番茄红素对脓毒血症大鼠心肌损伤的影响
2017-12-27,,,
,, ,
(1.皖南医学院第一附属医院 弋矶山医院 心功能科,安徽 芜湖 241001;2.皖南医学院 a.生理学教研室;b.病理生理学教研室,安徽 芜湖 241002)
·基础医学·
番茄红素对脓毒血症大鼠心肌损伤的影响
杨涛1,姜玉新2a,李伟2b,王国光2b
(1.皖南医学院第一附属医院 弋矶山医院 心功能科,安徽 芜湖 241001;2.皖南医学院 a.生理学教研室;b.病理生理学教研室,安徽 芜湖 241002)
目的:观察番茄红素对脓毒血症大鼠心肌损伤的影响。方法雄性SD大鼠随机分为正常对照组(NOR)、脓毒血症组(SEP)及番茄红素处理组(LYC)。大鼠通过腹腔注射内毒素(20 mg/kg)制备脓毒血症模型,番茄红素处理组大鼠在以番茄红素预处理2 h后腹腔注射内毒素,6 h后,收集血样品及心脏。检测血清炎症细胞因子水平、抗氧化酶活性及丙二醛(MDA)含量;观察心肌组织学变化;检测心肌组织炎症调节蛋白磷酸化核因子κB(p-NF-κB)、环氧酶-2(COX-2)的表达。结果与对照组比较,脓毒血症组大鼠血清炎症细胞因子肿瘤坏死因子-α(TNF-α)和白介素-6(IL-6)及MDA含量升高,超氧化物歧化酶(SOD)活性降低,心肌组织p-NF-κB和COX-2水平升高;番茄红素处理明显降低血清TNF-α和IL-6及MDA含量,提高SOD活性,降低心肌组织p-NF-κB和COX-2表达。形态观察可见脓毒血症组大鼠心肌纤维损伤、排列疏松,番茄红素处理减轻了心肌纤维损伤。结论番茄红素预处理可减轻脓毒血症大鼠的心肌损伤,其机制可能通过下调炎症信号NF-κB/COX-2实现。
番茄红素;脓毒血症;心肌损伤
脓毒血症是由感染引起的炎症失调而导致的全身炎症反应综合征,是临床高致死率的重要原因[1]。研究认为,炎症因子过度释放,在脓毒血症器官损伤中起着重要作用,并影响预后效果[2-4]。心肌是脓毒血症损伤的重要器官,是导致脓毒血病人死亡的重要原因[5]。番茄红素(lycopene)是一种不含氧的类胡萝卜素,广泛存在于番茄等红色水果中[6]。番茄红素有许多重要的生物学功能,研究表明,番茄红素有抗肿瘤及抗氧化等作用[7-9],并且其血清浓度与急性心肌梗死及心源性猝死相关[10-11]。本研究旨在观察番茄红素对脓毒血症大鼠心肌损伤的保护作用。
1 材料与方法
1.1 试剂与器材 番茄红素购自南京泽朗医药科技有限公司(NO. 502-65-8),内毒素(Escherichia coli 0111:B4)为Sigma公司产品,SOD和MDA检测试剂盒为南京建成生物工程研究所生产;TNF-α、IL-6特异度检测试剂盒购自合肥博美生物工程有限公司;β-actin、磷酸化核因子κB(phosphorylation of nuclear factor kappa B,p-NF-κB)、环氧酶-2(cyclooxygenase-2,COX-2)及核因子κB(nuclear factor-kappa B,NF-κB)多克隆抗体购自上海生物工程有限公司。
1.2 动物分组与实验方法
1.2.1 实验设计 雄性SD大鼠(体质量260~300 g)购自南京青龙山动物养殖场(许可证号:SCKK2009-0001)。在1周的自由饮水、进食适应后,大鼠被随机分为正常对照组(NOR)、脓毒血症组(SEP)及番茄红素处理组(LYC),每组10只。
1.2.2 动物模型复制 各组大鼠分别以腹腔注射戊巴比妥钠(45 mg/kg)麻醉,番茄红素处理组大鼠通过尾静脉注射番茄红素(50 mg/kg),正常对照及脓毒血症组大鼠尾静脉注射等体积溶剂。2 h后,番茄红素处理组及脓毒血症组大鼠通过腹腔注射内毒素(20 mg/kg),正常对照组大鼠腹腔注射生理盐水。注射6 h后,动脉插管收集血样品,并取心脏分别置于10%甲醛,-80℃保存。
1.2.3 SOD活性及MDA含量检测 血样品离心,取上清液检测血清SOD活性及MDA含量。黄嘌呤氧化酶法检测血清SOD活性,硫代巴比妥酸法检测血清MDA。
1.2.4 炎性因子检测 血清TNF-α、IL-6分别以TNF-α、IL-6特异ELISA试剂盒检测。操作按产品说明书步骤进行。
1.2.5 组织观察 取甲醛中固定的心肌,以石蜡包埋,制作5 μm切片,以苏木素-伊红(H-E)染色后,光学显微镜下观察心肌形态结构的变化。
1.2.6 Western blot 取-80℃保存的心肌置于有预冷组织裂解液的匀浆器中匀浆、裂解,转移至EP管,4℃下,以11 500 r/min转速离心20 min。将含等量蛋白量的上清液稀释至等体积,以10% SDS-PAGE电泳进行分离后,蛋白电泳转至硝酸纤维素膜。膜以5%的脱脂奶粉液封闭,加β-actin、p-NF-κB、COX-2及NF-κB抗体,4℃下杂交过夜,膜以TBS-T清洗后,与Ⅱ抗孵育杂交2 h,洗膜,以DAB显色。
2 结果
2.1 SOD活性及MDA的变化 3组大鼠血清SOD活性比较(F=47.03,P<0.01)及MDA含量比较(F=66.98,P<0.01)变化差异有统计学意义,脓毒血症组大鼠血清SOD活性较正常对照组降低[(183.41±13.60)U/mLvs.(132.23±10.15)U/mL,P<0.01],而MDA升高[(4.06±0.63)nmol/mLvs. (10.72± 1.74)nmol/mL,P<0.01](图1);番茄红素处理组较脓毒血症组SOD活性增强[(132.23±10.15)U/mLvs.(168.06±12.33) U/mL,P<0.01],而MDA含量降低[(10.72±1.74)nmol/mLvs. (7.16±1.24) nmol/mL,P<0.01](图1)。
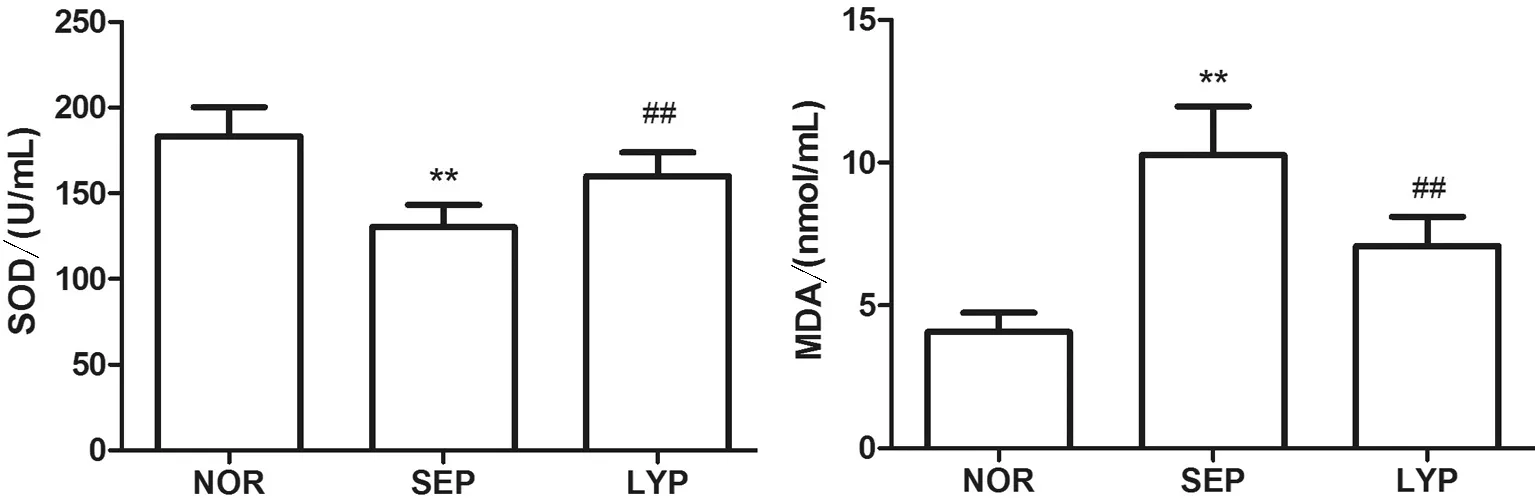
**P<0.01vs. NOR;##P<0.01vs. SEP。
图1 番茄红素对血清SOD活性和MDA含量的影响
2.2 番茄红素对炎性因子的影响 由图2可知,各组大鼠血清炎性因子TNF-α(F=19.53,P<0.01)及IL-6(F=13.63,P<0.01)变化差异有统计学意义,与对照组比较,脓毒血症组大鼠血清TNF-α和IL-6升高[(190.63±10.84 )ng/Lvs. (231.14±18.48) ng/L,(26.41±2.28) ng/Lvs. (32.91±3.15) ng/L,P<0.01];番茄红素预处理较脓毒血症组血清TNF-α和IL-6水平降低[(231.14±18.48) ng/Lvs. (193.28±13.12) ng/L,(32.91±3.15) ng/Lvs. (27.60±2.45) ng/L,P<0.01]。
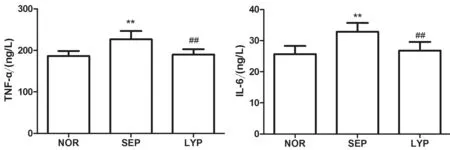
**P<0.01vs. NOR;##P<0.01vs. SEP。
图2 番茄红素对血清炎性因子的影响
2.3 组织学变化 对照组大鼠心肌排列规则且紧密;内毒素处理使大鼠心肌纤维断裂而出现空泡化,破坏心肌纤维排列使其疏松,心肌有炎症细胞浸润;番茄红素预处理改善心肌纤维排列,减轻心肌损伤。见图3。
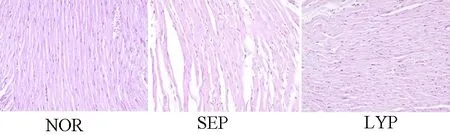
图3 心肌形态学变化
2.4 番茄红素对p-NF-κB和COX-2表达的影响 各组大鼠心肌p-NF-κB及COX-2表达差异有统计学意义,内毒素处理明显提高脓毒血症组大鼠心肌p-NF-κB、COX-2表达;番茄红素预处理降低内毒素诱导p-NF-κB、COX-2表达。见图4。
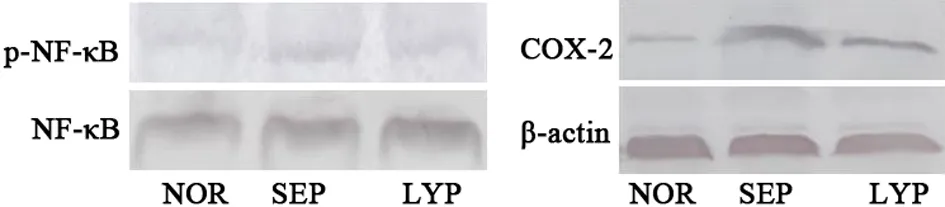
图4 番茄红素对心肌p-NF-κB和COX-2表达的影响
3 讨论
脓毒血症是由各种病原菌侵袭入血引起感染并释放毒素(如内毒素)而导致的过度全身炎症反应[12-13]。由于感染使炎性细胞激活释放大量的炎症因子,进而使炎症反应瀑布式爆发,累及全身组织器官,导致组织器官功能受损,心肌损伤而引起心功能障碍是脓毒血症常见的并发症,是脓毒血症高病死率的重要因素[14]。
内毒素在脓毒血症心肌损伤中起着非常重要的作用[15]。研究表明,感染时,大量释放的内毒素与膜表面Toll样受体(toll-like receptors,TLRs)结合,激活细胞内炎症信号,促进炎性细胞因子的表达和释放[16]。作为炎症标志物的TNF-α、IL-6及IL-1β是重要的炎性介质,在脓毒血症血清中升高[17]。这些炎性细胞因子参与脓毒血症的器官损伤作用,如TNF-α可作用于心肌,使心肌纤维发生损伤[18]。番茄红素是抗氧化作用最强的类胡萝卜素,近年研究显示,其还可抑制胆固醇的合成及增强机体免疫力[19-20]。本研究结果表明,注射内毒素大鼠血清TNF-α、IL-6水平升高,番茄红素预处理降低TNF-α、IL-6水平。NF-κB被内毒素激活,进而激活COX-2,NF-κB/ COX-2信号通路通过激活炎性细胞转录因子促进炎性介质的表达[16]。番茄红素预处理明显抑制COX-2及NF-κB的激活。结果提示番茄红素可能通过抑制NF-κB/COX-2信号降低炎性细胞因子释放而发挥抗炎作用。
脓毒血症释放的内毒素增加活性氧及自由基的产生,是脓毒血症器官损伤的重要环节。研究显示内毒素引起的炎症反应可诱发氧化应激,而氧化应激加强炎症反应[21]。因此,提高机体的抗氧化作用,有利于降低炎症反应及减轻组织损伤。作为抗氧化作用最强的类胡萝卜素,番茄红素降低血清MDA水平及SOD活性。结果表明,番茄红素可降低内毒素引起的氧化应激反应。
综上所述,番茄红素可减轻脓毒血症大鼠的心肌损伤,其作用机制可能是通过抑制NF-κB/COX-2信号,降低炎症反应而发挥作用,详细机制尚需进一步的研究。
[1] MAYR FB,YENDE S,ANGUS DC. Epidemiology of severe sepsis [J]. Virulence,2014,5(1):4-11.
[2] BANTEL H,SCHULZE-OSTHOFF K. Cell death in sepsis.:amatter of how,when,and where[J]. Crit Care,2009,13(4):173.
[3] MESSARIS E,MEMOS N,CHATZIGIANNI E,etal. Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis [J]. Crit Care Med,2004,32(8):1764-1770.
[4] PETER ME. Programmed cell death:Apoptosis meets necrosis [J]. Nature,2011,471(7338):310-312.
[5] ROMERO-BERMEJO FJ,RUIZ-BAILEN M,GIL-CEBRIAN J,etal. Sepsis-induced cardiomyopathy [J]. Curr Cardiol Rev,2011,7(3):163-183.
[6] YUE R,HU H,YIU KH,etal. Lycopene protects against hypoxia/reoxygenationinduced apoptosis by preventing mitochondrial dysfunction in primary neonatal mouse cardiomyocytes [J]. PloS one,2012,7(11):e50778.
[7] FUJITA K,YOSHIMOTO N,KATO T,etal. Lycopene inhibits ischemia/ reperfusion-induced neuronal apoptosis in gerbil hippocampal tissue [J]. Neurochemical research,2013,38(3):461-469.
[8] QIU X,YUAN Y,VAISHNAV A,etal. Effects of lycopene on protein expression in human primary prostatic epithelial cells [J]. Cancer Prevention Res,2013,6(5):419-427.
[9] TAKESHIMA M,ONO M,HIGUCHI T,etal. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines [J]. Cancer sci,2014,105(3):252-257.
[10] KARPPI J,LAUKKANEN JA,MAKIKALLIO TH,etal. Low serum lycopene and beta-carotene increase risk of acute myocardial infarction in men [J]. Eur J Public Health,2012,22(6):835-840.
[11] KARPPI J,LAUKKANEN JA,MAKIKALLIO TH,etal. Serum beta-carotene and the risk of sudden cardiac death in men:a population-based follow-up study [J]. Atherosclerosis,2013,226(1):172-177.
[12] 李攀,赵祎博,李小丽,等. 内毒素耐受状态小鼠对大肠埃希菌敏感性及促炎细胞因子水平变化的研究[J]. 中国临床药理学与治疗学,2015,20(3):261-265.
[13] LIU J,ZHAO S,TANG J,etal. Advanced glycation end products and lipopolysaccharide synergistically stimulate proinflammatory cytokine/chemokine production in endothelial cells via activation of both mitogen-activated protein kinases and nuclear factor-kappa B[J]. FEBS J,2009,276(16):4598-4606.
[14] ZHAC P,TURDI S,DONG F,etal. Cardiac-specific overexpression of insulin-like growth factor I(IGF-1) rescues lipopolysaccharide—induced cardiac dysfunction and activation of stress sign aling in murine cardiomyocytes[J].Shock,2009,32(1):100-107.
[15] BAUMGARTEN G,KNUEFERMANN P,NOZAKI N,etal. In vivo expression of proinflammatory mediators in the adult heart after endotoxin administration:the role of toll-like receptor-4[J]. J Infect Dis,2001,183:1617-1624.
[16] STANIMIROVIC D,SHAPIRO A,WONG J,etal. The induction of ICAM-1 in human cerebromicrovascular endothelial cells (HCEC) by ischemia-like conditions promotes enhanced neutrophil/HCEC adhesion [J].J Neuroimmunol,1997,76(1):193-205.
[17] 施孟如,南超,徐丽艳,等.TRPV1激活对内毒素血症小鼠肺组织炎症损伤的影响及机制[J].中国病理生理杂志,2013,29(12):2223-2228.
[18] WANG YY,YU XH,WANG FQ,etal. Yohimbine Promotes Cardiac NE Release and Prevents LPS-Induced Cardiac Dysfunction via Blockade of Presynaptic a2A-Adrenergic Receptor[J]. PLos One,2013,8(5):e63622.
[19] FUHMAN B,ELIS A,AVIRAM M. Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDLreceptor activity in macrophages [J]. Biochem Biophys Res Commun,1997,233(3):658-662.
[20] REIFEN R,NUR T,MATAS Z,etal. Lycopene supplementation attenuates the inflammatory status of colitis in a rat model[J].Int J Vitam Nutr Res,2001,71(6):347-351.
[21] MORSE D,PISCHKE SE,ZHOU Z,etal. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1 [J]. J Biol Chem,2003,278:36993-36998.
Effectsoflycopeneonmyocardialinjuryinratswithsepsis
YANGTao,JIANGYuxin,LIWei,WANGGuoguang
Department of Cardiac Function Test,The First Affiliated Hospital of Wannan Medical College,Wuhu 241001,China
Objective:To investigate the effects of lycopene on myocardial injury in rats with sepsis.Methods:Male Sprague-Dawley (SD) rats were randomly assigned to three groups:normal control (NOR),sepsis model (SEP),and lycopene treatment (LYC). Rat models of sepsis were induced by intraperitoneal injection of lipopolysaccharide (LPS,20 mg/Kg). Rats in LYC group were pre-treated with lycopene for 2 h,followed by intraperitoneal use of LPS. Then blood samples and hearts were collected 6 h after LPS treatment. Levels of inflammatory cytokines,including tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6),superoxide dismutase (SOD) activity,and malondialdehyde (MDA) content in serum were determined. Levels of cyclooxygenase-2 (COX-2) and phosphorylation of nuclear factor kappa B (p-NF-κB) in myocardial tissues were measured with Western blot. Myocardial tissues were processed for histological examination.Results:Compared with NOR,serum levels of TNF-α and IL-6,MDA content were significantly increased in SEP,yet SOD activity was decreased. LPS significantly led to increase of p-NF-κB and COX-2 expression in myocardial tissues,whereas lycopene pretreatment resulted in significantly decreased levels of inflammatory cytokines,and malondialdehyde (MDA) content in serum. Expressions of p-NF-κB and COX-2 in myocadial tissues were significantly decreased by lycopene. Morphological analysis indicated that lycopene was capable of alleviating the myocardial injury.Conclusion:Lycopene may protect the rats of sepsis from myocardial injury. The potential mechanisms may be associated with down regulation of the signaling of inflammatory cytokines(NF-κB/COX-2).
lycopene;sepsis;myocardial injury
1002-0217(2017)05-0-0413-04
国家自然科学基金项目(81671586)
2017-02-20
杨 涛(1986-),男,住院医师,(电话)15855995768,(电子信箱)tt520cg@126.com;王国光,男,教授,硕士生导师,(电子信箱)guoguangw1226@sina.com,通信作者。
R 285.5;R 459.7
A
10.3969/j.issn.1002-0217.2017.05.002
