Calix[4]Crowns类化合物在分离铯中的应用
2017-12-21董志敏黄永标张志宾王有群曹小红刘云海
董志敏,黄永标,张志宾,3,王有群,3,戴 荧,3,曹小红,3,刘云海,3
(1.东华理工大学 化学生物与材料科学学院,江西 南昌 330013; 2.广东省清远市公安局刑警支队,广东 清远 511518; 3.东华理工大学 核资源与环境省部共建国家重点实验室培育基地,江西 南昌 330013)
Calix[4]Crowns类化合物在分离铯中的应用
董志敏1,黄永标2,张志宾1,3,王有群1,3,戴 荧1,3,曹小红1,3,刘云海1,3
(1.东华理工大学 化学生物与材料科学学院,江西 南昌 330013; 2.广东省清远市公安局刑警支队,广东 清远 511518; 3.东华理工大学 核资源与环境省部共建国家重点实验室培育基地,江西 南昌 330013)
Calix[4]Crowns类化合物作为超分子识别材料能够有效地分离铯,并能很好地应用在溶剂萃取、液膜萃取、色谱分离等领域。介绍了Calix[4]Crowns类化合物及其复合超分子材料对铯离子的识别和分离效果,为寻求对Cs(Ⅰ)有优异配合能力的Calix[4]Crowns类有机配体结构及相应的功能材料提供参考。
Calix[4]Crowns类化合物;溶剂萃取;色谱分离;Cs(Ⅰ)
杯[4]芳烃冠醚类化合物(Calix[4]Crowns)通常由杯芳烃的酚醛氧和聚醚链结合而成,组分是杯芳烃(Calix[4]arenes)和冠醚[1-2],其中的大环是连接官能团的分子主链,侧链上酚醛空腔与碱金属阳离子相连,酚醛氧原子或有机分子通过CH—π或π—π键相连。尽管冠醚可络合金属离子,但杯芳冠醚的构型存在锥形、部分锥形、1,2-交替、1,3-交替等4种构型,不同构型对不同金属离子表现出不同的选择性。特别是,1,3-交替构型杯[4]芳烃冠-6衍生物能与Cs(Ⅰ)形成1∶1复合物,表现出较高的选择性,因此,能够很好地去除核废液中的Cs(Ⅰ)[3-4]。利用这些衍生物,通过溶剂萃取法可得到超过104和102的Cs/Na和Cs/K选择比[5]。
PUREX流程是传统的后处理流程,可将约99.3%的U和Pu分离并回收,但仍存在很大的局限性[6],其放射性废物毒性仅降低一个数量级,还存在许多毒性大、对环境污染严重的放射性核素,如135Cs、137Cs、90Sr、237Np、241Am、245Cm和99Tc等(含有这些元素的溶液称为高放废液(HLLW))[7]不能有效去除。其中,90Sr和137Cs半衰期分别是29、30年,两者皆为释热元素,在存储过程中容易破坏玻璃固化体,可能导致废液泄露[8]。135Cs半衰期长达2.3×106年,且容易迁移,是影响固化体安全处置的最危险元素之一[9]。若能将其分离,不但能减小玻璃固化体体积、缩短冷却时间和储存年限,还能简化地质处置工艺、节约成本[10-11]。因此,如何安全有效分离HLLW中的Cs(Ⅰ)是世界核能发展领域富有挑战性的课题之一。
目前,分离Cs(Ⅰ)的常用方法有溶剂萃取法(CSSX和NG-CSSX)[12-13]、色谱分离法(MAREC和SPEC)[14-15]、沉淀法[16-17]、离子交换法[18-19],以及多种方法的综合,而基于Calix[4]Crowns的识别材料对Cs(Ⅰ)有超强的识别能力,将Calix[4]Crowns及其复合材料用于处理HLLW,已开发的方法有溶剂萃取法、液膜萃取法和色谱分离法等。
1 溶剂萃取法
溶剂萃取法在近20年受到广泛关注[20-21]。在分离Cs(Ⅰ)过程中,超分子识别配体与杯[4]芳烃生成类似于“篮子”的主-配体配合物。杯芳烃只有在碱性条件下通过阳离子交换才能对Cs(Ⅰ)表现出良好的选择性,而在酸性条件下一般没有响应,因此这也限制了其作为萃取剂在分离放射性核素Cs(Ⅰ)方面的应用。但一种基于杯[4]芳烃的衍生物杯[4]芳烃冠醚化合物能够在酸性条件下对碱金属和碱土金属表现出很好的亲和性[22-23]。杯[4]芳烃冠醚的选择性取决于冠醚环的大小、构型、下沿取代基等。杯[4]芳烃-冠-4对碱金属离子Na(Ⅰ)的选择性最好[24],杯[4]芳烃-冠-5对碱金属离子K(Ⅰ)的选择性最好[25],杯[4]芳烃-冠-6对碱金属离子Cs(Ⅰ)的选择性最好[26],表明随冠醚环上氧原子数增加,杯[4]芳烃冠醚对半径更大的碱金属离子的选择性更大。,3交替构象比其他3种构象的Calix[4]arene-crown-6在萃取过程中对铯的识别能力和选择性更好。
双烷氧基杯[4]芳烃-冠-6下沿的酚羟基被烷基链取代,虽然降低了其在水中的溶解度,增大了对Cs(Ⅰ)的萃取容量,但Cs(Ⅰ)相对于Rb(Ⅰ)或K(Ⅰ)的分离系数却有所降低[3]。然而,下沿取代基不同的双烷氧基杯[4]芳烃-冠-6(如图1所示)对Cs(Ⅰ)的配位能力和选择萃取能力有很大影响。E.Chidini等[27]研究认为,当杯[4]芳烃-单冠-6下沿羟基被甲基取代时,由于构象不稳定易发生翻转,导致对Cs(Ⅰ)的选择性和识别能力都不高。U.Rocco等[28]研究表明:不同的取代基(如正辛基、正丙基和异丙基)分别取代杯[4]芳烃-单冠-6下沿羟基,随烷基链增长,材料对Cs(Ⅰ)的萃取率没有很大提高,但分离系数却大幅提高;当取代基的碳原子数相同时,具有支链烷基比直链烷基的化合物对Cs(Ⅰ)的萃取率和分离系数都要高。
相比于杯[4]芳烃-单冠-6,杯[4]芳烃-双冠-6具有2个冠醚桥联。理论上,杯[4]芳烃双冠-6与Cs(Ⅰ)的络合比为2∶1,但研究表明,Cs(Ⅰ)与杯[4]芳烃-双冠-6是通过与相邻的2个苯环上的π电子发生作用,还与其中1个冠醚环上的6个氧进行配位,从而使另一侧的冠醚环结构发生扭转,使其不再是一个适合Cs(Ⅰ)配位的构象,导致金属离子与杯[4]芳烃-双冠-6的配位比小于2。在冠醚环上引入2,3-萘基或1,2-亚苯基后,冠醚环的刚性得到提高,能够阻碍冠醚环与更小半径金属离子的配位,使其对Cs(Ⅰ)的萃取容量提高及Cs(Ⅰ)/Na(Ⅰ)分离系数提高[29]。
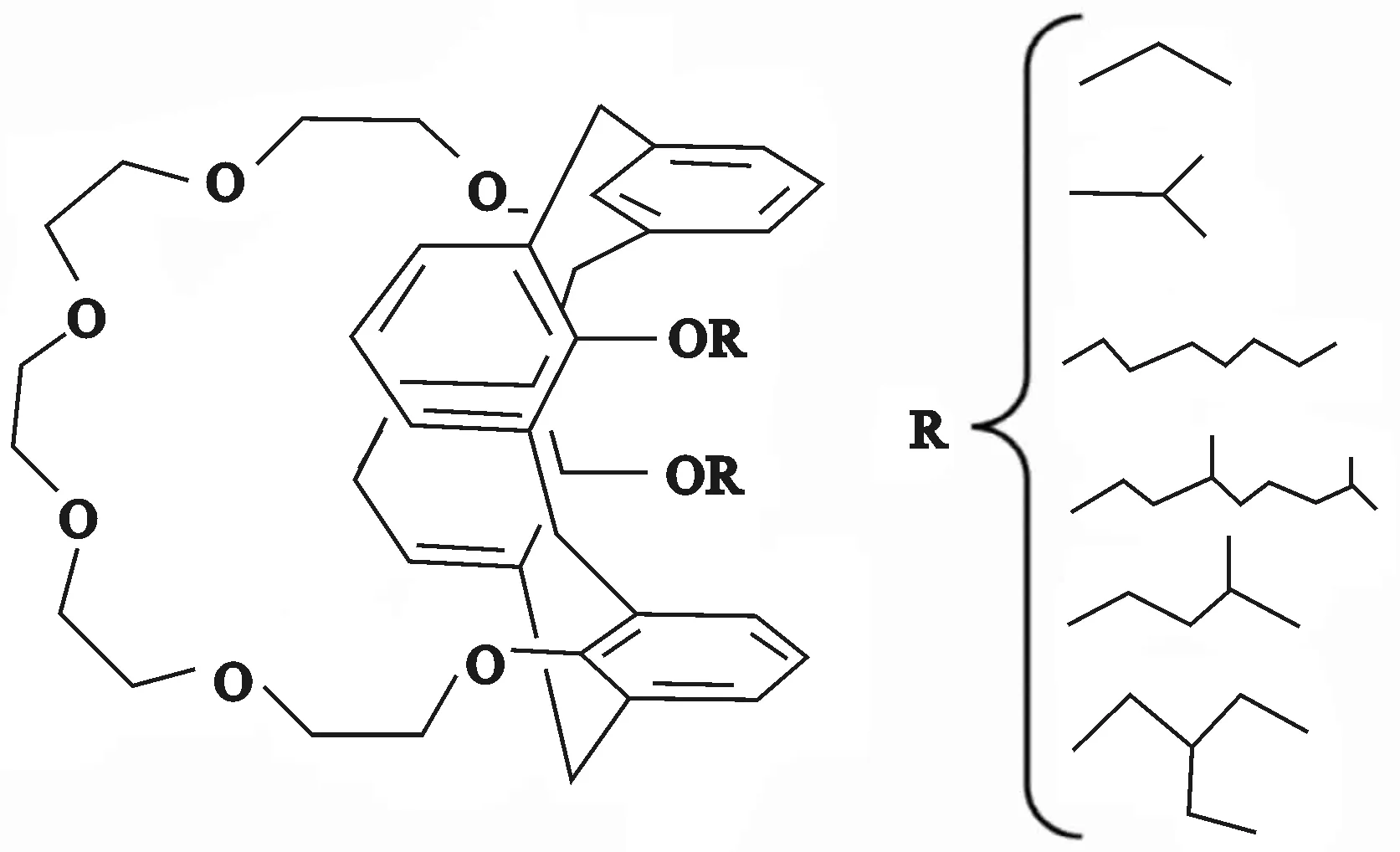
图1 不同取代基二烷氧基杯[4]芳烃-冠-6的化学结构
N.Simon等[30-31]认为,基于双烷氧基的杯[4]芳烃-冠-6对Cs(Ⅰ)有良好的选择萃取性,因此,提出了CCCEX(Cesium Separation by Calix-Crown Extraction)流程(如图2所示),并研究了2种不同取代基的双烷氧基杯[4]芳烃-冠-6体系在高放废液中对Cs(Ⅰ)的萃取。结果表明:以1,3-[(2,4-二乙基-庚基乙氧基)氧]-2,4-冠-6-杯[4]芳烃为萃取剂、甲基辛基-2-二甲基丁酸胺为修饰剂、TPH为稀释剂和以l,3-二辛氧基-2,4-冠-6-杯[4]芳烃为萃取剂、四丙基氢为稀释剂、磷酸三丁酯为修饰剂的2个体系对Cs(Ⅰ)的萃取率均高于99%,表明CCCEX流程是可行的。
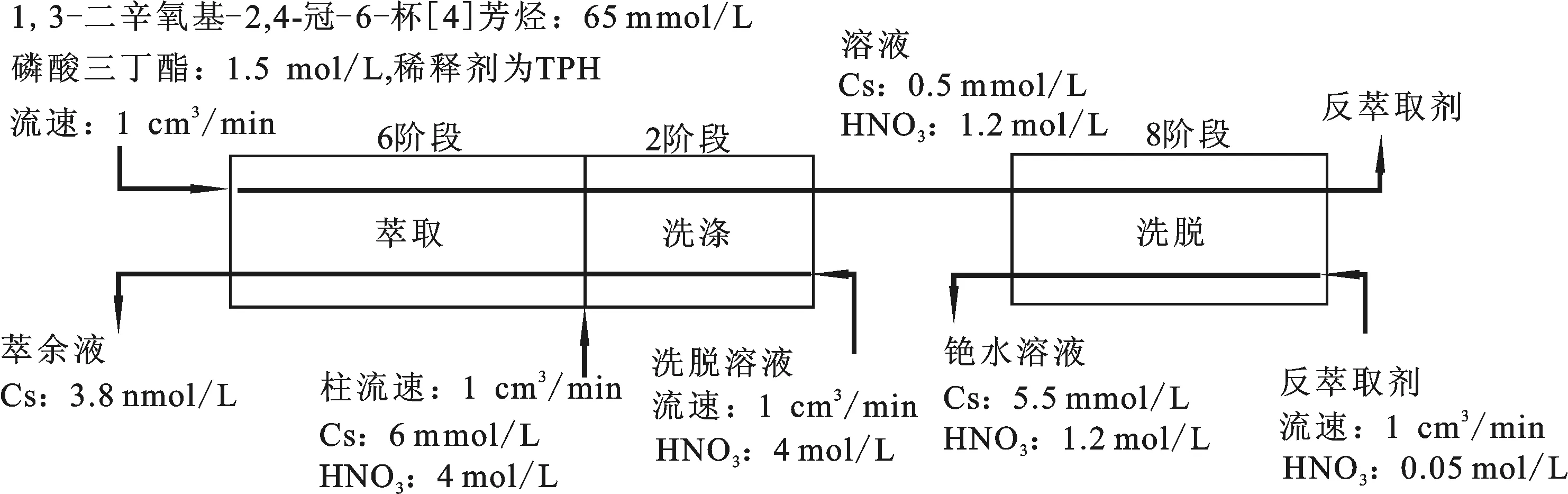
图2 CCCEX流程
美国橡树岭国家实验室(ORNL)[32]基于杯[4]芳烃-双(叔辛基苯并-冠-6)(BOB CalixC6)在碱性溶液中对Cs(Ⅰ)具有良好选择识别性,提出了CSSX(Caustic-Side Solvent-Extraction)流程,后又提出溶剂萃取Cs(Ⅰ)流程(CSEX),选取BOB Calix[4]C6作萃取剂,1-(2,2,3,3-四氟丙氧基)-3-(4-叔辛基酚)-2-丙醇(Cs-3)作修饰剂,用于从模拟SRS碱性高放废液中萃取Cs(Ⅰ)。但修饰剂Cs-3在52 ℃的SRS模拟碱性高放废液中存放15 d后有近40%发生溶解,稳定性较差,而且在萃取过程中容易形成第三相[33]。为了提高流程中杯芳双冠对Cs(Ⅰ)的萃取率,ORNL合成了5种性质稳定且适用于作有机溶剂修饰剂的烷基酚氧基氟化醇类物质,比较其有效性后发现,5种修饰剂中,1-(2,2,3,3-四氟丙氧基)-3-(4-仲丁基酚)-2-丙醇(Cs-7SB)最适合用于CSSX流程[34]。CSSX流程(试剂见表1)中的有机相组成为:萃取剂BOBCalix[4]C6,修饰剂Cs-7SB,抑制剂三正辛胺(TOA),稀释剂LIX79[35]。采用该流程,Cs(Ⅰ)分离净化系数可达4 000[12]。萃取剂组成可稍微调整,如为进一步优化流程对Cs(Ⅰ)的识别能力,可选取MaxCalix[4]C6作萃取剂,因其具有良好的溶解性,可代替BOBCalix[4]C6和BEHB Calix[4]C6。该流程被称为NG-CSSX,其对Cs(Ⅰ)的萃取容量远高于CSSX流程,因为抑制剂TiDG较TOA具有更好的热稳定性[36]。
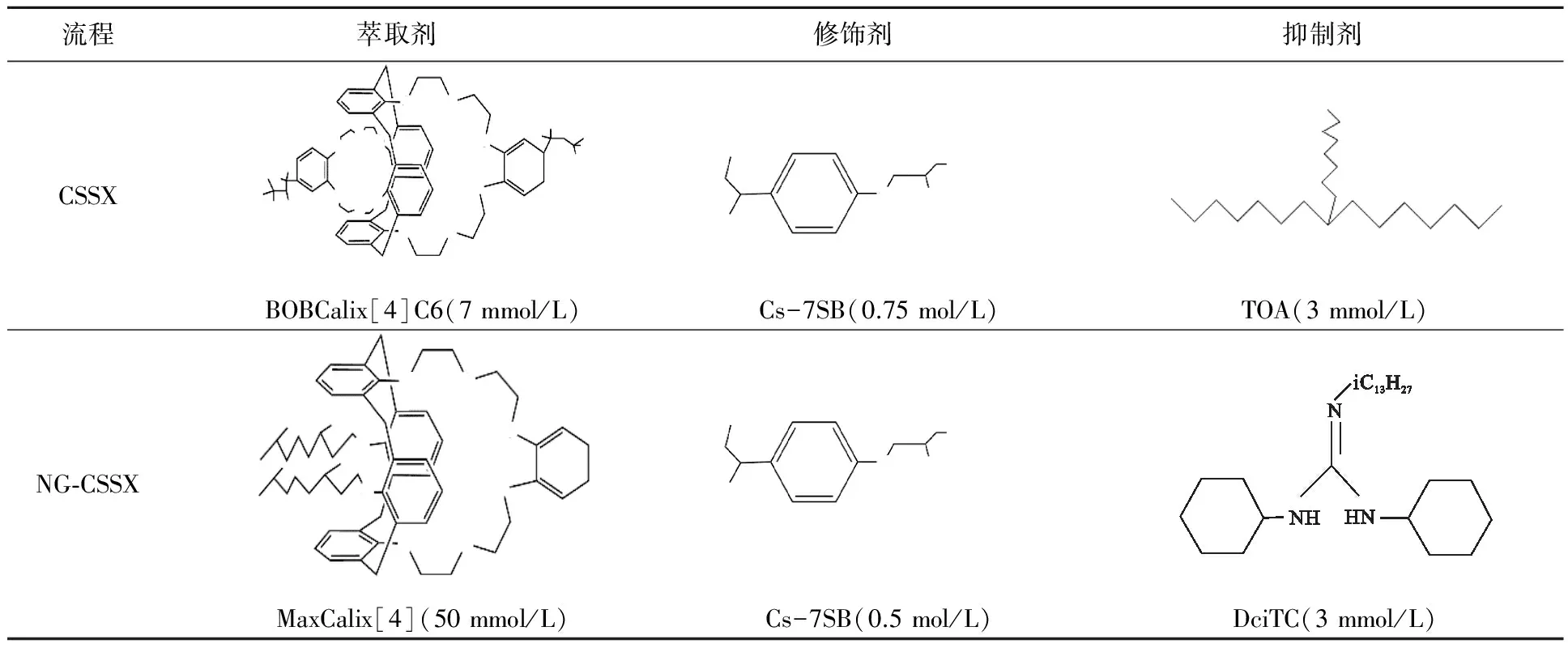
表1 溶剂萃取流程CSSX和NG-CSSX的组成比较
裂变产物的萃取(FPEX)可选取双-叔丁基环己基-18-冠-6(DtBuCH18C6)和杯[4]芳烃-双(叔辛基苯并-冠-6)(BOBCalix[4]C6)作萃取剂,可同时分离Cs(Ⅰ)和Sr(Ⅱ),对Cs(Ⅰ)和Sr(Ⅱ)的去除率均能达99.9%[37-38]。
2 液膜萃取法
液膜萃取法分离效率高、选择性好,设备简单、占地面积小,液膜比表面积大、厚度小,能够同时进行萃取、反萃取,但液膜不够稳定,使用时间短。
P.Kandwal等[39-40]研究了中空纤维支撑杯[4]-双(萘并)冠-6作为萃取剂回收高放射性废物中的铯离子。以正十二烷和邻硝基苯辛醚混合液作为稀释剂,硝酸铯溶液酸度为3 mol/L硝酸,水为洗脱剂。结果表明:膜传质系数为5.24×10-6cm/s,膜传质速度为传输Cs(Ⅰ)的主要控制步骤;在6 h内可回收溶液中99.9%以上的铯离子,在12 h内传输溶液中88%的铯离子;利用NaOH溶液和洗脱相的酸度,回收溶液中95%的铯离子是可能的。P.Jagasia等[41]研究了4种Calix-冠-6液膜在氟化稀释剂、苯基三氟甲基砜中对铯的萃取。其中,4种液膜传质速率不同,但总体来说都比相应的Calix-冠-6在硝基苯或邻硝基苯辛醚与正十二烷混合物中的传输速率快,分离效果好;但液膜的稳定性很短,5 d后传输效率明显降低。利用中空纤维支撑液膜作支撑体(HFSLM)[42],双(辛氧基)-杯[4]芳烃-冠-6(CMC)作萃取剂,分离酸性溶液中的放射性Cs(Ⅰ),在4 mol/L硝酸溶液中,HFSLM能在2 h内传输Cs+质量浓度为0.32 g/L的溶液300 mL,表明CMC在中空纤维中可以作为接触器来分离放射性废液中的铯,但负载量较低,有待进一步研究。
3 色谱萃取分离法
相比于传统的溶剂萃取法,色谱萃取法不仅具有设备简单、流程简单、成本低等优点,而且还兼有溶剂萃取法的高选择性及柱色谱法的简单多级,能够有效减少处理放射性废液过程中产生的废液量等优点,对后处理过程中分离铯和一些特定的裂变产物非常有利。
色谱分离法的色谱固定相一般是通过物理浸渍或化学接枝法将冠醚负载到固相载体上。最常用的载体包括硅胶、大孔树脂、介孔硅和大孔二氧化硅等。
1)硅胶。基于硅胶表面的多孔构造,将功能化合物通过物理或化学反应固定在多孔硅胶中,不仅能够加快溶液中金属离子扩散进入吸附剂的速率,而且还能提高吸附效率。G.Arena等[43]选取氯铂酸催化杯[4]芳烃-冠-5、杯[4]芳烃-冠-6,分别与三乙氧基硅烷发生缩聚反应得到硅烷化杯芳冠醚,然后用其作为色谱固相分离高放废液中的Cs(Ⅰ)。结果表明:在以水/甲醇(80/20)作为流动相、流速为0.2 mL/min的色谱柱中,单臂杯芳冠醚硅胶色固相能够完全分离Cs(Ⅰ)与Na(Ⅰ);但分离Cs(Ⅰ)与K(Ⅰ)的效果不好。合成的单臂杯芳烃冠醚硅胶虽产率高,但单臂长链柔性比较大,杯芳冠醚化合物容易缠绕在一起,降低了其在硅胶中的有效含量;而且,导致杯芳冠醚化合物构象发生旋转,进而降低了对其他离子的分离系数。L.L.Tavlarides等[44]同样通过溶胶-凝胶法将硅烷类前驱体在酸催化作用下与烷氧基硅发生聚合反应得到杯[4]芳烃-苯并-冠-6/硅胶和杯[4]芳烃-冠-6/硅胶2种色谱固定相,对Cs(Ⅰ)的吸附分配系数(Kd)分别为9.5 cm3/g和12.5 cm3/g。杯[4]芳烃-双冠-6与硅胶以化学键方式结合,杯[4]芳烃-双冠-6从吸附剂中溶解进入水相中的量较低,但杯[4]芳烃-双冠-6在硅胶中的排列较混乱,使得预组织成无序的聚集体,这使其吸附分配系数低于物理方法合成的色谱固定相。硅胶的优点是来源丰富、与识别材料负载方便,但硅胶通常颗粒较小,萃取时柱内压力较大,不利于分离。
2)大孔高分子树脂。大孔高分子树脂一般为聚乙烯、聚丙烯、聚苯乙烯等惰性聚合物,常用作固定相载体。A.S.Khan等[45]采用二环己基-18-冠-6(DCH18C6)与聚氨酯泡沫体物理复合得到萃取色谱树脂,用其填充色谱柱并分离Na+、Cs+、Rb+、K+,在4×10 mmol/L三硝基苯酚有机相中,萃取色谱树脂对碱金属(Na+∶Cs+∶Rb+∶K+)的分配系数比为 1∶3∶21∶40。〗M.L.Dietz等[46]将50 mmol/L BC6B溶解在二氯甲烷溶剂中并灌入到ArnberehromCG-7lm树脂中制备出冠醚色谱树脂,其负载量达40%,用其萃取分离Cs(Ⅰ),最佳酸度为2 mol/L,吸附分配系数超过100 cm3/g,能够有效地选择性分离Cs(Ⅰ)。Xiao C.L.等[47]合成了一种新型大孔聚合物基BiPCalix[4]C6超分子识别材料BiPCalix[4]C6/XAD-7,通过真空浸渍和共聚作用将BiPCalix[4]C6浸渍和固定到大孔XAD-7颗粒孔道中。BiPCalix[4]C6/XAD-7在含有典型裂变和非裂变产物,如Ru(Ⅲ)、Mo(Ⅵ)、K(Ⅰ)、Rb(Ⅱ)、Sr(Ⅱ)、Ba(Ⅱ)、La(Ⅲ)和Y(Ⅲ)中选择性吸附Cs(Ⅰ),其吸附Cs(Ⅰ)的最佳酸度为4.0 mol/L HNO3,分配系数为20.63 cm3/g。除对铷的分配系数为4.64 cm3/g外,对其他金属离子的分配系数皆低于1.35 cm3/g,表明BiPCalix[4]C6/XAD-7对铯具有非常好的选择性。但在分离Cs(Ⅰ)之前,HLLW的酸度要用硝酸调为4.0 mol/L,这会导致放射性废液量增加。Yi R.等[48]通过丙烯醛基杯[4]冠-6与丙烯酰胺类物质发生聚合反应得到PNIPAM-cl-calixcrown,这种水凝胶微球对Cs(Ⅰ)的选择系数分别为f(Cs/K)=20.0,f(Cs/Na)=34.0,在真正的海水中对Cs(Ⅰ)的去除率可达93.5%。
3)介孔硅。用F108作模板剂合成的介孔硅具有较大的比表面积、体积及有序的孔道,是具有优异性能的固体吸附剂基体。此外,有序介孔硅可用作固定支撑配体分子,不改变配体分子对捕获目标活性金属离子的功能。
高选择性配体固化介孔硅因比表面积大、孔容大、吸附容量大、吸附时间短和可多次重复使用而备受关注。介孔硅通过结合配体形成功能化纳米吸附剂,能有效分离和去除目标离子。冠醚被广泛用于萃取分离Cs(Ⅰ),分离过程主要基于Cs(Ⅰ)离子半径与冠醚配体的空腔尺寸相适应,且冠醚的苯环与阳离子发生π电子作用。但高成本和易产生严重二次污染是溶剂萃取分离铯的最大障碍。因此,用大环配体的双苯并-18-冠-6(DB18C6)固化介孔硅制备新型螯合吸附剂,试验结果表明:溶液pH在5.5~7.0范围内对吸附剂吸附Cs(Ⅰ)有影响;螯合吸附剂对Cs(Ⅰ)的最大吸附容量可达50.23 mg/g,远高于介孔硅对Cs(Ⅰ)的最大吸附容量27.40 mg/g[49];利用0.2 mol/L HCl溶液对吸附后的材料进行洗脱,洗脱率可达99.1%。M.R.Awual等[50]将DB24C8负载到有序介孔硅中,合成后的材料10 mg在25 ℃下对10 mL、0.015 mmol/L Cs+溶液的最佳吸附pH为7.0,吸附容量为70 mg/g,可实现对溶液中Cs+的完全去除。该材料有望应用于诸如福岛核事故泄露的放射性废液中的快速和高选择性去除Cs(在25 ℃下)。
4)大孔二氧化硅。Wei Y.Z.等[51]首次采用物理真空复合法将HDEHP和Cyanex301负载到大孔硅基SiO2-P中,获得HDEHP/SiO2-P和Cyanex 301/SiO2-P材料,并用以分离高放废液中的Ln(Ⅲ)、Am(Ⅲ)和Cm(Ⅲ)。结果表明:HDEHP/SiO2-P在0~1 mol/L HNO3溶液中的f(Am/Ln)仅仅为10,很难用于萃取色谱法;而Cyanex301/SiO2-P在pH为4~4.5范围内的f(Am/Ln)可达1 000,能选择性分离Am(Ⅲ)。Zhang A.Y.等[52]采用相同方法将Calix[4]arene-R14与SiO2-P复合获得超分子识别材料,用此材料从HLLW中分离Cs(25 ℃),其最佳酸度为4.0 mol/L HNO3,分配系数为50.46 cm3/g。
Chai Z.[15]分别将Calix[4]arene-R14和DtBuCH18C6与SiO2-P复合,制备出新型大孔硅基(杯芳)冠醚超分子识别材料,提出SPEC萃取色谱分离流程(Strontium/Cesium Partitioning from HLW by Extraction Chromatography),应用于HLLW中Cs(Ⅰ)和Sr(Ⅱ)的分离。试验结果表明,在(Calix[4]arene-R14+M)/SiO2-P和(DtBuCH18C6+M)/SiO2-P填充塔中,加入金属离子溶液酸度为4 mol/L硝酸溶液,以水作洗脱液,Cs(Ⅰ)和Sr(Ⅱ)的回收率分别为100.5%、99.6%;将CalixBNaphC负载到SiO2-P中,提出PCEC萃取色谱分离流程(Partitioning of Cesium by Extraction Chromatography),用以吸附分离HLLW中的Cs(Ⅰ)[53]。试验结果表明,以水作洗脱剂,经过6次吸附—解吸,Cs(Ⅰ)去除率超过99.1%。因为Cs(Ⅰ)与冠醚环的“尺寸效应”和与芳烃的“π电子效应”[54-55],Calix[4]arene-R14/SiO2-P对Cs(Ⅰ)的吸附能力和对Cs(Ⅰ)/Na(Ⅰ)的选择性更强。TBP、MODB和Octanol等修饰剂能够与Calix[4]arene-R14分子形成氢键,提高杯芳冠醚的疏水性,提高对Cs(Ⅰ)的吸附容量[56-57]。在4.0 mol/L HNO3溶液中,反应120 min,(Calix[4]+Oct)/SiO2-P对Cs(Ⅰ)的吸附分配系数高达94.11 cm3/g。Calix[4]arene-R14/SiO2-P对Cs(Ⅰ)的最佳吸附酸度为4.0 mol/L,与高放废液的酸度不符,且Calix[4]arene-R14的结构复杂,较难合成,产率低(低于15%),所以,基于HexylCalix[4]/SiO2-P、CalixBNapC6/SiO2-P和BiPCalix[4]C6的SPEC萃取色谱分离流程,对Cs(Ⅰ)的最佳吸附酸度为3.0 mol/L,分配系数分别达41.37、18.01 和59.43 cm3/g[58-59]。
Zhang A.等[60]将Calix[4]和DBC共同负载到SiO2-P中,创新性地提出GPSC萃取色谱分离流程(Group Partitioning of Strontium and Cesium by Extraction Chromatography),实现了HLLW中Cs(Ⅰ)、Sr(Ⅱ)的吸附分离。当溶液HNO3浓度为3.0 mol/L时,Calix[4]@DBC/SiO2-P对Cs(Ⅰ)和Sr(Ⅱ)的吸附分配系数分别高达89.93 cm3/g和75.91 cm3/g,对Cs(Ⅰ)、Sr(Ⅱ)的回收率分别为99.2%和99.7%。
4 展望
Calix[4]Crowns因其孔径大小与Cs(Ⅰ)的离子半径相适应,能够有效分离Cs(Ⅰ),被认为是一种很有前景的Cs(Ⅰ)萃取剂。然而,传统的溶剂萃取工艺存在一系列问题,如辐射分解退化,需要大量仪器设备,还有可能产生大量有机废液,等等,而色谱萃取技术因仪器简单、废液量小等优势,能够很好地解决上述问题。目前,许多固相萃取剂不可重复使用,并且回收处理可能会对环境有一定影响,所以,合成对Cs(Ⅰ)有强络合能力的新型Calix[4]Crowns材料以及性能更优异的固相载体仍是需要研究的课题之一。
[1] GEORGES W.Molecular dynamics of cation complexation and extraction[M]//ASFIERI C,BÖHMER V,HARROWFIELD J,et al.Calixarenes 2001.Dordrecht:Springer Netherlands,2001:312-333.
[2] DANIL De N,CLEVERLEY R M,ZAPATAORMACHEA M L.Thermodynamics of calixarene chemistry[J].Chemical Reviews,1998,98(7):2495-2526.
[3] SACHLEBEN R A,URVOAS A,BRYAN J C,et al.Dideoxygenated calix[4]arene crown-6 ethers enhanced selectivity for caesium over potassium and rubidium[J].Chemical Communications,1999,17:1751-1752.
[4] JI H F,DABESTANI R,BROWN G M,et al.Synthesis and sensing behavior of cyanoanthracene modified 1,3-alternate calix[4]benzocrown-6:a new class of Cs+selective optical sensors[J].Journal of the Chemical Society Perkin Transactions,2001,2(4):585-591.
[5] RUSSELL B C,WARWICK P E,CROUDACE I W.Calixarene-based extraction chromatographic separation of135Cs and137Cs in environmental and waste samples prior to sector field ICP-MS analysis[J].Analytical Chemistry,2014,86(23):11890-11896.
[6] GLATZ J.Demonstration of a TODGA based extraction process for the partitioning of minor actinides from a PUREX raffinate[J].Solvent Extraction and Ion Exchange,2009,27(1):26-35.
[7] ZHANG,DAI A,XU Y,et al.Extraction behavior of cesium and some typical fission and non-fission products with a new 1,3-di(1-decyloxy)-2,4-crown-6-calix[4]arene[J].Radiochimica Acta,2014,102(1/2):135-142.
[8] TSAI S C,OUYANG S,HSU C N.Sorption and diffusion behavior of Cs and Sr on Jih-hsing bentonite[J].Applied Radiation and Isotopes,2001,54(2):209-215.
[9] SHAHWAN T,ERTEN H N.Thermodynamic parameters of Cs+,sorption on natural clays[J].Journal of Radioanalytical and Nuclear Chemistry,2002,253(1):115-120.
[10] ZHANG A,CHEN C,WANG Y,et al.Adsorption of cesium and some typical elements on a novel solid-state macroporous silica-salix[4]arene material[J].Separation Science and Technology,2016,51(12):1962-1970.
[11] SENGUPTA P,SANWAL J,MATHI P,et al.Sorption of Cs and Sr radionuclides within natural carbonates[J].Journal of Radioanalytical & Nuclear Chemistry,2017,312(1):1-10.
[12] WALKER D D,NORATO M A,CAMPBELL S G,et al.Cesium removal from savannah river site radioactive waste using the caustic-side solvent extraction(CSSX) process[J].Separation Science and Technology,2005,40(1/2/3):297-309.
[13] MOYER B A,BONNESEN P V,DELMAU L H,et al.Development of the next-generation caustic-side solvent extraction(NG-CSSX) process for cesium removal from high-level tank waste[C]//Environmental Restoration and Waste Management.Conference:Waste Management 2011.Phoenix:[s.n.],2011:198-207.
[14] ZHANG A,WEI Y,KUMAGAI M,et al.Resistant behavior of a novel silica-based octyl(phenyl)-N,N-disobutyl carbamoylmethylpho shine oxide neutral extraction resin against nitric acid,temperature and gamma-radiation[J].Radiation Physics & Chemistry,2005,72(4):455-463.
[15] CHAI Z.SPEC:a new process for strontium and cesium partitioning utilizing two macroporous silica-based supramolecular recognition agents impregnated polymeric composites[J].Separation Science and Technology,2009,44(9):2146-2168.
[16] ROGERS H,BOWERS J,GATES-ANDERSON D.An isotope dilution-precipitation process for removing radioactive cesium from wastewater[J].Journal of Hazardous Materials,2012,243(12):124-129.
[17] ISAKSSON M,ERLANDSSON B,MATTSSON S.A 10-year study of the137Cs distribution in soil and a comparison of Cs soil inventory with precipitation-determined deposition[J].Journal of Environmental Radioactivity,2001,55(1):47-59.
[18] AND C S G,LUCA V.Ion-exchange properties of microporous tungstates[J].Cheminform,2016,36(9):4992-4999.
[19] DENG H,LI Y,WU L,et al.The novel composite mechanism of ammonium molybdophosphate loaded on silica matrix and its ion exchange breakthrough curves for cesium[J].Journal of Hazardous Materials,2017,324:348-356.
[20] RAIS J,TACHIMORI S,YOO E,et al.Extraction of radioactive Cs and Sr from nitric acid solutions with 25,27-bis(1-octyloxy)calix[4]-26,28-crown-6 and dicyclohexyl-18-Crown-6:effect of nature of the organic solvent[J].Separation Science and Technology,2015,50(8):1202-1212.
[21] LUDWIG R,DZUNG N T K.Calixarene-based molecules for cation recognition[J].Sensors,2002,2(10):397-416.
[22] BONNESEN P V.Fundamental studies regarding synergism between Calix[4]arene-bis(-octylbenzo-crown-6) and alcohol modifiers in the solvent extraction of cesium nitrate[J].Solvent Extraction and Ion Exchange,2005,23(1):23-57.
[23] KUMAR V,SHARMA J N,ACHUTHAN P V,et al.A new bisglycolamide substituted calix[4]arene-benzo-crown-6 for the selective removal of cesium ion:combined experimental and density functional theoretical investigation[J].Rsc Advances,2016,6(52):47120-47129.
[24] LIU X,SUROWIEC K,BARTSCH R A.Di-ionizable p-tert-butylcalix[4]arene-1,3-crown-4 ligands:synthesis and alkaline earth metal cation extraction[J].Cheminform,2009,65(31):5893-5898.
[25] KIM S,KIM H,NOH K H,et al.Potassium ion-selective membrane electrodes based on 1,3-alternate calix[4]crown-5-azacrown-5[J].Talanta,2003,61(5):709-716.
[27] GHIDINI E,UGOZZOLI F,UNGARO R,et al.Complexation of alkali metal cations by conformationally rigid,stereoisomeric calix[4]arene crown ethers:a quantitative evaluation of preorganization[J].Journal of Chromatography B Biomedical Applications,1990,676(1):131-140.
[28] ROCCO U,ALESSRO C,FRANCO U,et al.1,3-Dialkoxycalix[4]arenecrowns-6 in 1,3 alternate conformation:cesium-Selective ligands that exploit cation-arene Interactions[J].Angewandte Chemie International Edition in English,1994,33(14):1506-1509.
[29] THUÉRY P,NIERLICH M,ÉRONIQUE L,et al.Bis(crown ether) and azobenzocrown derivatives of Calix[4]arene:a review of structural information from crystallographic and modelling studies[J].Journal of Inclusion Phenomena and Macrocyclic Chemistry,2000,36(4):375-408.
[30] SIMON N,TOURNOIS B,EYMARD S,et al.Cs selective extraction from high level liquid waste with crown calixarene:where are today?[J].Atalante,2008,34(6):172-178.
[31] SIMON N,EYMARD S,TOURNOIS B,et al.Caesium extraction from acidic high level liquid wastes with functionalized calixarenes[J].DNa,2001,10(3):1-8
[32] DELMAU L H.Improved performance of the alkaline-side CSEX process for cesium extraction from alkaline high-level waste obtained by characterization of the effect of surfactant impurities[R].Tennessee:Oak Ridge National Laboratory.1999.
[33] BONNESEN P V,DELMAU L H,MOYER B A,et al.A robust alkaline-side CSEX solvent suitable for removing cesium from savannah river high level waste[J].Solvent Extraction and Ion Exchange,2000,18(6):1079-1107.
[34] MOYER B A.Development of effective solvent modifiers for the solvent extraction of cesium from alkaline high-level tank waste[J].Solvent Extraction and Ion Exchange,2003,21(2):141-170.
[35] HAVERLOCK T J.Alternatives to nitric acid stripping in the caustic-side solvent extraction(CSSX) process for cesium removal from alkaline high-level waste[J].Solvent Extraction and Ion Exchange,2009,27(2):172-198.
[36] HILL T G,ENSOR D D,DELMAU L H,et al.Thermal stability study of a new guanidine suppressor for the next-generation caustic-side solvent extraction process[J].Separation Science and Technology,2016,51(7):47-51.
[37] LAW J D.Fission product extraction(FPEX):development of a novel solvent for the simultaneous separation of strontium and cesium from acidic solutions[J].Solvent Extraction and Ion Exchange,2005,23(3):449-461.
[38] ELIAS G.FPEX γ-radiolysis in the presence of nitric acid[J].Solvent Extraction and Ion Exchange,2007,25(5):593-601.
[39] KANDWAL P,DIXIT S,MUKHOPADHYAY S,et al.Mass transport modeling of Cs(Ⅰ) through hollow fiber supported liquid membrane containing calix-[4]-bis(2,3-naptho)-crown-6 as the mobile carrier[J].Chemical Engineering Journal,2011,174(1):110-116.
[40] KANDWAL P,ANSARI S A,MOHAPATRA P K.Transport of cesium using hollow fiber supported liquid membrane containing calix[4]arene-bis(2,3-naphtho)crown-6 as the carrier extractant:Part Ⅱ:Recovery from simulated high level waste and mass transfer modeling[J].Journal of Membrane Science,2011,384(1):37-43.
[41] JAGASIA P,MOHAPATRA P K,RAUT D R,et al.Pertraction of radio-cesium from acidic feeds across supported liquid membranes containing calix-crown-6 ligands in a fluorinated diluent[J].Journal of Membrane Science,2015,487:127-134.
[42] JAGASIA P,ANSARI S A,RAUT D R,et al.Hollow fiber supported liquid membrane studies using a process compatible solvent containing calix[4]arene-mono-crown-6 for the recovery of radio-cesium from nuclear waste[J].Separation & Purification Technology,2016,170(2):208-216.
[43] ARENA G,CONTINO A,LONGO E,et al.Two calix-crown based stationary phases:synthesis,chromatographic performance and X-ray photoelectron spectroscopy investigation[J].Journal of Supramolecular Chemistry,2002,2(6):521-531.
[44] TAVLARIDES L L,LEE J S,NAM K H,et al.Sol-gel synthesized adsorbents for metal separation[J].清华大学学报自然科学版(英文版),2006,11(2):233-240.
[45] KHAN A S,BALDWIN W G,CHOW A.Extraction of alkali metal cations into polyester-based polyurethanef[J].Canadian Journal of Chemistry,2011,65(5):1103-1108.
[46] DIETZ M L,ENSOR D D,HARMON B,et al.Separation and preconcentration of cesium from acidic media by extraction chromatography[J].Separation Science and Technology,2006,41(10):2183-2204.
[47] XIAO C L,ZHANG A Y,CHAI Z F.Synthesis and characterization of a new polymer-based supramolecular recognition material and its adsorption for cesium[J].Solvent Extraction and Ion Exchange,2012,30(1):17-32.
[48] YI R,YE G,LV D,et al.Novel thermo-responsive hydrogel microspheres with calixcrown host molecules as cross-links for highly specific binding and controllable release of cesium[J].Rsc Advances,2015,5(68):55277-55284.
[49] AWUAL M R,YAITA T,TAGUCHI T,et al.Selective cesium removal from radioactive liquid waste by crown ether immobilized new class conjugate adsorbent[J].Journal of Hazardous Materials,2014,278(3):220-227.
[50] AWUAL M R,SUZUKI S,TAGUCHI T,et al.Radioactive cesium removal from nuclear wastewater by novel inorganic and conjugate adsorbents[J].Chemical Engineering Journal,2014,242(15):127-135.
[51] WEI Y Z,KUMAGAI M,TAKASHIMA Y,et al.Studies on the separation of minor actinide from high-level wastes by extraction chromatography using novel silica-based extraction resins[J].Nuclear Technology,2000,132(3):1472-1475.
[52] ZHANG A Y,WEI Y Z,HOSHI H,et al.Partitioning of cesium from a simulated high level liquid waste by extraction chromatography utilizing a macroporous silica-based supramolecular calix[4]arene-crown impregnated polymeric composite[J].Solvent Extraction and Ion Exchange,2007,25(3):389-405.
[53] ZHANG A,ZHANG W,WANG Y,et al.Effective separation of cesium with a new silica-calix[4]biscrown material by extraction chromatography[J].Separation & Purification Technology,2016,1719(3):17-25.
[54] ALI S M,JOSHI J M,SINGHADEB A K,et al.Dual mode of extraction for Cs+and Na+ions with dicyclohexano-18-crown-6 and bis(2-propyloxy)calix[4]crown-6 in ionic liquids:density functional theoretical investigation[J].Rsc Advances,2014,4(44):22903-22911.
[55] PRODI L,BOLLETTA F,MONTALTI M,et al.Photophysics of 1,3-alternate calix[4]arene-crowns and of their metal ion complexes:evidence for cation—π interactions in solution[J].New Journal of Chemistry,2000,24(24):155-158.
[56] ZHANG A,HU Q,CHAI Z.Chromatographic partitioning of cesium by a macroporous silica-calix[4]arene-crown supramolecular recognition composite[J].Aiche Journal,2010,56(10):2632-2640.
[57] ZHANG A,CHAI Z.Adsorption Property of cesium onto modified macroporous silica calix[4]arene-crown based supramolecular recognition materials[J].Industrial & Engineering Chemistry Research,2012,51(17):6196-6204.
[58] ZHANG A Y,HU Q H.Adsorption of cesium and some typical coexistent elements onto a modified macroporous silica-based supramolecular recognition material[J].Chemical Engineering Journal,2010,159(1/2/3):58-66.
[59] ZHANG A,HU Q,CHAI Z.Synthesis of a novel macroporous silica-calix[4]arene-crown polymeric composite and its adsorption for alkali metals and alkaline-earth metals[J].Industrial & Engineering Chemistry Research,2010,49(5):2047-2054.
[60] ZHANG A,LI J,YING D,et al.Development of a new simultaneous separation of cesium and strontium by extraction chromatograph utilization of a hybridized macroporous silica-based functional material[J].Separation & Purification Technology,2014,127(1):39-45.
ApplicationofCalix[4]CrownsCompoundsinSeparationCesium
DONG Zhimin1,HUANG Yongbiao2,ZHANG Zhibin1,3,WANG Youqun1,3,DAI Ying1,3, CAO Xiaohong1,3,LIU Yunhai1,3
(1.SchoolofChemistry,BiologicalandMaterialsScience,EastChinaUniversityofTechnology,Nanchang330013,China; 2.CriminalPoliceDetachmentofQingyuanPublicSecurityBureau,Qingyuan511518,China; 3.StateKeyLaboratoryBreedingBaseofNuclearResourcesandEnvironment,EastChinaUniversityofTechnology,Nanchang330013,China)
Calix[4]crowns compounds can be used as supermolecular recognition material to separate Cs(Ⅰ) and applied in solvent extraction,liquid membrane extraction and chromatographic separation.This paper introduces Calix[4]crowns compounds and homologous supermolecular recognition material and them recognition performances and adsorption effect for Cs(Ⅰ).The results provide a reference for seeking the Calix[4]crowns organic ligand structure and corresponding functional materials with excellent complexing ability for Cs(Ⅰ).
calix[4]crowns compounds;solvent extraction;chromatographic separation;Cs(Ⅰ)
O625.1
A
1009-2617(2017)06-0452-08
10.13355/j.cnki.sfyj.2017.06.003
2017-04-10
国家自然科学基金资助项目(21301028,21201033,11475044,41461070,21561002);校研究生创新基金资助项目(DHYC-2016009)。
董志敏(1991-),女,江西抚州人,硕士研究生,主要研究方向为放射性核素分离。
刘云海(1976-),男,江西南昌人,博士,教授,主要研究方向为放射性核素分离。E-mail:walton_liu@163.com。
