Gao S, Liu X, Liu G. 2005. Evaluation of biocontrol potential of a bacterial strain NCD-2 against cotton Verticillium wilt in field trials. Acta Phytopathologica Sinica,35, 451–455. (in Chinese)
Liu W, Hulett F M. 1998. Comparison of PhoP binding to the tuaA promoter with PhoP binding to other Pho-regulon promoters establishes a Bacillus subtilis Pho core binding site. Microbiology,144, 1443–1450.
Martin J F, Liras P. 2010. Engineering of regulatory cascades and networks controlling antibiotic biosynthesis in Streptomyces. Current Opinion in Microbiology,13,263–273.
Martin P, Lohr J, Dean D. 1981. Transformation of Bacillus thuringiensis protoplasts by plasmid deoxyribonucleic acid.Journal of Bacteriology,145, 980–983.
Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, Arpigny J L, Thonart P. 2007. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environmental Microbiology,9, 1084–1090.
Pragai Z, Harwood C R. 2002. Regulatory interactions between the Pho and sigma (B)-dependent general stress regulons of Bacillus subtilis. Microbiology,148, 1593–1602.
Ramachandran V K. 2008. Microarray Analysis of Rhizobium leguminosarum bv. Viciae 3841 Colonization of the Rhizosphere. University of Reading, United Kingdom.
Rebib H, Hedi A, Rousset M, Boudabous A, Limam F, Sad fi-Zouaoui N. 2012. Biological control of Fusarium foot rot of wheat using fengycin-producing Bacillus subtilis isolated from salty soil. African Journal of Biotechnology,11,8464–8475.
Sambrook J, Russell D W. 2001. Molecular Cloning: A Laboratory Manual. vol. 1–3. Cold Spring Harbor, Cold Spring Harbor Laboratory Press, New York.
Tao Y, Bie X, Lv F, Zhao H, Lu Z. 2011. Antifungal activity and mechanism of fengycin in the presence and absence of commercial surfactin against Rhizopus stolonifer. The Journal of Microbiology,49, 146–150.
Vanittanakom N, Loef fler W, Koch U, Jung G. 1986. Fengycin- A novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. The Journal of Antibiotics,39,888–901.
Wan J K, Chang B Y, Lin T P, Liu S T. 2009. Activation of the promoter of fengycin synthetase operon by the UP element.Journal of Bacteriology,191, 4615–4623.
Wang P, Guo Q, Ma Y, Li S, Lu X, Zhang X, Ma P. 2015.DegQ regulates the production of fengycins and bio film formation of the biocontrol agent Bacillus subtilis NCD-2.Microbiological Research,178, 42–50.
Wang T, Liang Y, Wu M, Chen Z, Lin J, Yang L. 2015. Natural products from Bacillus subtilis with antimicrobial properties.Chinese Journal of Chemical Engineering,23, 744–754.(in Chiense)
Yanez-Mendizabal V, Zeriouh H, Vinas I, Torres R, Usall J,de Vicente A, Perez-Garcia A, Teixido N. 2012. Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. European Journal of Plant Pathology,132,609–619.
Yoshida S, Hiradate S, Tsukamoto T, Hatakeda K, Shirata A. 2001. Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves.Phytopathology,91, 181–187.
13 February, 2017 Accepted 2 May, 2017
GUO Qing-gang, Tel: +86-312-5915677, E-mail: gqg77@163.com; Correspondence MA Ping, Tel: +86-312-5915678, E-mail:pingma88@126.com
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(17)61669-1
Section editor WAN Fang-hao
Managing editor ZHANG Juan
杂志排行
Journal of Integrative Agriculture的其它文章
- Climate change and agriculture: Impacts and adaptive responses in Iran
- Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
- Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
- ldenti fication of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao
- New clues concerning pigment biosynthesis in green colored fiber provided by proteomics-based analysis
- Constitutive expression of feedback-insensitive cystathionine γ-synthase increases methionine levels in soybean leaves and seeds
