Assessment of genetic effects on important breeding traits in reciprocal DH populations of winter oilseed rape (Brassica napus L.)
2018-01-04LaurencjaSzaTeresaCegielskaTarasElbietaAdamskaZygmuntKaczmarek
Laurencja Szała, Teresa Cegielska-Taras, Elżbieta Adamska, Zygmunt Kaczmarek
1 Plant Breeding and Acclimatization Institute, National Research Institute, Poznań 60-479, Poland
2 Institute of Plant Genetics, Polish Academy of Sciences, Poznań 60-479, Poland
RESEARCH ARTICLE
Assessment of genetic effects on important breeding traits in reciprocal DH populations of winter oilseed rape (Brassica napus L.)
Laurencja Szała1, Teresa Cegielska-Taras1, Elżbieta Adamska2, Zygmunt Kaczmarek2
1 Plant Breeding and Acclimatization Institute, National Research Institute, Poznań 60-479, Poland
2 Institute of Plant Genetics, Polish Academy of Sciences, Poznań 60-479, Poland
Here we present a study on the genetic effects of agronomic and seed quality traits in double haploid (DH) populations,which were developed by microspore culture from reciprocal F1hybrids produced between cultivar Californium and DH W-15. Each of the populations consisted of 25 DH lines. The field experiments were conducted in a randomized complete block design with three replications during three growing seasons. Field observations were recorded on seed yield and its structure: the number of branches and pods per plant, the number of seeds per pod, the 1 000-seed weight, the content of fat in the seeds, and three unsaturated fatty acids (oleic, linoleic, linolenic) in the seed oil. In order to investigate the in fluence of cross direction on the studied traits, parental effects were evaluated on the basis of differences between reciprocal DH populations. The maternal effect was revealed on the number of seeds per pod and the effect of the paternal form on linolenic acid content. The occurrence of transgression effects also depended on the direction of crossing and this was particularly noticeable in terms of the number of seeds per pod. The use of multivariate statistical methods allowed for the simultaneous characterization and grouping of tested lines in terms of several traits. Graphic images of the distribution of DH lines in the space of the two first canonical variates showed a great variation between the two reciprocal populations,both in terms of yield and its components, as well as fat and those unsaturated fatty acids.
DH line, reciprocal cross, oilseed rape, multivariate analysis, transgression
1. Introduction
In recent years, increased global interest in oilseed rape has focused on the widespread use of canola oil in different branches of industry. The dynamic development of the cultivation of oilseed rape was due to the enormous progress in breeding high quality varieties with a zero erucic acid and low-reduced glucosinolate content. Today, oilseed rape is not only a source of high nutritional quality oil with a balanced fatty acid composition, but also a valuable material for many other products ranging from methyl ester to industrial lubricants and hydraulic oils, tensides for detergent and soap production and biodegradable plastics (Friedt et al. 2007).
Modern breeding programs use state-of-the art biotechnology techniques. The doubled haploid (DH)method in winter oilseed rape, with the use of an in vitro androgenesis process, has become a routine way to produce completely homozygous and genetically fully stable lines within one generation. The bene fits of the DH lines mainly lie both in the accelerated breeding process and in increasing the ef ficiency of selection in the early generations.
Genetic information on the gene expression of quantitative traits in DH populations can help breeders to create an effective strategy for the development of new varieties of oilseed rape. Determination of the in fluence of the cytoplasmic and nuclear genetic maternal effects or the embryo effects for given traits allows the selection of the appropriate direction of crossing.
Transgressive segregation is a common mechanism in plant hybrids (Rieseberg et al. 1999) and is the result of complementary gene action (De Vincente and Taksley 1993; Monforte et al. 1997; Rieseberg et al. 2003). The frequency of occurrence of transgressive genotypes depends on the genetic differentiation of parental components and phenotypic similarity. Phenotypically similar species are more likely to produce transgressive hybrid offspring than dissimilar species (Stelkens and Seehausen 2009). However, Kuczyńska et al. (2007)reported that the occurrence of transgressive segregants in barley in a homozygous population depended on several factors characterizing parental genotypes, such as gene distribution, phenotypic diversity and genetic distance.
The breeding programs of many plant species have found a parallel improvement in a number of features that take into account both aspects of productivity, as well as the quality of the crop. The starting materials are evaluated for many of these traits in the early stages of the plant breeding process. Multivariate statistical methods provide a comprehensive assessment of breeding lines,while considering many important characteristics from an agronomic point of view. They also allow for the separation of groups of similar genotypes in respect to many traits and the identi fication of groups of varieties of high value. Analyses of this type are widely used for the characterization of germplasm collections of cultivated plants (Nassir and Ariyo 2007; Tucak et al. 2009) and in the search for parents in the breeding of many species of plants. Multivariate analysis of variance has been applied to the culture, for example, of peppers (Lahbib et al. 2013)and barley (Setotaw et al. 2010) or soybean resistance breeding (Gravina et al. 2004). In a study of oilseed rape,multivariate statistical methods were applied by Kaczmarek et al. (2005) for evaluation of the DH lines in terms of the content of five fatty acids, while Adamska et al. (2007)evaluated the combining ability of DH lines in terms of the characteristics of yield and fat content and Shara fiet al.(2015) assessed 28 winter oilseed cultivars for genetic variation and investigated relationships between 11 agromorphological characters. Based on a multi-trait analysis,Bocianowski et al. (2016) assessed the genetic crossing potential of eight inbred lines of the spring oilseed rape.
In the present study, univariate and multivariate statistical methods were used to estimate the effect of cross direction on the seed yield and the yield structure, the fat content as well as three fatty acids. This estimation was based on the differences between two reciprocal DH populations without the F1generation, and on the frequency of occurrence of transgressive DH lines in the reciprocal populations.
2. Materials and methods
2.1. Plant materials
The materials consisted of two DH populations obtained from F1hybrids of reciprocal crosses between cultivar Californium and DH W-15 line derived from cultivar Wotan and the parental forms. The population, marked as CW, consisted of 25 DH lines and was derived from a F1hybrid, cultivar Californium×DH W-15. The population marked as WC also consisted of 25 DH lines and was derived from a F1hybrid, DH W-15×cultivar Californium.
2.2. Experimental conditions
This study was performed in the growing seasons of 2010-2011, 2011-2012 and 2012-2013 at the Experimental Station of the Polish Academy of Sciences in Cerekwica near Poznań, Poland. The soil type at Cerekwica is sandy clay. Mean temperature for the growing season at the experimental site was 9.2, 8.5 and 7.0°C for the first, the second and the third years, respectively. Precipitation was 442.8 mm from August 2010 to July 2011, 354.5 mm from August 2011 to July 2012, and 303.1 mm from August 2012 to July 2013. Full chemical protection of plants was applied.Other agronomical practices were similar to the commercial planting in the area.
Field experiments including 50 DH lines and parental forms were conducted in a randomized complete block design with three replications and a plot size of 1.2 m×5.0 m.The following observations and evaluations of seed yield and its structure were performed: the number of branches and pods per plant, the number of seeds per pod and the 1 000-seed weight and the content of fat in the seeds and of the three unsaturated fatty acids with 18-carbon atoms in the seed oil.
Approximately three weeks before harvesting, five plants were randomly selected from each plot to record data of the number of branches and pods per plant. Twenty- five welldeveloped pods were taken from the middle main stems of each material to measure the number of seeds per pod.Two months after the harvest, the 1 000-seed weight was calculated as the average of five replicate samples from the mixed seeds for each plot.
The fat content in dry seeds (about 5% moisture)was determined via pulsed nuclear magnetic resonance(NMR, 7005 MOA Oxford Instruments, UK). The fatty acid composition of the oil was determined by gas chromatography (GC, Agilent Technologies 6890N, USA).
2.3. Statistical analysis
The experimental data were analyzed with uni- and multivariate statistical methods (Morrison 1976; Gomez and Gomez 1984). Two-factor analysis of variance was carried out to test the hypotheses of no differences between genotypes and between years. The F-statistic was used to compare the average value for traits between the populations and the parental forms for an examination of the direction crossing effect on given traits. Each DH line from the CW and WC populations was compared with both parents to identify those DH lines with advantageous and signi ficant effects of transgression for the studied traits. For seed yield, yield structure and fat content, positive effects of transgression were sought as well as for oleic and linoleic acid, and negative transgression effects were investigated for linolenic acid content.
Multivariate analysis of variance (MANOVA) was used to study the differences and interrelations between genotypes and to formulate the characteristics of the DH lines of both studied populations in terms of two sets of traits. The first set consisted of yield and its components, and the second consisted of the content of fat and the three fatty acids: oleic, linoleic and linolenic acid. Canonical variate analysis enabled the creation of a graphical representation on a plane of the location of the CW and WC DH lines and their relation to parental forms. In order to determine the characteristics with the most powerful discrimination in the study, the simple correlation coef ficients between the first two canonical variates and the values of the individual original traits were calculated.
3. Results
3.1. Mean values of characteristics of the DH populations and parental forms
The mean values of the studied characteristics of the parental forms (cultivar Californium and DH W-15), and the two populations of the DH lines (CW and WC) are presented in Table 1. The general hypothesis of no difference between genotypes was rejected for all traits at least P=0.05. The effect of years on the expression on these traits turned out to be signi ficant as well, except for the number of seeds per pod and fat content. Hypotheses concerning no genotype by year interaction were rejected only for yield of seeds,number of pods per plant and number of seeds per pod.
3.2. Maternal and paternal effects
The estimation and the results of testing of the means for yield and yield components between CW and WC populations and parental forms are presented as contrasts(Table 2). Signi ficant contrast values were found for all the studied traits, except for the number of branches. CW and WC populations did not differ signi ficantly from one another in terms of yield, number of branches or 1 000-seed weight.A signi ficantly higher number of pods per plant and fewer seeds per pod characterized the CW population compared to the WC population, but the direction of crossing revealed an in fluence of maternal effects only on the number of seeds per pod.
Among traits of the second set, signi ficant values for the contrast between the studied DH populations were obtained for the contents of linoleic acid and linolenic acid (Table 3). The estimates for the CW population were signi ficantly positive in terms of the results of the contrast with the WC population for linoleic acid content, while they were signi ficantly negative for the results of the contrast with the WC population for linolenic acid content. CultivarCalifornium contained more linoleic acid than the line DH W-15, and therefore linolenic acid content continued to be signi ficantly in fluenced by the paternal parent.
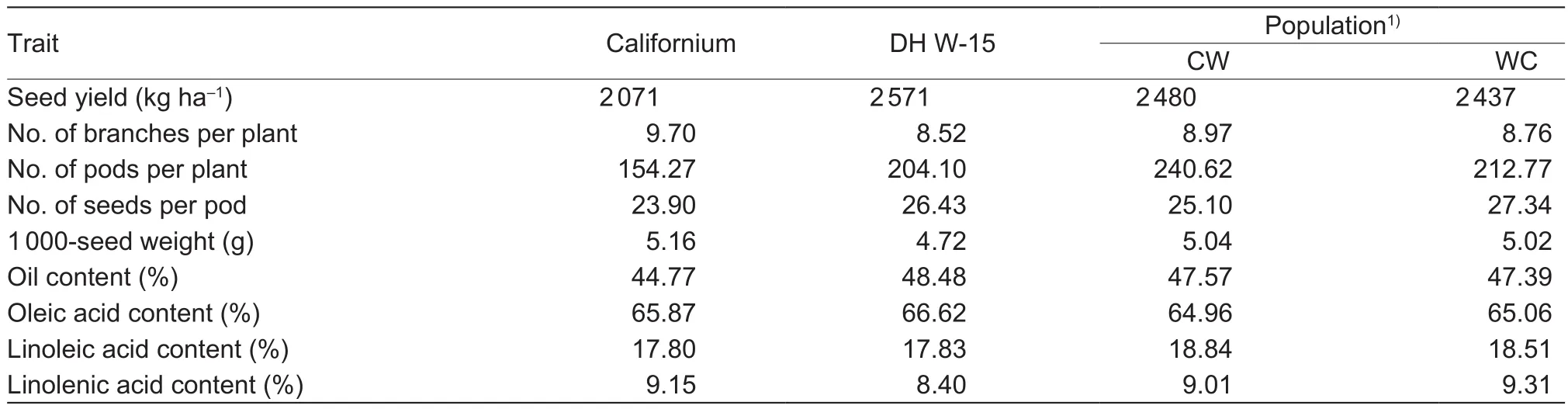
Table 1 Mean values of studied traits for cultivar Californium, DH W-15, and CW and WC populations
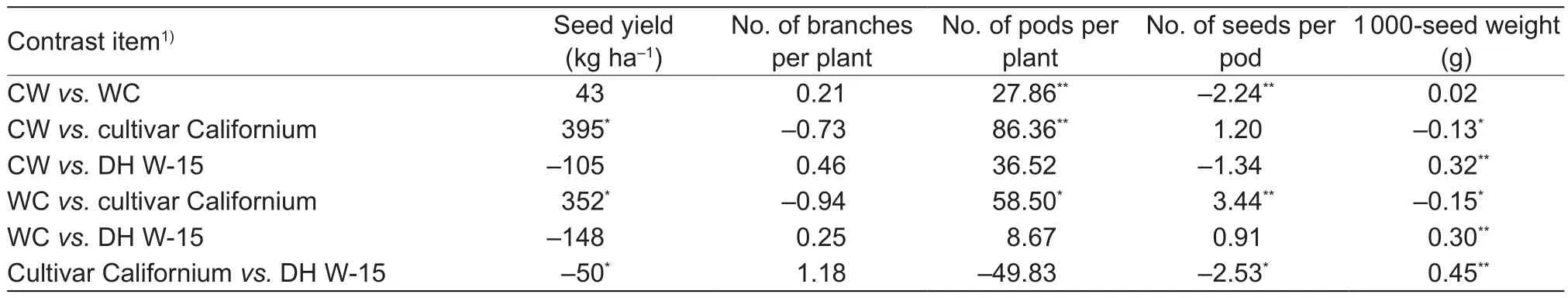
Table 2 Estimates and results of testing the contrast estimation among CW and WC populations, cultivar Californium, and DH W-15 for yield and yield components
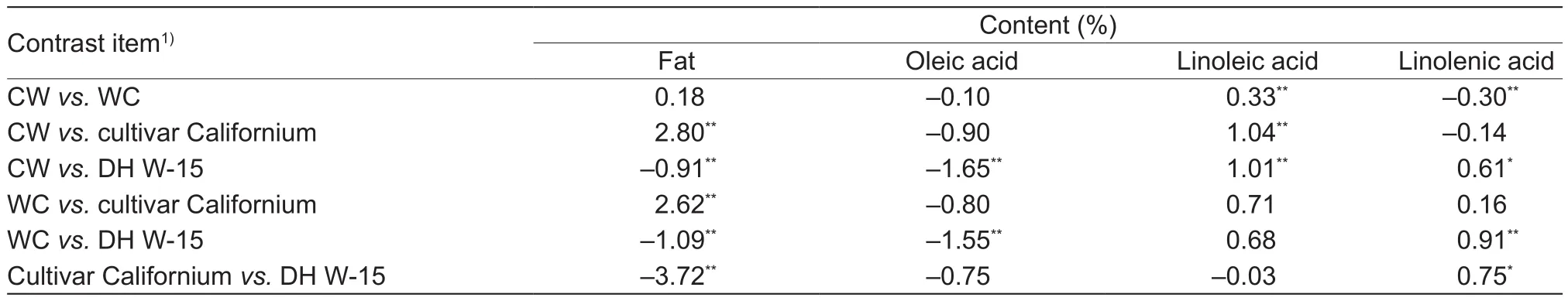
Table 3 Estimates and results of contrasts test between each pair of CW and WC populations, cultivar Californium, and DH line W-15 for fat and three fatty acid contents
3.3. Transgression effects
Each DH from the CW and WC populations was compared with the better parent to identify the transgressive DH lines(Table 4). Among the traits of yield structure, the most transgressive lines were found in terms of the 1 000-seed weight, seven out of the 50 DH lines tested. In the case of the number of pods per plant, six DH lines with positive effects of transgression were found. For the number of seeds per pod, six transgressive DH lines were also recorded. In terms of yield, only three DH lines signi ficantly exceeded their better parent (DH W-15). The direction of crossing played a certain role in the frequency of transgression effects; this was particularly so in the case of the number of seeds per pod. Positive effects of transgression were observed only in the WC population where the female parent contained more seeds per pod. The in fluence of maternal effects was also revealed in the case of the 1 000-seed weight. Out of the seven transgressive lines demonstrating this effect, five belonged to the CW population, where the maternal form was cultivar Californium.
Among the biochemical traits, 13 DH lines showed positive effects of transgression on the content of linoleic acid, eight DH lines belonged to the CW population, and five to the WC population. Signi ficantly lower linolenic acid content was found in two DH lines, one from each population.The increase in linoleic acid and reduction in linolenic acid content, although statistically signi ficant, did not lead to any important change in the fatty acid pro file in the tested lines from the point of view of the breeding. Five DH lines were characterized by signi ficantly increased fat content.
In the analyzed DH populations, two lines can be particularly distinguished, line WC-53 with the effects of transgression on yield, fat content and the 1 000-seed weight and line CW-36, in which there were positive effects of transgression in terms of yield, number of pods per plant,fat and linoleic acid content simultaneously.
3.4. Simultaneous multitrait evaluation of DH populations
Using multivariate statistical analysis, the tested DH lines and their parental forms were simultaneously characterized in terms of several traits.
The first set of traits included yield and yield structure.The first canonical variate (V1) and the second canonical variate (V2) explained 77.81 and 13.57% of the total variation, respectively. The first two canonical variates explained jointly 91.38% of the total variation of the studied traits, allowing for the arrangement of DH on the planewith only minimal loss of information. The values of the correlation coef ficients between the analyzed characteristics of the first two canonical variates (Table 5) indicate that V1 was highly positively correlated with the 1 000-seed weight(r=0.992***) and signi ficantly negatively correlated with the number of branches (r=-0.423**), number of pods per plant(r=-0.422**) and number of seeds per pod (r=-0.398**).However, V2 was in fluenced by the number of seeds per pod(r=0.681**) and slightly lower and negatively by the number of branches (r=-0.484**) and the number of pods per plant(r=-0.381*). Graphic presentation of the distribution of lines CW (Fig. 1) and WC (Fig. 2) according to the first two canonical variates indicated more diverse CW lines than WC lines in terms of the 1 000-seed weight. However, in the population of WC, variation in the number of seeds per pod was greater than that in the CW population.
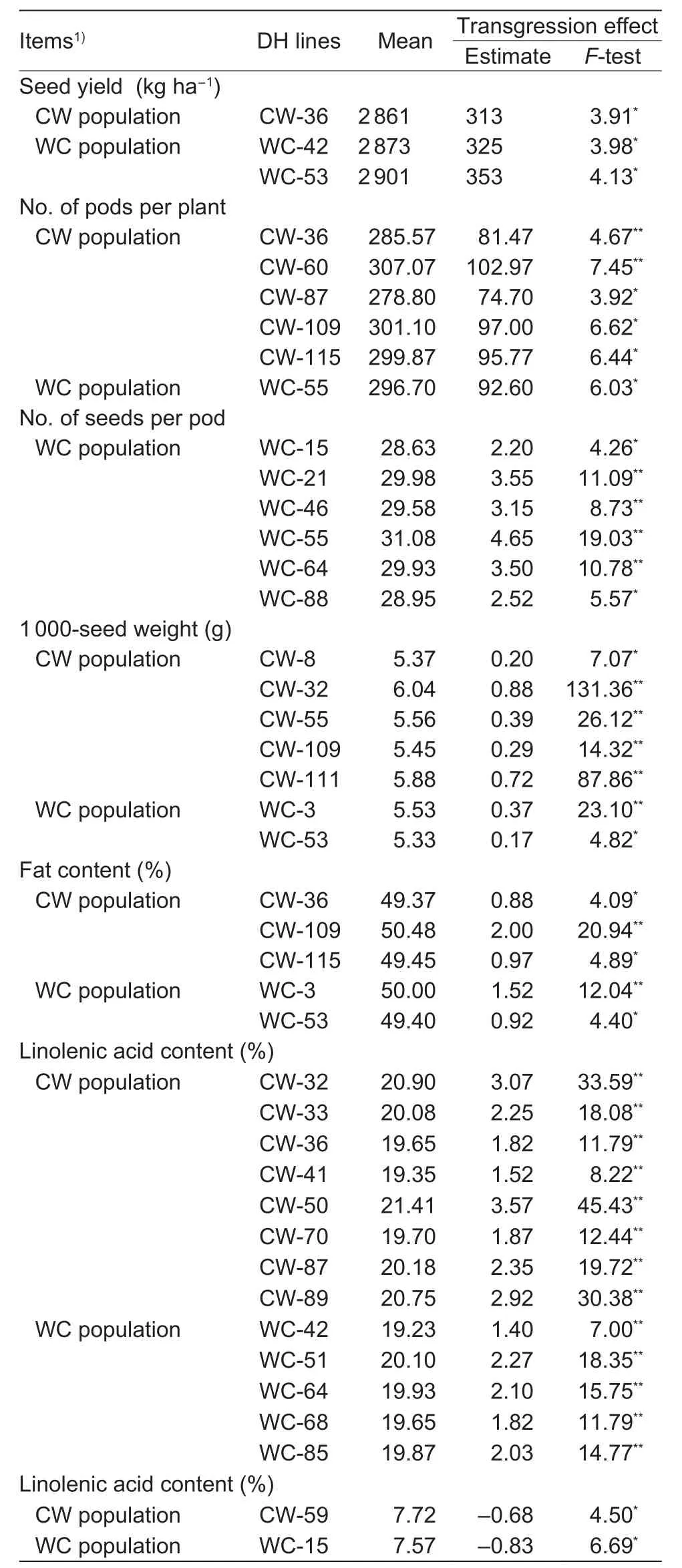
Table 4 Transgression effects in the CW and WC populations
In the second set of traits, characterized jointly for fat and three fatty acid contents, V1 explained 50.14% of the total variation, and V2 explained 33.11%, V1 and V2 both explaining together 83.25% of the total variability of this set of features. V1 was highly positively correlated with the fat content, r=0.940***(Table 6). There was also a positive correlation between this variate and the content of linolenic acid (r=0.334*) and a negative correlation with the content of linoleic acid (r =–0.334*). In the case of V2, the greatest in fluence on the distribution of genotype objects was shown by linoleic acid (r=–0.568**) and linolenic acid(r=0.533**) in oil, with a smaller effect in fat (r=–0.324*). Thus,both variates simultaneously depend on the same traits.Linolenic acid content had a positive effect, linoleic acid had a negative effect, and oleic acid had no signi ficant effect on either variate. The dominant in fluence on the distribution of the tested lines on the graph was shown by fat content level, whereby on the right side of the graph were lines of the highest fat content, with the left-hand lines indicating the lowest content of this compound. Multivariate assessment including fat and three fatty acids simultaneously indicated a slightly different arrangement of the DH lines from the CW population (Fig. 3) compared to DH lines from the WC population (Fig. 4) in terms of the parental forms in the system of the first two canonical variates.
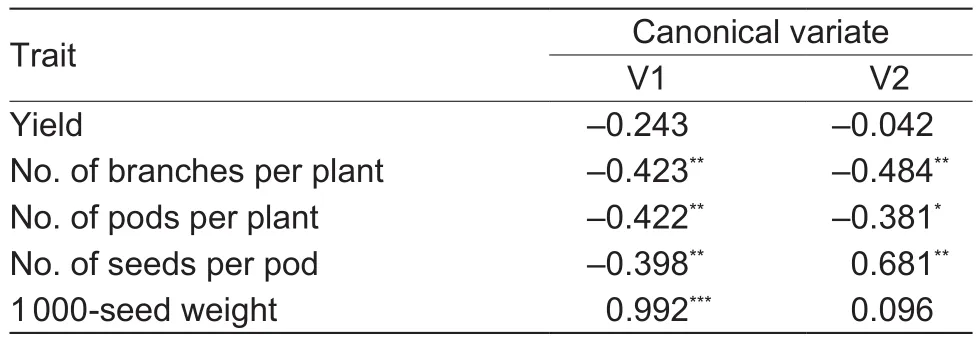
Table 5 Correlation coef ficients between two first canonical variates (V1 and V2) and yield and yield components
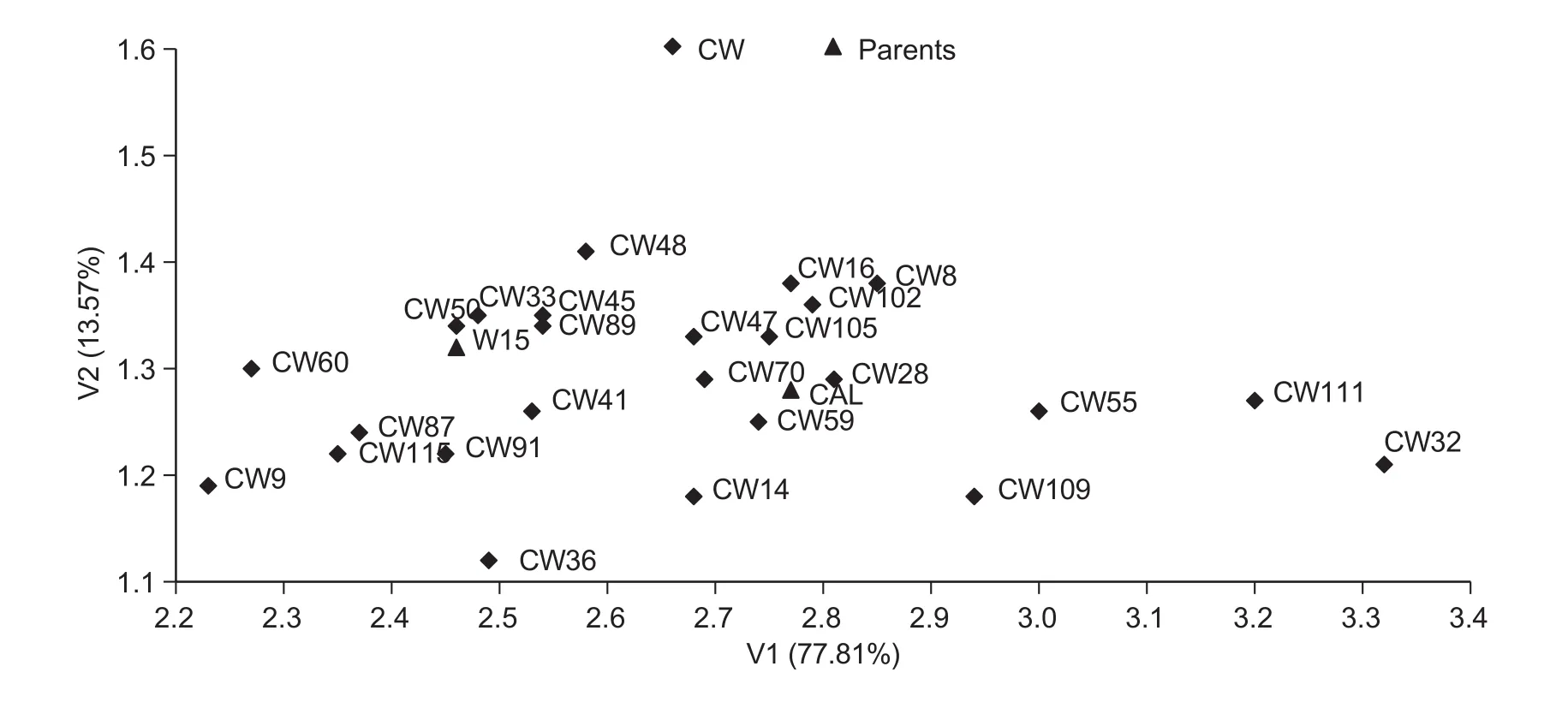
Fig. 1 Distribution of double haploid (DH) lines from CW population (consisted of 25 DH lines and was derived from a F1 hybrid,cultivar Californium×DH W-15) characterised by yield and its components in the space of the two first canonical variates (V1 and V2).
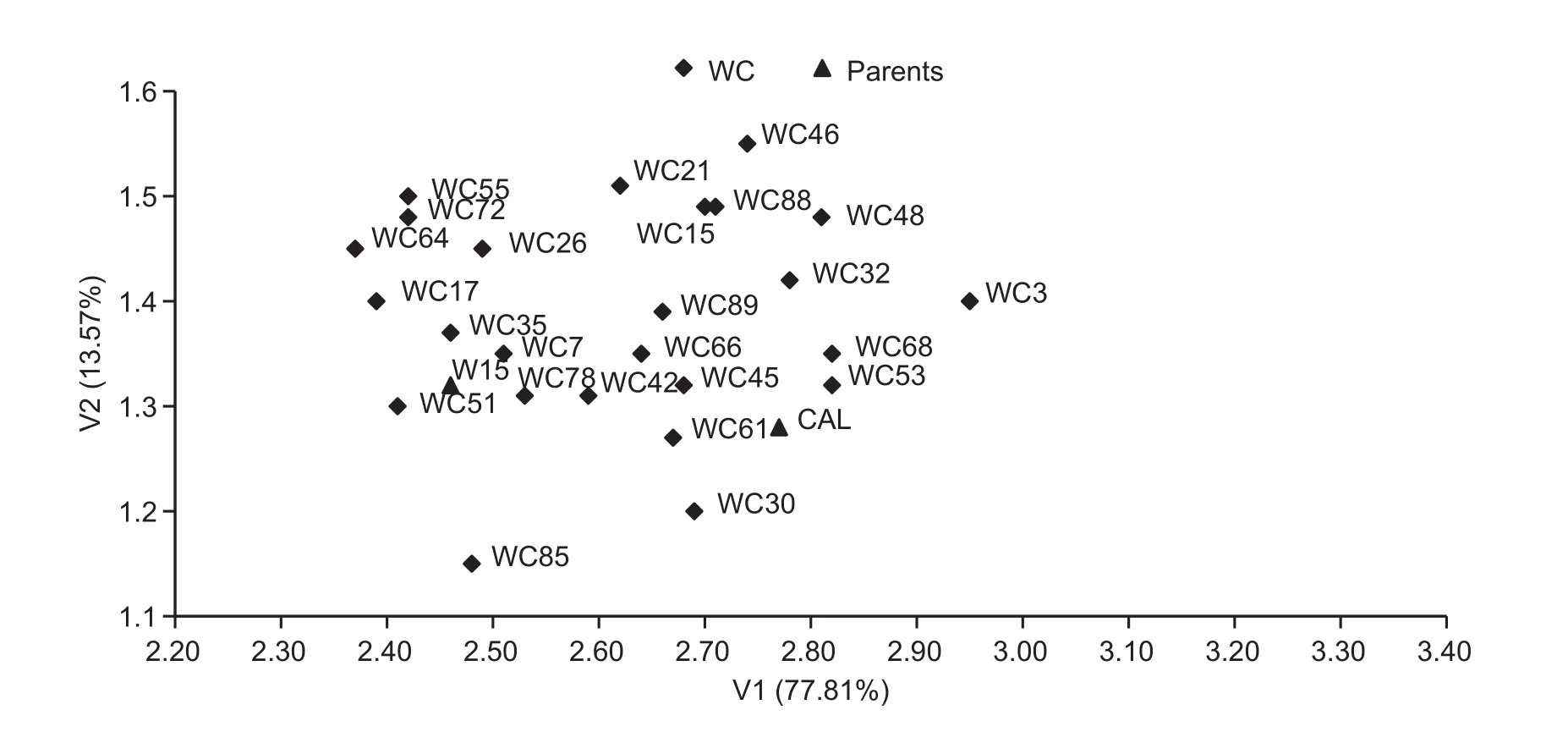
Fig. 2 Distribution of double haploid (DH) lines from WC population (consisted of 25 DH lines and was derived from a F1 hybrid, DH W-15×cultivar Californium) characterised by yield and its components in the space of the two first canonical variates (V1 and V2).
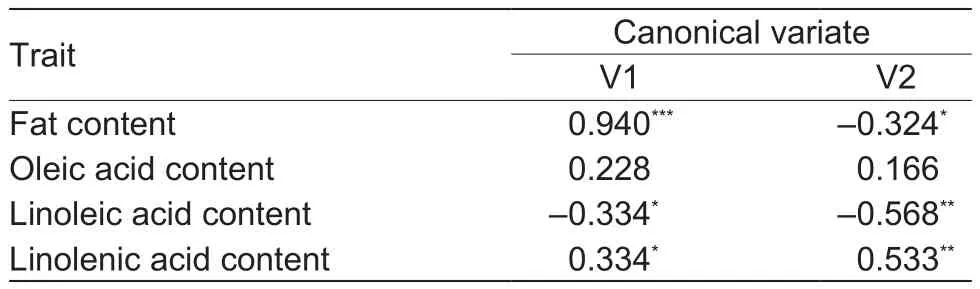
Table 6 Correlation coef ficients between the two first canonical variates (V1 and V2) for fat and three fatty acid contents
4. Discussion
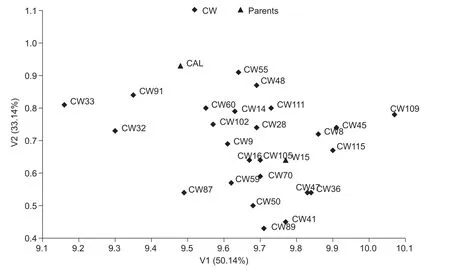
Fig. 3 Distribution of double haploid (DH) lines from CW population (consisted of 25 DH lines and was derived from a F1 hybrid,cultivar Californium×DH W-15) characterised by fat and three fatty acid contents in the space of the two first canonical variates(V1 and V2).
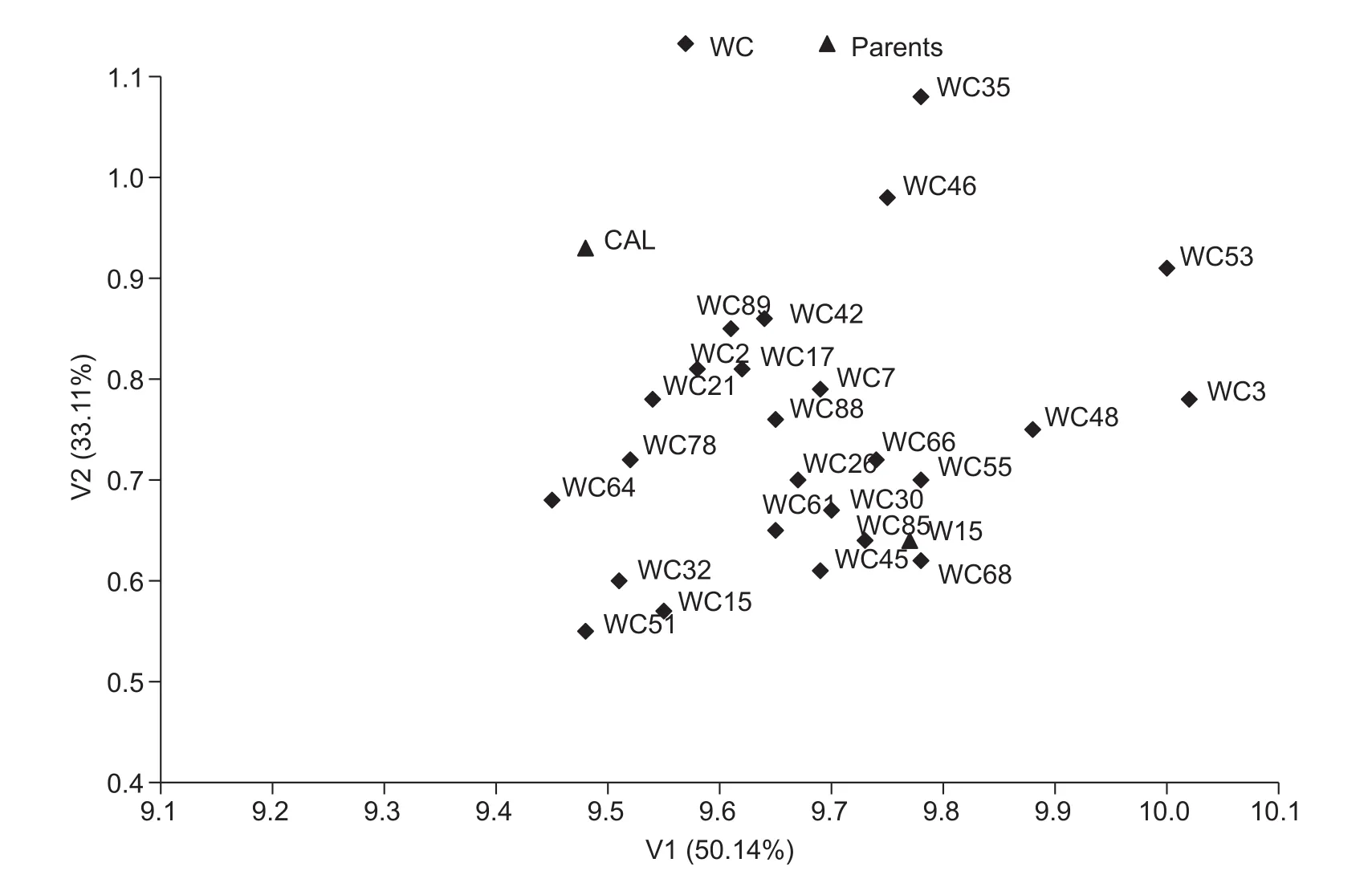
Fig. 4 Distribution of double haploid (DH) lines from WC population (consisted of 25 DH lines and was derived from a F1 hybrid,DH W-15×cultivar Californium) characterised by fat and three fatty acid content in the space of the two first canonical variates (V1 and V2).
Based on the obtained results, an estimation was made of the effect of the cross on yield, its components and some biochemical characteristics. In order to conduct such an estimation, note had to be taken of both the signi ficant variation between parental forms in the trait, and the fact that the basis for inferences about the in fluence of maternal or paternal effects was the statistically signi ficant differences between the mean values of the DH lines of the two populations. Both the pair of parental forms and the pair of DH line populations revealed signi ficant differences in the number of seeds per pod, and linolenic acid content.Maternal effects have been revealed for only the number of seeds per pod. Xing et al. (2014) showed that the number of seeds per pod is controlled by maternal genotype,as well as the 1 000-seed weight. They also detected paternal inheritance of pods per plant and seed yield using the contribution ratio of maternal and paternal general combining ability (GCA) variance as well as correlations between GCA and heterosis levels. However, there are few reports about the in fluence of the direction of crossing on the expression of yield and yield components. Rameah et al. (2003) observed signi ficant maternal effects for yield and yield components, except for pod length. Therefore,they considered the direction of crosses to be important for these traits and in crosses with signi ficant negative reciprocal effects. However, the results of the study by Sincik et al. (2011) indicated that the direction of crossing did not in fluence the number of seeds per pod, seed yield per plant or 1 000-seed weight. The yield is a complex trait controlled by multiple genes, also strongly subject to the in fluence of the environment. In a study of genotype by environment interaction for seed yield in oilseed rape conducted by Nowosad et al. (2016), the sum of squares for environment as the main effect represented up 69.82% of the total variation, while the differences between genotypes explained only 13.67% of the total yield variation. It is possible that this is why it is dif ficult to determine the genetic effects for this trait from field trial data.
Differences in linolenic acid content between two reciprocal DH populations and parental forms show that paternal parents have in fluenced this trait. This indicates that nuclear genes may be the main factor controlling expression of this feature. Inheritance of linolenic acid in oilseed rape, however, has been a matter of controversy as the results obtained by different groups of researchers have varied (Rajcan et al. 2002). Pleines and Friedt (1989) noted that linolenic acid concentration is mainly under the control of the nuclear genes of the embryo. They found signi ficant differences in reciprocal F1, BC1, and BC2indicating maternal control, which is realized by interaction between maternal genotypes and nuclear genes of the embryo, but in F2no reciprocal differences were detectable. Similar results were obtained by Rajcan et al. (2002), who observed cytoplasmic effects in the inheritance of linolenic acid concentrations in two reciprocal DH populations based on signi ficant differences between them. However, they did not find any signi ficant differences between the means of this trait in recreated F2populations produced from the same pair of parents. The results of the present study suggested that maternal effects did not exert a distinct effect on the content of the analyzed fatty acid in reciprocal DH populations.Likewise, Coonrod et al. (2008) observed the lack of maternal effects for any of five long chain fatty acid contents in progeny obtained from two parental lines that differed in terms of erucic and oleic acid contents. However, in studies on the heredity of the oleic acid content of oilseed rape,Zhang et al. (2004) concluded that this was simultaneously controlled by diploid embryo nuclear, cytoplasmic and diploid maternal plant nuclear genes.
The direction of the cross did not affect the percentage of oil. Thus, it can be concluded that nuclear genes, rather than extra nuclear determinants, condition the expression of this trait. Similar results have been reported by Rajcan et al. (2002), who compared two DH populations produced from reciprocal F1made between the cultivar Reston and line LL09. However, there are different models to explain the genetics of oil content in B. napus, because it is a typical quantitative trait controlled by multiple genes and in fluenced by various environmental factors (Si et al. 2003;Zhao et al. 2005; Delourme et al. 2006). Some studies have indicated that oil concentration in F1hybrid seeds is mainly controlled by the maternal genotype (Wang et al.2010). Using two oilseed rape lines with contrasting oil contents, Hua et al. (2012) described an important role of pod wall photosynthesis in the regulation of the oil content of F1seeds in terms of maternal effects. However, in the F2generation derived from reciprocal cross F1seeds, the difference in oil content disappeared. Also in the study by Xing et al. (2014), the contribution of the maternal parent was slightly higher than that of the paternal parent with regard to the fat content. Other studies have indicated that embryo genetic, cytoplasmic and maternal effects as well as genotype×environment interaction effects (Wu et al. 2006;Variath et al. 2010) simultaneously controlled oil content in oilseed rape.
In both the studied populations, the highest number of transgressive segregants was observed for linoleic acid in oil. No transgressive line was detected for the number of branches per plant or oleic acid content. Average values for the number of branches as well as the oleic and linoleic acid contents of parental lines were similar. Therefore, in this case, the frequency of occurrence of transgressive lines did not depend on phenotypic similarity. The direction of crossing played a role in the frequency of transgression effects, and this was particularly so in terms of the number of seeds per pod. Positive transgression effects were observed only among the WC lines- in population, which forms the mother, there are more seeds per pod. The in fluence of maternal effects also manifested itself in relation to the 1 000-seed weight. Of the seven transgressive lines in terms of the features, five belonged to the CW population. Multidimensional estimation of the DH lines of winter oilseed rape was conducted in terms of two sets of features. On the basis of canonical variate analysis, it could be concluded that the feature which exerted a very large in fluence on the differentiation of multivariate genotypes of both populations in the first set was the 1 000-seed weight and this was negatively correlated with the number of seeds per pod. In the second set of traits, fat content had the most discriminative power and this was positively correlated with the content of linoleic acid. Multidimensional estimation of DH in terms of the parents showed their high variation in terms of the characteristics of each set. Graphical distribution lines in the system of the first two canonical variables showed recombinant variability, which appeared in the doubled haploid lines. The biggest difference in terms of the studied traits was shown by DH lines: CW-32 and CW-109, WC-3 and WC-64.
5. Conclusion
Populations DH lines created from reciprocal F1hybrids provided good material for the assessment of genetic effects associated with the direction of crossing using statistical methods. In our study, the statistical analysis of two reciprocal populations demonstrated the maternal effects on the number of seeds per pod and the paternal effect on the content of linolenic acid. Based on canonical variate analysis, it could be concluded that the 1 000-seed weight was the character which had most in fluence on multivariate variation of genotypes of both populations in the first set of traits, while the fat content was key in the second set of traits. Graphic images of the distribution of DH lines in the space of the two first canonical variates showed a greater differentiation between the lines in the CW population than in the WC population in terms of both sets of traits.This information may be useful for oilseed rape breeding programs. The direction of cross should be considered by oilseed rape breeders for developing genotypes with a large number of seeds per pod and a modi fied content of linolenic acid in Brassica napus L.
Adamska E, Szała L, Cegielska-Taras T, Kaczmarek Z.2007. Multidimensional GCA and SCA effects in doubled haploid lines of winter rape in the analysis of yield structure characteristics and oil content of F1line×tester crosses.Rośliny Oleiste - Oilseed Crops,28, 97–108.
Bocianowski J, Nowosad K, Bujak H, Łuczkiewicz T, Piesik D.2016. Evaluation of the breeding value of the spring oilseed rape (Brassica napus L.) inbred lines based on a multi-trait analysis. Indian Journal of Genetics and Plant Breeding,76, 284–289.
Coonrod D, Brick M A, Byrne P F, DeBonte L, Chen Z. 2008.Inheritance of long chain fatty content in rapeseed (Brassica napus L.). Euphytica,164, 583–592.
Delourme R, Falentin C, Huteau V, Clouet V, Horvais R, Gandon B, Specel S, Hanneton L, Dheu J E, Deschamps M, Margal E, Vincourt P, Renard M. 2006. Genetic control of oil content in oilseed rape (Brassica napus L.). Theoretical Applied Genetics,113, 1331–1345.
Friedt W, Snowdon R, Ordon F, Ahlemeyer J. 2007. Plant breeding: Assessment of genetic diversity in crop plants and its exploitation in breeding. Progress in Botany,168,152–177.
Gomez K A, Gomez A A. 1984. Statistical Procedures for Agricultural Research. 2nd ed. John Wiley and Sons, New York, USA.
de Gravina G A, Sediyama C S, Martins Filho S, Moreira M A, de Barros E G, Cruz C D. 2004. Multivariate analysis of combining ability for soybean resistance to Cercospora sojina Hara. Genetics and Molecular Biology,27, 395–399.
Hua W, Li R J, Zhan G M, Li J, Wang X F, Liu G H, Wang H Z. 2012. Maternal control of seed oil content in Brassica napus: the role of silique wall photosynthesis. The Plant Journal,69, 432–444.
Kaczmarek Z, Adamska E, Cegielska-Taras T, Szała L. 2005.Multivariate statistical methods used for evaluation of DH lines of winter oilseed rape on account of various fatty acid compositions. Rośliny Oleiste - Oilseed Crops,26,325–334.
Kuczyńska A, Surma M, Kaczmarek Z, Adamski T. 2007.Relationship between phenotypic and genetic diversity of parental genotypes and frequency of transgression effects in barley (Hordeum vulgare L.). Plant Breeding,126, 361–368.
Lahbib K, Bnejdi F, El Gazzah M. 2013. Selection of pepper parent from a collection of Capsicum annuum landraces based on genetic diversity. Journal of Plant Breeding and Crop Science,5, 68–72.
Monforte A J, Asins M J, Carbonell E A. 1997. Salt tolerance in Lycopersicon species. V. Does genetic variability at quantitative trait loci affect their analysis? Theoretical Applied Genetics,95, 284–293.
Morrison D F. 1976. Multivariate Statistical Methods. McGraw-Hill Kogakusha Ltd., Tokyo.
Nasir A L, Ariyo O J. 2007. Multivariate analysis of the variation of field-planted upland rice (Oryza sativa L.) in a tropical habitat. Malaysian Applied Biology,36, 47–57.
Nowosad K, Liersch A, Popławska W, Bocianowski J. 2016.Genotype by environment interaction for seed yield in rapeseed (Brassica napus L.) using additive main effects and multiplicative interaction model. Euphytica,208, 187–194.
Pleines S, Friedt W. 1989. Genetic control of linolenic acid concentration in seed oil of rapeseed (Brassica napus L.).Theoretical Applied Genetics,78, 793–797.
Rajcan I, Kasha K J, Kott L S, Beversdorf W D. 2002. Evaluation of cytoplasmic effects on agronomic and seed quality traits in two doubled haploid populations of Brassica napus L.Euphytica,123, 401–409.
Rameah V, Rezai A, Saeidi G. 2003. Estimation of genetic parameters for yield, yield components and glucosinolate in rapeseed (Brassica napus L.). Journal of Agricultural Science and Technology,5, 143–151.
Rieseberg L H, Archer M A, Wayne R K. 1999. Transgressive segregation, adaptation and speciation. Heredity,83,363–372.
Rieseberg L H, Widmer A, Arntz A M, Burke J M. 2003.The genetic architecture necessary for transgressive segregation is comma in both natural and domestical populations. Philosophical Transactions of the Royal Society B,358, 1141–1147.
Setotaw T A, dos Santos Diaz L A, Missio R F. 2010. Genetic divergence among barley accessions from Ethiopia. Crop Breeding and Applied Bioechnology,10, 116–123.
Shara fiY, Majidi M M, Jafarzadeh M, Mirlohi A. 2015.Multivariate analysis of genetic variation in winter oilseed rape (Brassica napus L.) cultivars. Journal of Agricultural Science and Technology,17, 1319–1331.
Si P, Mailer R J, Galwey N, Turner D W. 2003. In fluence of genotype and environment on oil and protein concentration of canola (Brassica napus L.) grow across southern Australia. Australian Journal of Agricultural Research,54,397–407.
Sincik M, Goksoy A T, Turan Z M. 2011. The heterosis and combining ability of diallel crosses of rapeseed inbred lines. Notulae Botanicae Horti Agrobotanici Cluj-Napoca,39, 242–248.
Stelkens R, Seehausen O. 2009. Genetic distance between species predicts novel trait expression in their hybrids.Evolution,63–64, 884–897.
Tucak M, Popović S, Čupić T, Šimić G, Gantner R, Meglić V. 2009. Evaluation of alfalfa germplasm collection by multivariate analysis based on phenotypic traits. Romanian Agricultural Research,26, 47–52.
Variath M T, Wu J, Li Y, Chen G, Shi C. 2010. Temporal patterns of maternal, cytoplasmic and embryo genetics effects for thousand-seed weight and oil content in F1hybrid rapeseed(Brassica napus L.). Crop Pasture Science,61, 945–955.
De Vincente M C, Tanksley S D. 1993. QTL analysis of transgressive segregation in an interspeci fic tomato cross.Genetics,134, 585–596.
Wang X, Liu G, Yang Q, Hua W, Liu J, Wang H. 2010. Genetic analysis on oil content in rapeseed (Brassica napus L.).Euphytica,173, 17–24.
Wu J G, Shi C H, Zhang H Z. 2006. Partitioning genetic effects due to embryo, cytoplasm and maternal parent for oil content in oilseed rape (Brassica napus L.). Genetic Molecular Biology,29, 533–538.
Xing N, Fan C, Zhou Y. 2014. Parental selection of hybrid breeding based on maternal and paternal inheritance of traits in rapeseed (Brassica napus L.). PLOS ONE,9,e103165.
Zhang H, Shi C, Wu J, Ren Y, Li C, Zhang D, Zhang Y. 2004.Analysis of genetic and genotype×environment interaction effects from embryo, cytoplasm and maternal plant for oleic acid content of Brassica napus L. Plant Science,167, 43–48.
Zhao J, Becker H C, Zhang D, Zhang Y, Ecke W. 2005. Oil content in a European×Chinese rapeseed population:QTL with additive and epistatic effects and their genotypeenvironment interactions. Crop Science,45, 51–59.
9 December, 2016 Accepted 6 March, 2017
Correspondence Laurencja Szała, Tel: +48-618-464237, Fax:+48-618-233721, E-mail: lszala@nico.ihar.poznan.pl
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(17)61776-3
Managing editor SHI Hong-liang
杂志排行
Journal of Integrative Agriculture的其它文章
- Climate change and agriculture: Impacts and adaptive responses in Iran
- Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
- Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
- ldenti fication of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao
- New clues concerning pigment biosynthesis in green colored fiber provided by proteomics-based analysis
- Constitutive expression of feedback-insensitive cystathionine γ-synthase increases methionine levels in soybean leaves and seeds
