Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
2018-01-04XIAOWumingPENGXinLUOLixinLIANGKeqinWANGJiafengHUANGMingLIUYongzhuGUOTaoLUOWenlongYANGQiyunZHUXiaoyuanWANGHuiCHENZhiqiang
XIAO Wu-ming, PENG Xin, LUO Li-xin, LIANG Ke-qin, WANG Jia-feng, HUANG Ming, LIU Yongzhu, GUO Tao, LUO Wen-long, YANG Qi-yun, ZHU Xiao-yuan, WANG Hui, CHEN Zhi-qiang
1 National Engineering Research Center of Plant Space Breeding, South China Agricultural University, Guangzhou 510642,P.R.China
2 Plant Protection Research Institute, Guangdong Academy of Agricultural Sciences/Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Guangzhou 510640, P.R.China
RESEARCH ARTICLE
Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
XIAO Wu-ming1*, PENG Xin1*, LUO Li-xin1, LIANG Ke-qin1, WANG Jia-feng1, HUANG Ming1, LIU Yongzhu1, GUO Tao1, LUO Wen-long1, YANG Qi-yun2, ZHU Xiao-yuan2, WANG Hui1, CHEN Zhi-qiang1
1 National Engineering Research Center of Plant Space Breeding, South China Agricultural University, Guangzhou 510642,P.R.China
2 Plant Protection Research Institute, Guangdong Academy of Agricultural Sciences/Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Guangzhou 510640, P.R.China
Blast resistance and grain quality are major problems in hybrid rice production in China. In this study, two resistance (R)genes, Pi46 and Pita, along with the gene Wxb, which mainly affects rice endosperm amylose content (AC), were introgressed into an elite indica restoring line, R8166, which has little blast resistance and poor grain quality through marker-assisted selection (MAS). Eight improved lines were found to have recurrent genome recovery ratios ranging from 88.68 to 96.23%.Two improved lines, R163 and R167, were selected for subsequent studies. R167, which has the highest recovery ratio(96.23%), showed no signi ficant differences in multiple agronomic traits. In contrast, R163 with the lowest recovery ratio(88.68%) exhibited signi ficant differences in heading date and yield per plant compared with the recurrent parent. At two developmental stages, R163 and R167 had greatly enhanced resistance to blast over the recurrent parent. Similar trends were also observed for agronomic traits and blast resistance in R163- and R167-derived hybrids when compared with the counterparts from R8166. In addition, R163, R167, and their derived hybrids signi ficantly improved the grain quality traits,including amylose content (AC), gel consistency (GC), chalky grain rate (CGR), and degree of endosperm chalkiness (DEC).It con firmed the success of ef ficiently developing elite restoring lines using MAS in this study.
rice, restoring line, blast resistance, grain quality, MAS
1. Introduction
Rice, one of the most important food crops, feeds nearly half of the global population. Maintaining stable rice production is extremely important to feed the constantly growing human population, particularly in some Asian and African countries(RoyChowdhury et al. 2012; Tsukaguchi et al. 2016). Hybrid rice, which gives signi ficantly higher yields than the inbred lines due to its heterosis or hybrid vigor, has been widely cultivated in China. Currently, more than half of the total rice growing areas in China are planted with hybrid rice cultivars(Peng et al. 2009; Duan et al. 2012). However, whether a hybrid cultivar could be planted widely or not depends upon many factors. Among them, the resistance to blast disease and the grain quality play a very important role.
The extensive cultivation of a few hybrids on a large scale not only narrows down the genetic diversity but also increases the risk of blast disease epidemicity as it favors the speedy spread of virulent races of M. oryzae fungus(Xiao et al. 2015). The fungus can infect all aerial parts of the plant and cause rice blast, which is one of the most devastating and destructive diseases of rice worldwide (Couch and Kohn 2002). Commonly, the disease reduces yield by 10–30%, and even up to 80% under suitable environmental conditions (Skamnioti and Gurr 2009). A serious yield loss was witnessed in 2006 due to severe seedling and neck blast, which was reported in approximately 20% of the hybrid rice fields in China (Liu et al. 2010). Furthermore,more than 1 500 and 500 ha of hybrid rice in Guangdong Province, China was seriously damaged by blast disease in 2008 and 2011, respectively. Therefore, adequate blast resistance is a primary criterion for a new rice cultivar to be of ficially certi fied and released in several provinces of China (Xiao et al. 2012). It is generally believed that utilization of resistant cultivars is an effective and economical way of combating the disease. Moreover, it is documented that integrating multiple resistance (R) genes is the most advocated strategy to develop new cultivars with broad-spectrum resistance to blast (Hittalmani et al.2000; Tacconi et al. 2010). Thus far, more than 100 blast R genes conferring resistance to various M. oryzae races have been reported. Among them, only a small portion have been cloned (Xu et al. 2014; Ramkumar et al. 2015).Molecular markers that are tightly linked to these R genes have been developed and are universally used to transfer R genes into rice through MAS to improve resistance to blast disease (Ni et al. 2015; Ramkumar et al. 2015; Ellur et al. 2016; Xiao et al. 2016).
Currently, rice cultivars with superior cooking and eating quality are preferred in China. It is well documented that amylose content (AC) is the major determinant of rice cooking and eating quality (Yi et al. 2009; Jin et al. 2010;Hori et al. 2016). The Waxy (Wx) gene, which encodes a granule-bound starch synthase (GBSS) and is located on chromosome 6 of rice, plays a key role in amylose synthesis(Wang et al. 1995; Smith et al. 1997). Many studies have proved that the Waxy region on chromosome 6 has major effects not only on AC, but also on gel consistency (GC),which re flects the gel length (Tan et al. 1999; Zhou et al.2003; He et al. 2006; Wang et al. 2007). Two Waxy gene alleles, Wxaand Wxb, have traditionally been associated with the contents of GBSS and AC in rice endosperm. There is more transcription from the Wxaallele, thus leading to higher levels of GBSS and thus AC, which results in firm and nonsticky cooked rice. However, the Wxballele usually results in low AC and thus tender and sticky cooked rice, which is favored by most Asian consumers (Chen et al. 2008; Jairin et al. 2009). A polymorphic (CT)nmicrosatellite, locating in the 5´-untranslated region of the Wx gene, was identi fied by Bligh et al. (1995) and subsequently renamed RM190(Temnykh et al. 2000). The marker RM190 has been reported to be signi ficantly associated with the AC and GC(Ayres et al. 1997; Zhou et al. 2003; Bao et al. 2006), and it has been intensively used to improve the qualities of rice cultivars using marker-assisted selection (MAS) (Yi et al.2009; Jin et al. 2010; Jantaboon et al. 2011).
In previous studies, a restoring line named R8166 was found that showed a strong combining ability. Although R8166 has been widely utilized in hybrid rice breeding programs, its susceptibility to blast disease is a potential threat to its application. The other defects of R8166 are its high AC and hard GC due to its Wxagenotype, leading to dry and fluffy cooked rice that tends to become hard after cooling. As a result, the poor rice quality negatively affects its application. Therefore, the improvement of R8166 has focused on increasing blast disease resistance,reducing AC and improving GC without damaging its elite agronomic traits and combining ability. Here, we reported the introgression of the genes Pi46, Pita, and Wxbinto R8166 by marker-assisted backcross breeding (MABB).Eventually, two improved lines, R163 and R167 with the lowest and highest recovery ratios of the recurrent genome,respectively, were developed and used for generating corresponding hybrid combinations with enhanced blast resistance and improved grain quality.
2. Materials and methods
2.1. Plant materials
R8166, an elite indica restoring line, was chosen as the recurrent parent. Although it has good combining ability,it has poor resistance against blast and its grain is of poor quality due to the relatively high AC and hard GC. The indica rice accession H4, conferring broad-spectrum resistance to blast at both the seedling and adult stages, was used as the donor parent in this study. H4 was found to carry at least two major R genes, with Pi46 on the long arm of chromosome 11 (Xiao et al. 2011) and Pita on chromosome 12 (Xiao et al. 2016). The R gene Pi46 was con firmed to be a different allele of the Pik/Pi1 locus, for several single nucleotide polymorphisms (SNPs) that can discriminate them were identi fied (data not shown). Besides, H4 has low AC and soft and sticky cooked rice mainly due to its Wxballele.Two sterile lines including the cytoplasmic male-sterile(CMS) line Ning A and the thermo-sensitive genic male sterility (TGMS) line Shen 08S, were used to produce six F1hybrids by crossing with the recurrent parent, R8166, and the improved versions, R163 and R167 (described below),respectively. The F1hybrids were subsequently evaluated for disease resistance and grain quality.
2.2. Molecular marker analysis
Three simple sequence repeat (SSR) markers were used for foreground selection. The SSR markers RM224 and RM179 were used to detect Pi46 and Pita, respectively.Marker RM224 is tightly linked with Pi46 at ~1.0 cM (Xiao et al. 2011). Whereas marker RM179, which is located near the centromere of chromosome 12, is tightly linked with Pita (data not shown). The SSR marker RM190 was used for tracing the Wxballele derived from H4. Information about these three markers is listed in Table 1. For further background selection, the other 253 SSR markers distributed evenly on the 12 chromosomes, were used for polymorphism surveys between the donor and recurrent parent, and polymorphic markers were used to recover the genetic background of R8166. Genomic DNA was extracted from frozen leaf materials using the cetyl triethylammnonium bromide (CTAB) method (Murray and Thompson 1980) with minor modi fications. Each 20 μL PCR reaction consisted of 1×PCR buffer (10 mmol L–1Tris, pH 8.4, 50 mmol L–1KCl, 1.8 mmol L–1MgCl2), 0.05 mmol L–1dNTPs, 5 pmol of each primer, 1.0 U of Taq polymerase, and 50 ng genomic DNA. All ampli fications were performed using an applied biosystems (ABI, Shanghai, China) thermal cycler under the following pro file: 94°C for 5 min; 32 cycles of 30 s at 94°C,30 s at 55°C, and 1 min at 72°C; and an extension of 5 min at 72°C. The PCR products for all markers were separated in 8% non-denatured polyacrylamide gel (PAGE) in 1.0×Tris borate EDTA (TBE) buffer followed by silver stains.
2.3. The MABB procedure and crossing
A cross was made between R8166 (female parent) and H4(male parent) to generate F1hybrids during the early crop season (March to July) of 2008 at Guangzhou, Guangdong Province, China. The F1plants were backcrossed to R8166 using mixed pollen to generate the BC1F1generation.Consecutive backcrossing was conducted until the BC3F1generation was produced. Pedigree selection was then performed to obtain the target BC3F3lines (Fig. 1). The recurrent parent and the two stable improved lines R163 and R167, which are simultaneously homozygous for the Pi46, Pita, and Wxbgenes, were selected to cross with the male sterile lines (Ning A and Shen 08S) to produce the corresponding F1hybrids for agronomic assessment,disease evaluation, and grain quality tests.
2.4. Evaluation of blast resistance
The resistance spectra of the donor parent, recurrent parent,improved lines, and six F1hybrids derived from R163, R167,and R8166 were determined at the seedling stage. In total,34 highly diverse M. oryzae isolates, which were collected from different ecological areas in the Guangdong Province,China across many years, were used for arti ficial inoculation of leaf blast at the seedling stage. Two-week-old seedlings were spray-inoculated with spore suspensions (1×105spores mL–1) and were cultured in a dew growth chamber for 24 h in darkness at 26°C. The inoculated seedlings were subsequently transferred into a semi-temperature-controlled greenhouse where the temperature and relative humidity were maintained for six days at around 24–30°C and 90%,respectively. The most serious disease lesions on the inoculated rice leaves were rated on a scale of 0–9, with rating of 0–3 considered as resistant (R) and 4–9 as susceptible(S) according to the Standard Evaluation System for Rice(IRRI 2013).
Neck blast severity was evaluated by growing the tested lines at two natural blast nurseries with a severe blast epidemic, Conghua (23°57´N, 113°55´E) and Yangjiang(21°50´N, 111°58´E), in the Guangdong Province, China during the early crop season (March to July) of 2013.Both sites are characterized with the microclimate (proper temperature and high humidity) favorable for outbreaks of numerous M. oryzae races each year during the rice growing season. Each entry was planted in four rows with five hills (3–4 plants per hill) per row at a planting density of 20 cm×20 cm. The variety CO39, which is highly susceptible, was planted around each plot as a spreader to maintain the pathogen population diversity and to enhance natural infection. Panicle blast resistance was measured usingthe 0–9 scale of the Standard Evaluation System for Rice(IRRI 2013).

Table 1 Molecular markers for marker-assisted selection (MAS) of foreground selection

Fig. 1 Schematic work flow of marker-assisted backcross breeding. MAS, marker-assisted selection.
2.5. Assessment of agronomic traits
The donor parent, recurrent parent, improved lines and six F1hybrids were grown using a randomized complete block design with three replications at the experimental field of South China Agricultural University, Guangzhou, during the late crop season (July to November) of 2013. Each plots consisted of six rows with six plants per row at a planting density of 20 cm×20 cm. Only four plants in the middle of each plot were used to measure agronomic traits. The measured traits included the heading date (days to 50%flowering), plant height, panicles per plant, panicle length,total grains per panicle, seed setting rate, 1 000-grain weight,and yield per plant. Water and fertilizer were managed regularly. Statistical analysis was performed with independent samples using the least signi ficance difference (LSD)software (Ni et al. 2015).
2.6. Evaluation of grain quality parameters
Rice grains of the tested lines were harvested at physiological maturity and dried naturally to a moisture content of 12–14% in a greenhouse. The dried grains were stored at room temperature for 1 month prior to the evaluation of grain quality parameters. Grain samples of 100 g were taken from each replicate and combined. A total of 50 g of mixed samples were then used as the materials for the grain quality test. Grain samples were dehulled to brown rice (Kett Electric Laboratory, Tokyo, Japan) and milled to polished rice using a Satake Rice Machine (Satake Corp,Japan), and then grounded to powder using a minipolisher(Qianjiang Yiqishebei Corp, Hangzhou, China). AC and GC were evaluated following the procedures described by Lanceras et al. (2000). A standard curve made using the rice samples with known AC was used to estimate the AC of each sample. The GC was determined by measuring the length of grain starch slurry in a culture tube of cold gel. The length of the gel, the distance from the bottom of the tube to the front of the gel migration, was measured in millimetres after one hour. The longer gel is considered to be softer than the shorter gel.
The morphological features of rice grains include the degree of endosperm chalkiness (DEC), chalky grain rate(CGR), and the size and shape of the kernel. Ten seeds of milled rice kernel were selected for measurements. The length and breadth were measured using a vernier calliper,from which the length/breadth ratio (L/B) was calculated.CGR was determined manually using 100 grains of polished head rice each time. The DEC of polished grains was measured using a CanoScan 5600F (Canno Inc., Japan). The evaluation of the above-mentioned grain quality parameters was replicated three times and conducted at South China Agricultural University in December of 2013(1 month after harvest). Statistical analysis was performed with independent samples using the LSD software (Ni et al. 2015).
3. Results
3.1. MAS of the BC1F1, BC2F1, BC3F1, and BC3F2 generations
In the early crop season of 2009, 27 BC1F1individuals were firstly assayed with RM190 and 14 plants were identi fied as being heterozygous state at the Wx locus. Subsequently,these 14 plants were genotyped using RM224, and six showed heterozygosity for Pi46. Finally, genotyping with RM179 revealed two individuals as being heterozygous at the Pita locus. Therefore, these two plants, which simultaneously carried Wxb, Pi46, and Pita in heterozygous state,were backcrossed with R8166 using mixed pollen to generate the BC2F1generation. Similarly, by using RM190, 17 plants heterozygous at the Wx locus were firstly identi fied from the 35 BC2F1individuals. Among these 17 plants, nine were con firmed to be heterozygous for Pi46, and then four were further found to be heterozygous for Pita. These four plants were used to generate 41 BC3F1individuals. Only five individuals in the BC3F1population were identi fied to be simultaneously heterozygous for Wxb, Pi46, and Pita simultaneously. The BC3F2population was produced from these five individuals through self-pollination.
The BC3F2population was cultivated in the early crop season of 2010, and a total of 500 plants at the young seedling stage were genotyped firstly using marker RM190 for the Wx alleles. As a result, 112 plants were homozygous for Wxa, 269 were heterozygous for Wxa/Wxb, and 119 were homozygous for Wxb, respectively. The segregation ratio of 112:269:119 was in accordance with 1:2:1 segregation according to the Chi-square test, χ2=3.08<χ(20.05,2)=5.99.The 119 plants were then genotyped using RM224, and 31 individuals were identi fied as homozygous for Pi46. Finally,seven individuals among the 31 were identi fied as being homozygous for Pita. Therefore, a total of seven plants simultaneously carrying homozygous Wxb, Pi46, and Pita were identi fied.
3.2. Producing stable improved lines and genetic background analysis
A total of 200 individuals of the BC3F3generation derived from the above seven BC3F2individuals described above were planted side by side with the recipient parent R8166 in the late season of 2010. At maturity stage, only eight plants,labelled as T1 to T8, were selected based on their closest phenotypic resemblance to the recurrent parent. This work was performed by a panel of four experienced experts in rice breeding. Subsequently, a total of 253 SSR markers were used to assay the genetic background recovery of the eight plants. Although 217 markers gave valid ampli fication products, only 53 markers were polymorphic between the two parents, R8166 and H4 (Appendix A).
The frequency of the R8166 alleles at non-target loci for the eight plants ranged from 88.68 to 96.23%, with an average ratio of 92.93%, which was very close to the expected value (93.75%). Among the eight plants, four showed higher recovery percentages of the recurrent genome than the theoretical value (Table 2). It implied that the evaluation of performance apparently took work to a degree in the recovery of recipient’s genome. For subsequent studies,the individuals T3 and T7, which had the lowest and highest recovery ratio of recurrent genome (88.68 and 96.23%),respectively, were selfed to generate the stable BC3F4lines,which were designated as R163 and R167.
3.3. Blast resistance of the improved lines and their hybrids
To test the resistance spectrum at the seedling stage, the tested lines were inoculated with 34 different M. oryzae isolates. The resistance spectrum of both R163 and R167 reached 94.1%. This is an increase of 58.8% compared with the recurrent parent, which had a resistance spectrum of 35.3% (Table 3). In other words, they conferred resistance to 20 more M. oryzae isolates than R8166. Additionally, all of the hybrids derived from R163 and R167 showed broader resistance spectrum than the corresponding hybrids derived from R8166. For example, both Ning A/R163 and Ning A/R167 were resistant to 32.4% more M. oryzae isolates than Ning A/R8166, whereas both Shen 08S/R163 and Shen 08S/R167 were resistant to 26.5% more isolates compared with Shen 08S/R8166. The different increasing ranges were observed obviously. This may be attributed to the R gene(s) in different sterile lines, which could interact with the R gene(s) in the restoring lines and resulted in different resistance reactions. The donor parent H4 conferred a full-spectrum resistance, indicating that it was resistant to more isolates than the recurrent parent, the improved lines and all of the hybrids.
Regarding the severity of neck blast, the improved lines R163 and R167 performed markedly better than the recurrent parent R8166 (Table 4). Likewise, the hybrids derived from R163 and R167 also showed better resistance against neck blast than the corresponding hybrids from R8166. This suggests that the introgression of the two major R genes, Pi46 and Pita, played critical roles in enhancing the resistance of R163 and R167 and the derived hybrids. Among the 10 tested lines, six performed better at the Yangjiang nursery than at the Conghua nursery in terms of disease response (Table 4). This indicated that there probably existed more virulent races at the Conghua nursery or that conditions at the Conghua nurseryincreased susceptibility to neck blast.
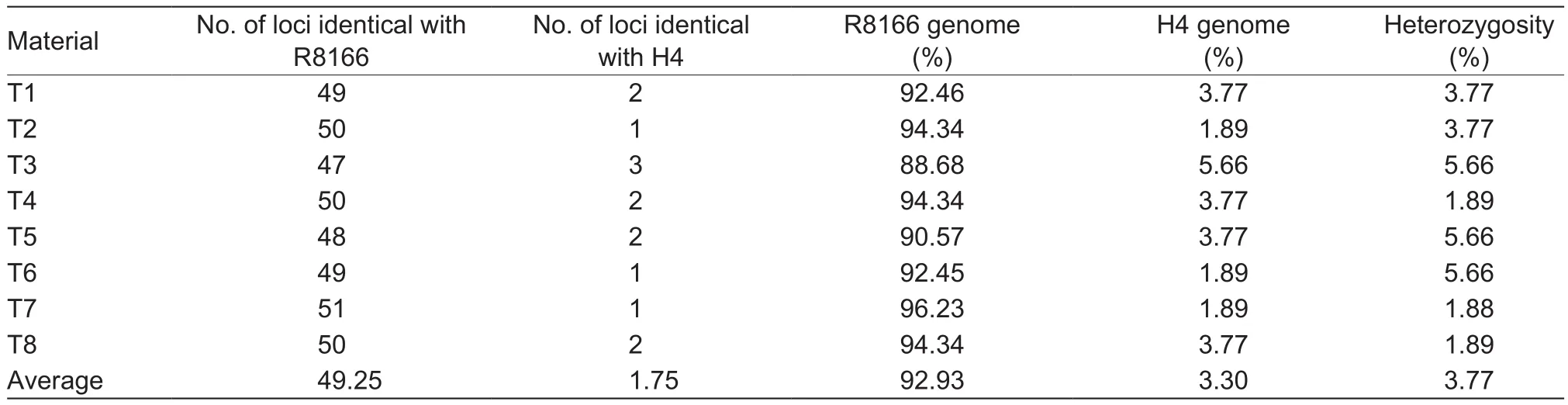
Table 2 Ratio of genetic background recovery of the eight plants using 53 polymorphic simple sequence repeat (SSR) markers
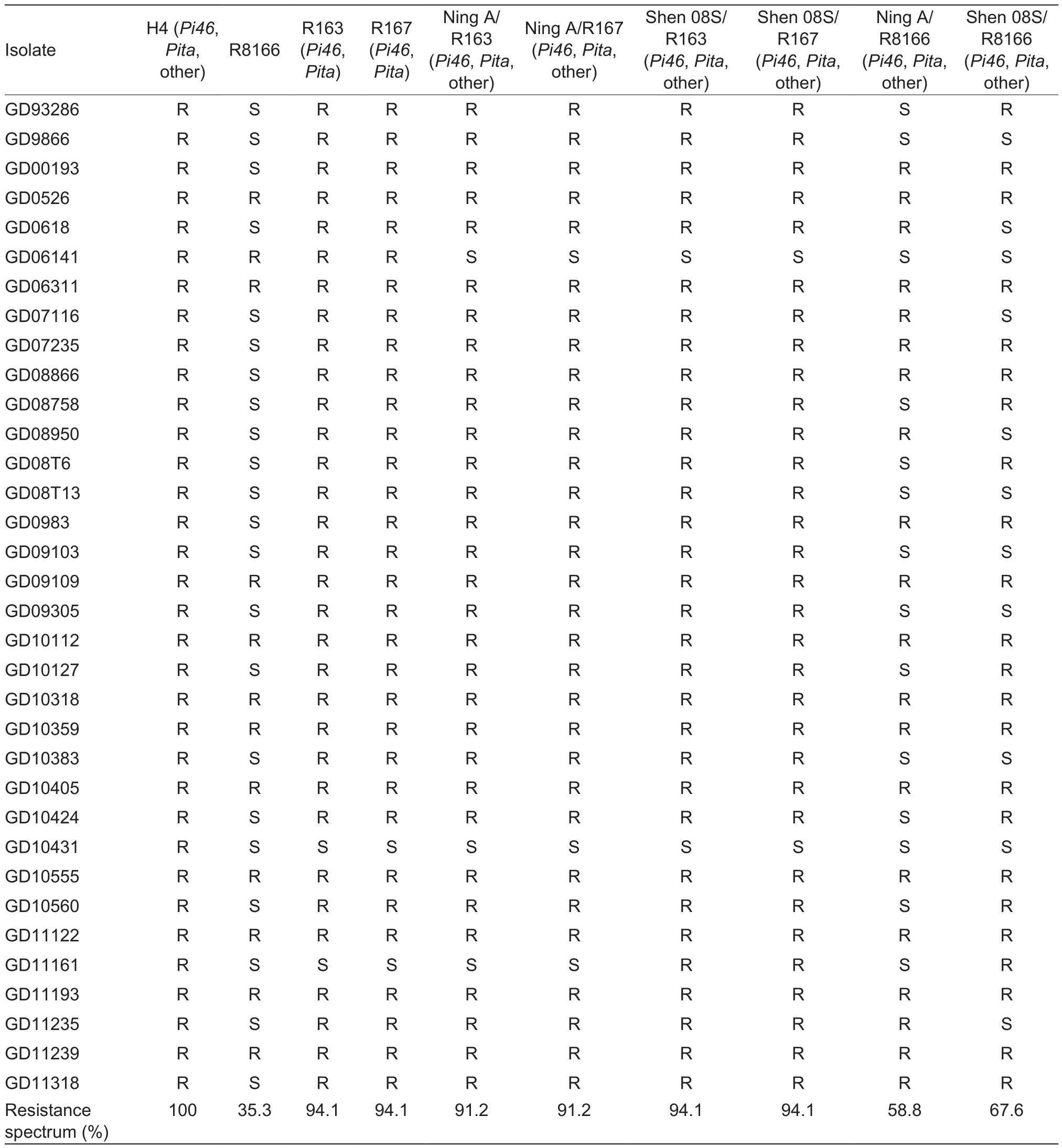
Table 3 Resistance reactions of varieties of H4, R8166, R163, R167, and their derived hybrids to 34 Magnaporthe oryzae isolates1)
3.4. Agronomic performance of the improved lines and their derived hybrids
We examined the agronomic traits for the tested lines planted in Guangzhou where there was no disease stress.Compared with the recipient parent, the improved version R163 had signi ficantly decreased heading date and yield per plant, but signi ficantly increased seed setting rate. The decrease in heading date indicated that R163 matured earlier,which was desirable. Except for markedly increased panicle length, the improved version R167 showed no signi ficant differences from the recipient parent in other agronomic traits,indicating that it shared the closest phenotypic resemblance with the recipient parent. Therefore, the two improved lines and the recurrent parent can be recognized as a panel of near isogenic lines (NIL), because they almost shared the same background. Both R163 and R167 were phenotypically distinct from the donor parent H4, exhibiting signi ficant differences in multiple traits. In comparison to the recurrent parent, the donor parent showed signi ficant decreases in heading date, grains per panicle, 1 000-grain weight, and yield per plant, respectively. Nevertheless, the donor parent showed markedly high seed setting rate compared with the recipient parent (Table 5). The reduction of heading date in R163 could thus be ascribed to the genomic segments inherited from the donor parent. However, the reduced yield per plant of R163 could be attributed to the mixed results of slightly less panicles, grains, and grain weight.
Regarding hybrids, both Ning A/R163 and Shen 08S/R163 showed a signi ficant reduction in heading date compared with their counterparts Ning A/R8166 and Shen 08S/R8166 (Table 6), indicating that the early heading date of R163 could be passed to its hybrids. However, the reduced range from Ning A/R163 to Ning A/R8166 was wider than that from Shen 08S/R163 to Shen 08S/R8166. This may be due to the interactions of R163 with different sterile lines. Ning A/R163 had signi ficantly reduced panicle length compared with Ning A/R8166. However, Ning A/R163 had a markedly increased seed setting rate, which could compensate for the fewer grains per panicle. Consequently, no signi ficant difference in yield per plant was detected between Ning A/R163 and the control Ning A/R8166. Similarly, Ning A/R167 exhibited no signi ficant differences with Ning A/R8166 in all of the tested traits, which re flected the closet phenotypic resemblance. Shen 08S/R163 had dramatically reduced yield per plant, which may be due to the reduction in panicles per plant, grains per panicle, and 1 000-grain weight compared with Shen 08S/R8166. Shen 08S/R167 showed a signi ficant increase in plant height over Shen 08S/R8166.Nevertheless, there was not a signi ficant increase in yield per plant because plant height is not a yield components.Generally, the hybrids derived from the improved version R167 per se showed superiority over the hybrids from R163 in yield per plant.

Table 4 Neck blast resistance reactions of varieties of H4, R8166, R163, R167, and the derived hybrids at two natural nurseries1)

Table 5 Comparison of agronomic traits between the two improved versions, the donor parent and the recurrent parent

Table 6 Comparison of agronomic traits between hybrids derived from the two improved versions and the recurrent parent
3.5. Grain quality of the improved lines and their hybrids
As expected, the improved lines R163 and R167 had an AC of ~15.03 and 15.37%, respectively, which is close to the AC of the recipient parent (14.24%) but markedly lower than that of the recurrent parent (25.58%). The CGR of R163 and R167 were reduced to ~8.00 and 10.33%, respectively, in comparison to R8166. Signi ficant reduction of DEC was also observed in R163 and R167 compared with R8166. Conversely, the GC of R163 and R167 was increased over R8166 by 16.73 and 15.56 mm, respectively(Table 7). However, all three lines had similar L/B, which indicates that the two improved versions retained a similar grain shape as the recurrent parent. Overall, R163 and R167 have improved grain quality in terms of AC, GC, CGR,and DEC simultaneously.
Both Ning A/R163 and Ning A/R167 had signi ficantly lower AC, CGR, and DEC but markedly higher GC compared with the control Ning A/R8166. This proved that Ning A/R163 and Ning A/R167 remarkably improved in multiple quality parameters. The same trend was also observed for Shen 08S/R163 and Shen 08S/R167 compared with Shen 08S/R8166 (Table 8). However, the hybrids derived from Shen 08S displayed more reduction in AC, CGR, and DEC and more increase in GC than the corresponding hybrids derived from Ning A. In conclusion, Shen 08S/R163 and Shen 08S/R167 showed superior grain quality compared with Ning A/R163 and Ning A/R167, respectively. This is mainly due to the effects of the different sterile lines on the grain quality of the F1hybrids.
4. Discussion
MABB, which includes two steps (i) MAS for the target gene(s), known as foreground selection and (ii) MAS for recovery of the recurrent parent genome, known as background selection (Hospital et al. 1992), is the most effective way of integrating speci fic gene(s) into an elite line to breed new lines that have the desired donor allele(s) but otherwise look identical to the recurrent parent (Basavaraj et al. 2010).It is the outstanding advantages that make MAS prevail over conventional breeding and has been utilized widely for decades. However, the accuracy of MAS depends mainly on the distance between the target gene and its closest marker.In this study, we used the marker RM190 for MAS breeding of rice lines with improved grain quality. Because RM190 is a functional marker of the Wx gene, there is no doubt about the accuracy of MAS. The marker RM224, which is linked with the R gene Pi46 at ~1.0 cM (Xiao et al. 2011),is close enough to be used for MAS. In addition, Pi46 is located in a cross-cold region, this reduces recombination between this R gene and the marker RM224. Although several functional markers have been developed for Pita (Jia et al. 2002; Wang et al. 2007), they are dominant markers that cannot discriminate heterozygous from homozygous genotypes. In our previous research, we found that it is very ef ficient to use the marker RM179 to tag Pita (data not shown) because RM179 is located near the centromere of chromosome 12 where Pita is covered (Bryan et al. 2000).It is well-known that recombination case seldom occurs in the region spanning centromere (Nakamura et al. 1997).Therefore, RM179 is also tightly-linked with Pita. Actually,for better accuracy, all the individuals heterozygous for Pita in the BC1F1(two plants), BC2F1(four plants), and BC3F1( five plants) generations and the individuals homozygous for Pita in the BC3F2population (seven plants) based on RM179 genotypic assays, were subjected to a pathogenic assay at the seedling stage with an M. oryzae isolate that is avirulent to Pita. This con firmed the accuracy of using RM179 for the MAS of Pita.
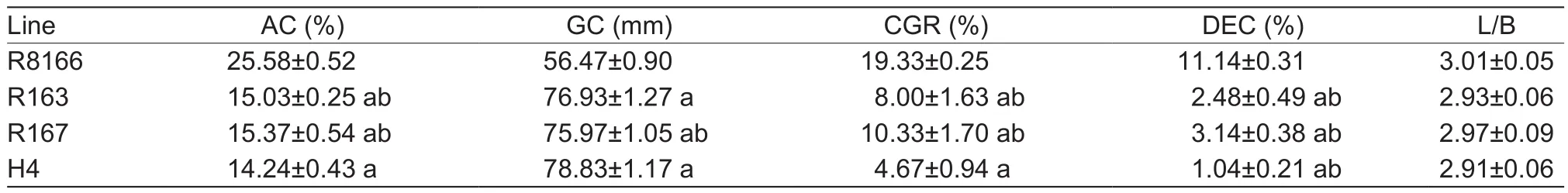
Table 7 Comparison of grain quality between the two improved versions, the donor parent and the recipient parent1)
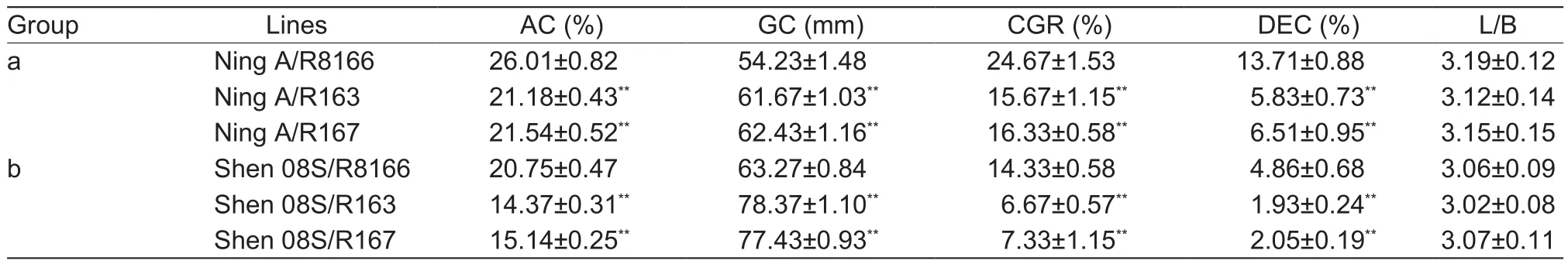
Table 8 Comparison of grain quality between hybrids derived from the two improved versions and the recurrent parent1)
In this study, both the improved versions and their derived hybrids showed broad resistance spectrum at the seedling stage and enhanced resistance to neck blast at the adult stage. The resistance of R163, R167 and their derived hybrids was dramatically enhanced, reaching our anticipated target. Our results showed that the combination of Pi46 and Pita really works to improve blast resistance.Before developing the improved lines, we deeply considered whether we should integrate Pi46 or Pita. Eventually, we chose to introduce both genes because resistance due to a single R gene tends to be overcome by the M. oryzae races which are complicated and dynamic in natural nurseries.The findings revealed that several classic cultivars with broad-spectrum resistance, including Moroberekan, Tetep,IR64, Sanhuangzhan 2, and LAC 23, harbored multiple (at least two) R genes simultaneously (Mackill and Bonman 1992; Chen et al. 1999; Sallaud et al. 2003; Barman et al.2004; Liu et al. 2004). We have found that the presence of only Pi46 or only Pita could not condition good neck blast resistance (Xiao et al. 2016). In addition, we speculated that R8166 carries other R gene(s) because it had a resistance spectrum of 35.3%, conferring resistance to 12 isolates among the tested 34. Therefore, it can be concluded that the broad-spectrum resistance of the improved lines (R163 and R167) resulted from the introduction of Pi46, Pita, and other R gene(s) from R8166. Similarly, the enhanced resistance of the hybrids derived from the improved lines may be due to the interaction between R genes in the improved lines and those in the different sterile lines. In fact, we could witness that isolate GD11161 was virulent to R163 and R167, but avirulent to Shen 08S/R163 and Shen 08S/R167.This is because the sterile line Shen 08S was resistant to the isolate and the resistance could be transferred to its F1hybrids (Table 3). Therefore, the improved versions could be used to cross with sterile lines with different R gene(s)to obtain broader resistance.
Currently, there is a strong emphasis in China on improving the grain quality of hybrid rice varieties, especially the quality of indica hybrids. The most serious problems lie in the eating and cooking quality as well as appearance. It is known that the eating and cooking quality of rice is largely determined by AC of the endosperm, which is mainly controlled by the Wx locus (Zhou et al. 2003; Yi et al. 2009).Thus, it is feasible to improve the grain quality using MAS for Wx. In this study, the Wxbgene in the donor parent was successfully integrated into the recipient parent. The improved versions showed dramatically lower AC and higher GC than the recipient parent. This con firms the effectiveness of improving the grain quality of the restoring line itself.However, our ultimate aim was to improve the grain quality of the derived hybrids, which would be commercialized. It is well known that the grain quality of a hybrid cultivar depends on both the maternal and paternal parents. Although all the hybrids derived from R163 and R167 displayed signi ficantly improved grain quality compared with their counterparts,the hybrids Ning A/R163 and Ning A/R167 still showed poor grain quality owing to the high AC (21.18 and 21.54%) and hard GC (61.67 and 62.43 mm). This could be attributed to the inferior grain quality of the sterile line Ning A. Since the Wxaallele is a dominant allele and leads to high AC (Jairin et al. 2009). The Ning A/R163 and Ning A/R167 hybrids,which had the genotype of Wxa/Wxb, had high AC and hard GC similar to Ning A. Therefore, it is necessary to cross the improved lines with the sterile lines displaying good grain quality to generate hybrids with superior grain quality. To take advantage of the two improved lines, the development of new hybrids using them is in progress.
Chalkiness, a critical factor in fluencing grain appearance,has also attracted wide attention in hybrid rice development.Translucent rice, which is preferred by consumers, is usually characterized by low chalkiness (Zhou et al. 2015). In this study, we observed that the CGR and DEC decreased along with the reduction of AC in the improved lines and their hybrids in this study (Tables 7 and 8). Consistent with our finding, Zhou et al. (2003) found that AC decreased in parallel with the reduction in grain opacity, when Wx from Minghui 63 was introduced to Zhenshan 97. Although there is no direct evidence that AC contributes to the occurrence of endosperm chalkiness, variation in AC was reported to be associated with the formation of grain chalkiness(Cheng et al. 2005; Cai et al. 2013), indicating that AC and chalkiness may be co-regulated by certain factors. Further studies are needed to determine the relationship between AC and chalkiness.
Based on our findings in the present study, we selected the two lines with the lowest and highest recovery ratio of the recurrent genome for further studies. When performing MABB, we usually try to obtain improved versions with the highest recovery ratio to maintain the closest phenotypic resemblance with the recurrent parent. Therefore, R167 was naturally the aim of the improvement plan. Considering the low recovery ratio of R163, we hoped to obtain a line not only showing improved blast resistance and grain quality, but also showing distinct agronomic traits from the recurrent parent to increase genetic diversity. R163 showed signi ficantly earlier maturity and low yield. Besides, its derived hybrids were inferior to the hybrids derived from R167 in general. This indicates that R163 inherited some genomic segments with linkage drag from the donor parent,which might account for the earlier maturity and low yield of R163 compared with the recipient parent. Therefore, it was undesirable to select the lines with a large percentage of genetic background from the donor parent in this study. This is because the donor parent (H4) actually showed poorer agronomic traits and lower yield compared with the recipient parent (R8166). However, if the donor parent is better than the recurrent parent in agronomic traits, it would be feasible to select improved lines with the incorporation of different proportion of the donor parent’s genetic background.
5. Conclusion
Two restoring lines R163 and R167 were developed by incorporating three genes, Pi46, Pita, and Wxb, through MABB and agronomic selection. Both of them acquired enhanced blast resistance and improved grain quality. Besides, the hybrids derived from the two improved lines also obtained remarkable improvement in blast resistance and grain quality compared with their counterparts. The two improved lines could, therefore, be used to develop elite hybrids.
Acknowledgements
We thank Dr. Wu Kunsheng, Monsanto Company, St. Louis,Missouri, USA for critical reading of the manuscript. This research was supported by the grant from the State Scholarship Fund of China (20153069), the the National Key R&D Program of China (2016YFD0101100) and by the earmarked fund for China Agriculture Research System (CARS-01-12).
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
Ayres N M, McClung A M, Larkin P D, Bligh H F J, Jones C A, Park W D. 1997. Microsatellites and a single-nucleotide polymorphism differentiate apparent amylose classes in an extended pedigree of US rice germ plasm. Theoretical Applied Genetics,94, 773–781.
Bao J S, Corke H, Sun M. 2006. Microsatellites, single nucleotide polymorphisms and a sequence tagged site in starchsynthesizing genes in relation to starch physicochemical properties in nonwaxy rice (Oryza sativa L.). Theoretical Applied Genetics,113, 1185–1196.
Barman S R, Gowda M, Venu R C, Chattoo B B. 2004.Identi fication of a major blast resistance gene in the rice cultivar ‘Tetep’. Plant Breeding,123, 300–302.
Basavaraj S H, Singh V K, Singh A, Anand D, Yadav S, Ellur R K, Singh D, Krishnan S G, Nagarajan M, Mohapatra T,Prabhu K V, Singh A K. 2010. Marker-assisted improvement of bacterial blight resistance in parental lines of Pusa RH10,a super fine grain aromatic rice hybrid. Molecular Breeding,26, 293–305.
Bligh H F J, Till R I, Jones C A. 1995. A microsatellite sequence closely linked to the Waxy gene of Oryza sativa. Euphytica,86, 83–85.
Bryan G T, Wu K S, Farrall L, Jia Y L, Hershey H P, McAdams S A, Faulk K N, Donaldson G K, Tarchini R, Valent B. 2000.A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta.The Plant Cell,12, 2033–2045.
Cai H, Xie W, Lian X. 2013. Comparative analysis of differentially expressed genes in rice under nitrogen and phosphorus starvation stress conditions. Plant Molecular Biological Reports,31, 160–173.
Chen D H, Vina M, Inukai T, Mackill D J, Ronald P C, Nelson R J. 1999. Molecular mapping of the blast resistance gene,Pi44(t) in a line derived from a durably resistant rice cultivar.Theoretical Applied Genetics,98, 1046–1053.
Chen M H, Bergman C, Pinson S, Fjellstrom R. 2008. Waxy gene haplotypes: Associations with apparent amylose content and the effect by the environment in an international rice germplasm collection. Journal of Cereal Science,47,536–545.
Cheng F M, Zhong L J, Wang F, Zhang G P. 2005. Differences in cooking and eating properties between chalky and translucent parts in rice grains. Food Chemistry,90, 39–46.
Couch B C, Kohn L M. 2002. A multilocus gene genealogy concordant with host preference indicates segregation of a new species Magnaporthe oryzae, from M. grisea.Mycologia,94, 683–693.
Duan Y L, Guan H Z, Zhuo M, Chen Z W, Li W T, Pan R S,Mao D W, Zhuo Y C, Wu W R. 2012. Genetic analysis and mapping of an enclosed panicle mutant locus esp1 in rice(Oryza sativa L.). Journal of Integrative Agriculture,11,1933–1939.
Ellur R K, Khanna A, Yadav A, Pathania S, Rajashekara H,Singh V K, Gopala Krishnan S, Bhowmick P K, Nagarajan M, Vinod K K, Prakash G, Mondal K K, Singh N K, Vinod Prabhu K, Singh A K. 2016. Improvement of Basmati rice varieties for resistance to blast and bacterial blight diseases using marker assisted backcross breeding. Plant Science,242, 330–341.
He Y, Han Y, Jiang L, Xu C, Lu J, Xu M. 2006. Functional analysis of starch synthesis genes in determining rice eating and cooking qualities. Molecular Breeding,18, 277–290.
Hittalmani S, Parco A, Mew T V, Zeigler R S, Huang N. 2000.Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice. Theoretical Applied Genetics,100, 1121–1128.
Hori K, Suzuki K, Iijima K, Ebana K. 2016. Variation in cooking and eating quality traits in Japanese rice germplasm accessions. Breeding Science,66, 309–318.
Hospital F, Chevalet C, Mulsant P. 1992. Using markers in gene introgression breeding programs. Genetics,132,1199–1210.
IRRI (International Rice Research Institute). 2013. Standard Evaluation System (SES) for Rice. 5th ed. International Rice Research Institute, Manila. pp. 18–19.
Jairin J, Teangdeerith S, Leelagud P, Kothcharerk J, Sansen K, Yi M, Vanavichit A, Toojinda T. 2009. Development of rice introgression lines with brown planthopper resistance and KDML105 grain quality characteristics through markerassisted selection. Field Crops Research,110, 263–271.
Jantaboon J, Siangliw M, Immark S, Jamboonsri W, Vanavichit A, Toojind T. 2011. Ideotype breeding for submergence tolerance and cooking quality by marker-assisted selection in rice. Field Crops Research,123, 206–213.
Jia Y, Wang Z, Singh P. 2002. Development of dominant rice blast Pi-ta resistance gene markers. Crop Science,42,2145–2149.
Jin L, Lu Y, Shao Y A, Zhang G, Xiao P, Shen S Q, Corke H,Bao J S. 2010. Molecular marker assisted selection for improvement of the eating, cooking and sensory quality of rice (Oryza sativa L.). Journal of Cereal Science,51,159–164.
Lanceras J C, Huang Z L, Naivikul O, Vanavichit A, Ruanjaichon V, Tragoonrung S. 2000. Mapping of genes for cooking and eating qualities in Thai jasmine rice (KDML 105). DNA Research,7, 93–101.
Liu B, Zhang S H, Zhu X Y, Yang Q Y, Wu S Z, Mei M T, Mauleon R, Leach J, Mew T, Leung H. 2004. Candidate defense genes as predictors of quantitative blast resistance in rice.Molecular Plant Microbe Interactions,17, 1146–1152.
Liu J L, Wang X J, Mitchell T, Hu Y J, Liu X L, Dai L Y, Wang G L.2010. Recent progress and understanding of the molecular mechanisms of the rice - Magnaporthe oryzae interaction.Molecular Plant Pathology,11, 419–427.
Mackill D J, Bonman J M. 1992. Inheritance of blast resistance in near-isogenic lines of rice. Phytopathology,82, 746–749.
Murray M G, Thompson W F. 1980. Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Research,8,4321–4325.
Nakamura S, Asakawa S, Ohmido N, Fukui K, Shimizu N,Kawasaki S. 1997. Construction of an 800-kb contig in the near-centromeric region of the rice blast resistance gene Pi-ta2using a highly representative rice BAC library. Molecular Genetics Genomics,254, 611–620.
Ni D, Song F, Ni J, Zhang A, Wang C, Zhao K, Yang Y, Wei P,Yang J, Li L. 2015. Marker-assisted selection of two-line hybrid rice for disease resistance to rice blast and bacterial blight. Field Crops Research,184, 1–8.
Peng S B, Tang Q Y, Zou Y B. 2009. Current status and challenges of rice production in China. Plant Production Science,12, 3–8.
Ramkumar G, Prahalada G D, Hechanova S L, Vinarao R,Jena K K. 2015. Development and validation of SNP-based functional codominant markers for two major disease resistance genes in rice (O. sativa L.). Molecular Breeding,35, 129.
RoyChowdhury M, Jia Y L, Jackson A, Jia M H, Fjellstrom R,Cartwright R D. 2012. Analysis of rice blast resistance gene Pi-z in rice germplasm using pathogenicity assays and DNA markers. Euphytica,184, 35–46.
Sallaud C, Lorieux M, Roumen E, Tharreau D, Berruyer R,Svestasrani P, Garsmeur O, Ghesquiere A, Notteghem J L.2003. Identi fication of five new blast resistance genes in the highly blast-resistant rice variety IR64 using a QTL mapping strategy. Theorrtical Applied Genetics,106, 794–803.
Skamnioti P, Gurr S J. 2009. Against the grain: Safeguarding rice from rice blast disease. Trends in Biotechnology,27,141–150.
Smith A M, Denyer K, Martin C. 1997. The synthesis of the starch granule. Annual Review Plant Physiology Plant Molecular Biology,48, 67–87.
Tacconi G, Baldassarre V, Lanzanova C, Faivre-Rampant O,Cavigiolo S, Urso S, Lupotto E, Vale G. 2010. Polymorphism analysis of genomic regions associated with broad-spectrum effective blast resistance genes for marker development in rice. Molecular Breeding,26, 595–617.
Tan Y F, Li J X, Yu S B, Xing Y Z, Xu C G, Zang Q. 1999. The three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid,Shanyou 63. Theoretical Applied Genetics,99, 642–648.
Temnykh S, Park W D, Ayres N, Cartinhour S, Hauck N,Lipovich L, Cho Y G, Ishii T, McCouch S R. 2000. Mapping and genome organization of microsatellite sequences in rice (Oryzan sativa L.). Theoretical Applied Genetics,100,697–712.
Tsukaguchi T, Nitta S, Matsuno Y. 2016. Cultivar differences in the grain protein accumulation ability in rice (Oryza sativa L.). Field Crops Research,192, 110–117.
Wang L Q, Liu W J, Xu Y, He Y Q, Luo L J, Xing Y Z, Xu C G, Zhang Q. 2007. Genetic basis of 17 traits and viscosity parameters characterizing the eating and cooking quality of rice grain. Theoretical Applied Genetics,115, 463–476.
Wang Z, Jia Y, Rutger J N, Xia J. 2007. Rapid survey for presence of a blast resistance gene Pi-ta in rice cultivars using the dominant DNA markers derived from portions of the Pi-ta gene. Plant Breeding,126, 36–42.
Wang Z Y, Zheng F Q, Shen G Z, Gao J P, Snustad D P, Li M G, Zhang J L, Hong M M. 1995. The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. The Plant Journal,7, 613–622.
Xiao W M, Luo L X, Wang H, Guo T, Liu Y Z, Zhou J Y, Zhu X Y, Yang Q Y, Chen Z Q. 2016. Pyramiding of Pi46 and Pita to improve blast resistance and to evaluate the resistance effect of the two R genes. Journal of Integrative Agriculture,15, 2290–2298.
Xiao W M, Yang Q Y, Sun D Y, Wang H, Guo T, Liu Y Z, Zhu X Y, Chen Z Q. 2015. Identi fication of three major R genes responsible for broadspectrum blast resistance in an indica rice accession. Molecular Breeding,35, 49.
Xiao W M, Yang Q Y, Wang H, Duan J, Guo T, Liu Y Z, Zhu X Y,Chen Z Q. 2012. Identi fication and fine mapping of a major R gene to Magnaporthe oryzae in a broad-spectrum resistant germplasm in rice. Molecular Breeding,30, 1715–1726.
Xiao W M, Yang Q Y, Wang H, Guo T, Liu Y Z, Zhu X Y, Chen Z Q. 2011. Identi fication and fine mapping of a resistance gene to Magnaporthe oryzae in a space-induced rice mutant. Molecular Breeding,28, 303–312.
Xu X, Hayashi N, Wang C T, Fukuoka S, Kawasaki S, Takatsuji H, Jiang C J. 2014. Rice blast resistance gene Pikahei-1(t),a member of a resistance gene cluster on chromosome 4,encodes a nucleotide-binding site and leucine-rich repeat protein. Molecular Breeding,34, 691–700.
Yi M, Nwea K T, Vanavichit A, Chai-arree W, Toojinda T. 2009.Marker assisted backcross breeding to improve cooking quality traits in Myanmar rice cultivar Manawthukha. Field Crops Research,113, 178–186.
Zhou L J, Liang S S, Ponce K, Marundon S, Ye G Y, Zhao X Q. 2015. Factors affecting head rice yield and chalkiness in indica rice. Field Crops Research,172, 1–10.
Zhou P H, Tan Y F, He Y Q, Xu C G, Zhang Q. 2003.Simultaneous improvement for four quality traits of Zhenshan 97, an elite parent of hybrid rice, by molecular marker-assisted selection. Theoretical Applied Genetics,106, 326–331.
12 December, 2016 Accepted 11 May, 2017
XIAO Wu-ming, Mobile: +86-15914508647, E-mail: heredity24@126.com; Correspondence WANG Hui, Tel: +86-20-85283237,Fax: +86-20-85285772, E-mail: wanghui@scau.edu.cn; CHEN Zhi-qiang, Tel: +86-20-85283237, Fax: +86-20-85285772, E-mail:chenlin@scau.edu.cn
*These authors contributed equally to this study.
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(17)61684-8
Section editor ZHANG Xue-yong
Managing editor WANG Ning
杂志排行
Journal of Integrative Agriculture的其它文章
- Climate change and agriculture: Impacts and adaptive responses in Iran
- Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
- ldenti fication of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao
- New clues concerning pigment biosynthesis in green colored fiber provided by proteomics-based analysis
- Constitutive expression of feedback-insensitive cystathionine γ-synthase increases methionine levels in soybean leaves and seeds
- Characterization of groundnut (Arachis hypogaea L.) collection using quantitative and qualitative traits in the Mediterranean Basin
