雷帕霉素对大鼠神经病理性疼痛及脊髓背角神经元凋亡的影响
2017-12-19黄凤贞深圳市宝安区中心医院麻醉科深圳518102通讯作者mail374379335qqcom
冯 涛,陈 功,丁 洁,黄凤贞(深圳市宝安区中心医院麻醉科,深圳 518102;通讯作者,E-mail:374379335@qq.com)
雷帕霉素对大鼠神经病理性疼痛及脊髓背角神经元凋亡的影响
冯 涛*,陈 功,丁 洁,黄凤贞
(深圳市宝安区中心医院麻醉科,深圳 518102;*通讯作者,E-mail:374379335@qq.com)
目的 研究鞘内注射雷帕霉素对大鼠神经病理性疼痛及脊髓背角神经元凋亡的影响。 方法 雄性SD大鼠30只随机分为假手术组(sham组)、脊神经结扎组(SNL组)和雷帕霉素组(Rap组),每组10只。各组大鼠L4-5鞘内置管后,SNL组和Rap组大鼠结扎左侧腰5脊神经建立神经病理性疼痛模型,sham组大鼠则仅分离暴露左侧腰5神经。Rap组大鼠于术后30 min鞘内注射雷帕霉素0.1 μg/10 μl,sham组和SNL组则给予注射等容溶剂(5% DMSO)。每日1次,连续7 d。各组大鼠测量术后1,3,5,7 d机械痛阈(paw withdrawal threshold,PWT)和热痛阈(paw withdrawal latency,PWL)。术后第7天处死大鼠并立即取左侧L5节段脊髓背角,电镜下观察神经元自噬体(n=3); Western blot法检测自噬相关蛋白LC3、神经元特异性蛋白NeuN以及凋亡相关蛋白a-caspase3表达(n=7)。 结果 与sham组比较,SNL组大鼠PWT和PWL在术后第1天和第3天开始分别明显降低,且持续到术后第7天(P<0.05),术后第7天 PWT、PWL分别为(12.5±1.0)g和(7.8±0.4)s,较sham组分别降低67%和44%(P<0.05);而脊髓背角LC3 Ⅱ和a-caspase3表达分别增高96%和359%(P<0.05), NeuN表达降低21%(P<0.05);与SNL组比较,Rap组大鼠各相应时间点PWT和PWL明显增高(P<0.05),术后第7天 PWT、PWL分别为(24.9±1.6)g和(11.8±0.8)s,较SNL组分别增加99%和51%(P<0.05);而脊髓背角LC3 Ⅱ和NeuN表达增高44%和19%(P<0.05), a-caspase3表达降低27%(P<0.05)。电镜下Rap组脊髓背角神经元自噬体较SNL组可见较多自噬体。 结论 雷帕霉素能减轻神经病理性疼痛,其机制可能与雷帕霉素增加脊髓背角神经元自噬,减少神经元凋亡有关。
雷帕霉素; 神经病理性疼痛; 自噬; 细胞凋亡
神经病理性疼痛(neuropathic pain, NP)是指外周或中枢神经系统受到损害后引起的疼痛,常表现为自发性疼痛、痛觉异常、痛觉过敏等[1],由于发病机制异常复杂,其治疗一直是个世界性难题。近年来有学者发现NP与脊髓背角神经元凋亡有关,而通过减少脊髓背角神经元凋亡能缓解NP[2, 3]。自噬是真核细胞通过溶酶体途径降解细胞内受损的细胞器及蛋白等物质,从而在维持细胞自身稳态方面起着保护作用的关键机制[4]。雷帕霉素是常用的自噬诱导剂,我们的前期研究发现雷帕霉素能通过诱导脊髓背角神经元细胞自噬及减少脊髓背角白介素1β水平从而有效减轻NP[5, 6]。然而雷帕霉素对NP脊髓背角神经元凋亡的影响,目前少见报道。本实验旨在研究雷帕霉素对NP大鼠的镇痛作用及对脊髓背角神经元凋亡的影响,为其临床应用提供理论依据。
1 材料和方法
1.1 主要试剂和仪器
雷帕霉素(Rapamycin)、二甲基亚砜(DMSO)和兔抗微管相关轻链蛋白3(microtubule-associated protein 1-light chain 3,LC3)多克隆抗体均购自美国Sigma公司,兔抗神经元特异性核蛋白(Neuronal Nuclei,NeuN))多克隆抗体(Abcam,美国),兔抗甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)、兔抗活化型半胱氨酸-天冬氨酸酶3(activated caspase3,a-caspase3)多克隆抗体(Sant Cruz,美国),聚乙烯PE-10导管(anilab,宁波),蛋白提取试剂盒及蛋白定量试剂盒(南京,凯基)。大鼠爪机械痛阈测定仪及热辐射痛阈测量仪(UGO BASILE,意大利),DYY-6C型电泳仪(北京六一仪器厂),Bio-Rad化学发光荧光成像系统(美国),透射电镜(型号Tecnai G2 12,荷兰)。
1.2 实验动物及分组
本实验得到华中科技大学同济医学院伦理委员会批准。雄性清洁级SD大鼠30只,8周龄,体质量200 -220 g,由武汉大学实验动物中心提供(许可证号SCXK(鄂)2008-0004)。大鼠采用随机数字表法分为假手术组(sham组),脊神经结扎组(SNL组)和雷帕霉素组(Rap组)(n=10)。各组大鼠首先行腰4-5间隙经鞘内向头端方向置入PE-10导管约3 cm,即脊髓腰膨大处。置管成功后,SNL组和Rap组大鼠行左侧腰5脊神经结扎建立神经病理性疼痛模型,sham组大鼠则仅分离左侧腰5脊神经不结扎。Rap组大鼠于术后30 min经鞘内置管注射雷帕霉素0.1 μg(10 μl),SNL组大鼠给予等容溶剂(5% DMSO),各组注射完毕后再给予15 μl生理盐水冲洗导管,以保证药物或溶剂全部进入鞘内,每日1次,连续7 d。动物模型制备及药物剂量的选择参考我们的预实验及之前的研究[5]。大鼠自由进食水,室温保持20-25 ℃。
1.3 大鼠痛阈测量
各组大鼠于术前测定基础痛阈(baseline),然后在术后1,3,5,7 d测量机械痛阈和热痛阈。机械痛阈测量方法为将大鼠置入底板为网格的有机玻璃箱内,将金属针头连接测痛仪后,针头缓慢匀速上升刺激大鼠患侧(左侧)后肢足底,当大鼠感到疼痛并收缩后爪时记录此压力值(paw withdrawal threshold,PWT)。热痛阈测量方法为将大鼠置于3 mm厚的玻璃板上,使用热辐射仪光源照射患侧(左侧)后肢足底,记录从照射开始到大鼠因疼痛而缩爪的时间(paw withdrawal latency,PWL)。各组连续测定3次,每次间隔时间为5 min。
1.4 透射电镜观察脊髓背角自噬
术后7 d各组大鼠各取3只采用水合氯醛350 mg/kg腹腔注射麻醉,用含0.25%戊二醛和4%多聚甲醛的0.1 mol/L磷酸缓冲液经心脏灌注后取脊髓L4-5节段左侧脊髓背角,2.5%戊二醛过夜,1%四氧化锇固定,丙酮脱水,环氧树脂包埋。标本制成超薄切片后以3%枸橼酸铅染色,然后在透射电镜下观察神经元自噬情况。
1.5 蛋白标本采集及检测

1.6 统计学分析
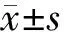
2 结果
2.1 大鼠机械痛阈和热痛阈
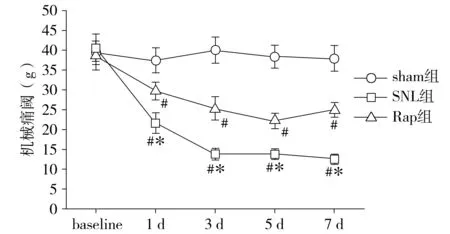
所有大鼠均未见明显伤口感染。sham组大鼠活动未见明显异常,痛阈无明显变化(P>0.05)。SNL组和Rap组大鼠术后出现术侧(左侧)后爪呈内收畸形,行走轻度异常。与sham组大鼠相比,SNL组大鼠术后1 d出现机械痛阈(PWT)明显降低(P<0.05),术后3 d热痛阈(PWL)明显降低(P<0.05),且持续到7 d;与SNL组相比,Rap组大鼠术后各相应时间点PWT和PWL明显增高(P<0.05,见图1,2)。
2.2 透射电镜观察脊髓背角自噬体
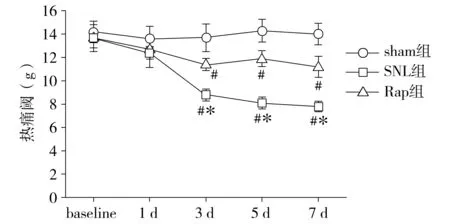
术后7 d,透射电镜下sham组神经元细胞核、内质网、线粒体和溶酶体形态基本正常,自噬体少见。SNL和Rap组神经元可见细胞核皱缩,线粒体变形肿胀,溶酶体形态异常,在胞质中可见双层膜组成的自噬囊泡,有的自噬囊泡中可见高电子密度的自噬体。与SNL组相比,Rap组自噬体较多,且细胞器受损情况好于SNL组(见图3)。
与sham组比较,#P<0.05;与SNL组比较,*P<0.05图1 各组大鼠术后7 d机械痛阈比较Figure 1 Comparison of mechanical pain(PWT) of rats between three groups (±s,n=10)
与sham组比较,#P<0.05;与SNL组比较,*P<0.05图2 各组大鼠术后7 d热痛阈(PWL)比较Figure 2 Comparison of thermal pain(PWL) of rats between three groups (±s,n=10)
图3 各组大鼠术后7 d脊髓背角神经元透射电镜下形态 (scale bars=1 μm,箭头为自噬体)Figure 3 Morphology of autophagosomes in neurons of the spinal cord at 7 d after surgery in each group under transmission electron microscope (scale bars=1 μm)
2.3 大鼠脊髓背角LC3蛋白水平比较
与sham相比,SNL组大鼠在术后7 d脊髓背角LC3 Ⅱ和a-caspase3表达明显增高(P<0.05),NeuN表达明显下降(P<0.05);与SNL组比较,Rap组LC3 Ⅱ和NeuN表达明显增高(P<0.05),而a-caspase3表达明显下降(P<0.05,见图4-6)。
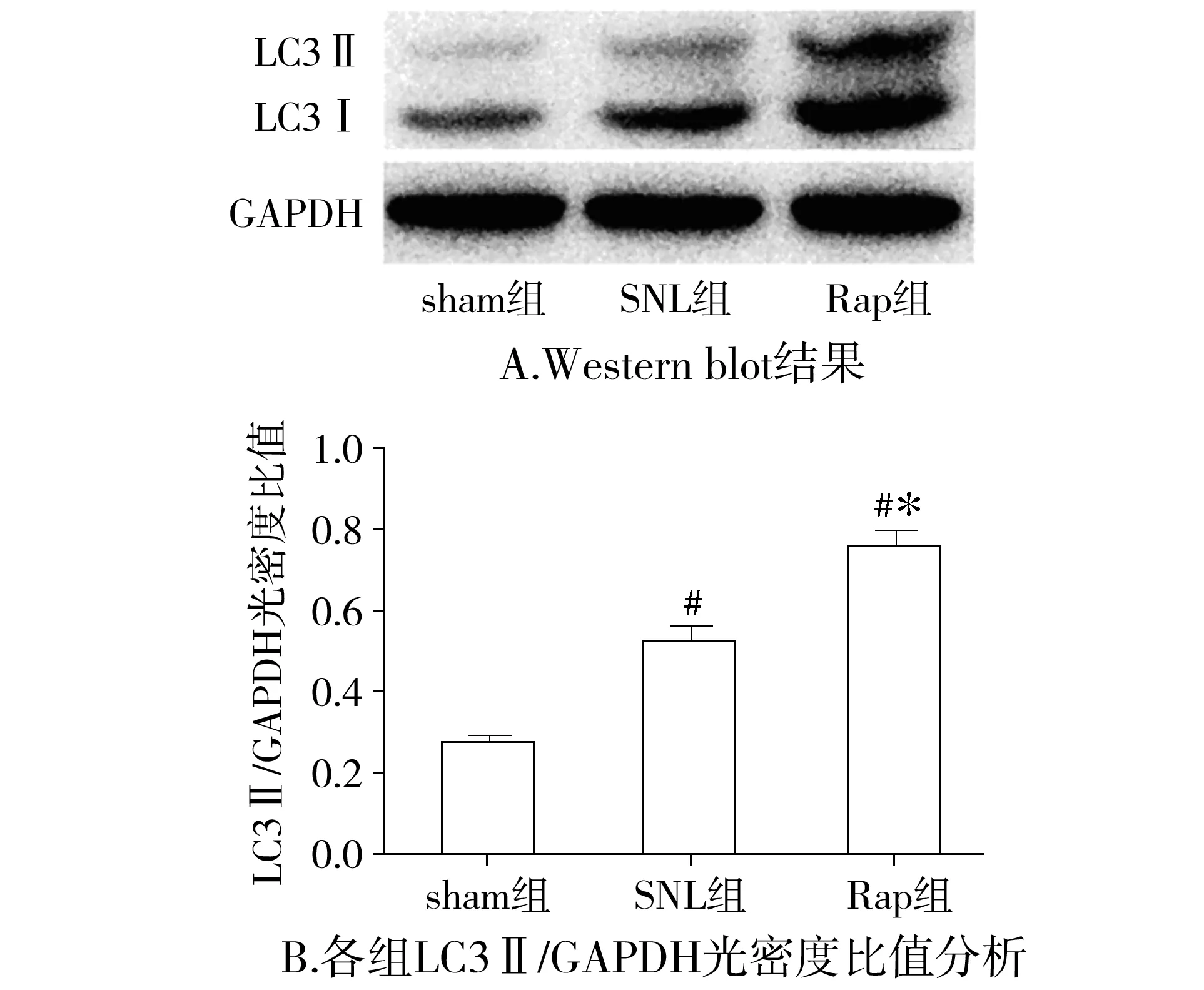
与sham组比较,#P<0.05;与SNL组比较,*P<0.05图4 各组大鼠术后7 d脊髓背角LC3表达比较Figure 4 The expression of LC3 in L5 spinal cord dorsal horn at 7 d after surgery in each group (±s,n=7)
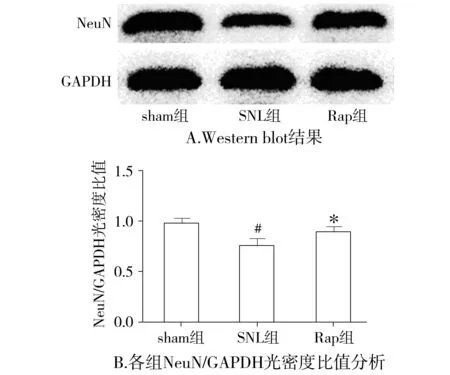
与sham组比较,#P<0.05;与SNL组比较,*P<0.05图5 各组大鼠术后7 d脊髓背角NeuN表达比较Figure 5 The expression of NeuN in L5 spinal cord dorsal horn at 7 d after surgery in each group (±s,n=7)
3 讨论
NP的发病机制非常复杂,近年来的一些研究逐步揭示了NP与脊髓背角神经元的凋亡之间的关系。Scholz等[7]在坐骨神经结扎、脊神经结扎、选择性外周神经损伤三种外周神经损伤引起的NP动物模型中,均发现伴有脊髓背角γ氨基丁酸(γ-ami-nobutyric acid,GABA)能神经元凋亡。GABA能神经元为抑制性中间神经元,由于其凋亡导致GABA释放减少,从而使神经兴奋性增加,进而参与了NP;而使用caspase广泛抑制剂zVAD,能减少GABA能神经元凋亡,并明显减轻神经病理性疼痛。此外有研究发现坐骨神经结扎模型大鼠给予caspase3的特异性抑制剂Z-DEVD-FMK后能明显缓解疼痛,其机制与减少脊髓背角神经元凋亡和抑制神经生长相关蛋白(GAP-43)有关[8]。GAP-43是神经元轴突生长标记物,与神经元轴突芽生和神经元可塑性有关,而神经元轴突芽生和神经元可塑性是神经病理性疼痛的重要机制。此外另有研究也发现通过减少脊髓背角神经元凋亡能缓解神经病理性疼痛[9,10],阻断或减轻脊髓背角神经元凋亡,有望成为治疗NP的一个新思路。
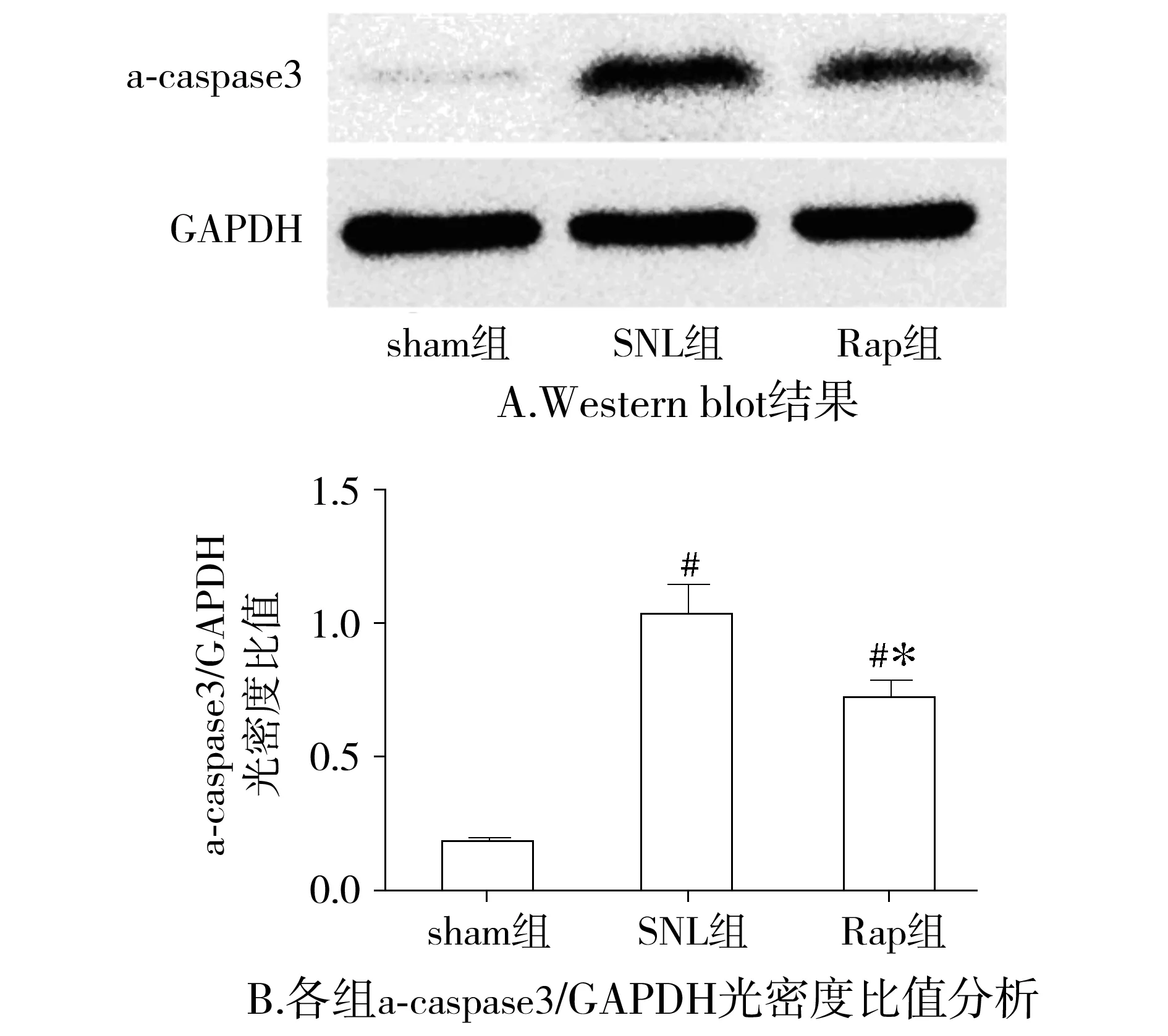
与sham组比较,#P<0.05;与SNL组比较,*P<0.05图6 各组大鼠术后7 d脊髓背角a-caspase3表达比较Figure 6 The expression of a-caspase3 in L5 spinal cord dorsal horn at 7 d after surgery in each group
与sham组比较,SNL大鼠机械痛阈和热痛阈明显降低,说明我们成功建立了神经病理性疼痛模型。为了研究神经病理性疼痛时自噬的变化,本课题组检测了脊髓背角蛋白微管相关轻链蛋白3(microtubule-associated protein light chain 3, LC3),并在电镜下观察脊髓背角神经元自噬体。LC3是自噬体的形成的关键蛋白,当自噬被激活时,细胞质里的LC3(LC3 Ⅰ)在泛素样反应酶的作用下与磷脂酰乙醇胺生成LC3 Ⅱ,LC3 Ⅱ由于特异定位于自噬体的内外膜,因此LC3 Ⅱ是反映自噬的一个可靠的指标[5]。与sham组大鼠相比,SNL组大鼠术后第7天脊髓背角LC3 Ⅱ表达增高,说明其自噬水平增加,这与其电镜下发现脊髓背角神经元自噬体轻度增多相一致,反映了脊髓背角对脊神经结扎这一病理刺激的保护性反应;而反映凋亡指标的蛋白a-caspase3表达明显增高,神经元的特异性标志物神经特异核蛋白(NeuN)表达明显减少,说明脊髓背角神经元发生了凋亡并参与了NP,这与已有报道相符[7]。与SNL组大鼠相比,Rap组大鼠机械痛阈和热痛阈明显增加,说明雷帕霉素能明显缓解神经病理性疼痛。Rap组大鼠LC3 Ⅱ表达较SNL组明显增加,电镜下观察可见脊髓背角神经元自噬增加,说明鞘内给予雷帕霉素确实增加了Rap组大鼠脊髓背角神经元自噬水平。同时Rap组大鼠a-caspase3表达下降,NeuN表达增加,说明雷帕霉素减少了Rap组大鼠脊髓背角神经元凋亡。近来有多项研究发现当中枢系统受损如脑缺血时,增加自噬能减少神经元的凋亡从而起到神经保护作用[11,12],由于神经病理性疼痛的根本原因在于中枢或外周神经受损,因此雷帕霉素减轻神经病理性疼痛的机制可能与其增加脊髓背角自噬,减少神经元凋亡有关。
综上所述,本实验发现SNL大鼠给予雷帕霉素后,脊髓背角神经元自噬增加,凋亡明显减少,雷帕霉素可能通过上调影响脊髓背角神经元自噬、减少神经元凋亡从而减轻神经病理性疼痛。
[1] Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment[J]. Lancet Neurol,2010,9(8):807-819.
[2] Gradl G, Herlyn P, Finke B,etal. A pan-caspase inhibitor reduces myocyte apoptosis and neuropathic pain in rats with chronic constriction injury of the sciatic nerve[J]. Anesth Analg,2013,116(1):216-223.
[3] Hu Q, Fang L, Li F,etal. Hyperbaric oxygenation treatment alleviates CCI-induced neuropathic pain and decreases spinal apoptosis[J]. Eur J Pain,2015,19(7):920-928.
[4] Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues[J]. Cell,2011,147(4):728-741.
[5] Feng T, Yin Q, Weng ZL,etal. Rapamycin ameliorates neuropathic pain by activating autophagy and inhibiting interleukin-1beta in the rat spinal cord[J]. J Huazhong Univ Sci Technolog Med Sci,2014,34(6):830-837.
[6] 冯涛, 翁泽林. 张建成, 等. 自噬在大鼠神经病理性痛形成中的作用[J]. 中华麻醉学杂志,2013,33(11):1362-1364.
[7] Scholz J, Broom DC, Youn DH,etal. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury[J]. J Neurosci,2005,25(32):7317-7323.
[8] Wu F, Miao X, Chen J,etal. Down-regulation of GAP-43 by inhibition of caspases-3 in a rat model of neuropathic pain[J].Int J Clin Exp Pathol,2012,5(9):948-955.
[9] Kaeidi A, Esmaeili-Mahani S, Sheibani V,etal. Olive (Oleaeuropaea L.) leaf extract attenuates early diabetic neuropathic pain through prevention of high glucose-induced apoptosis: in vitro and in vivo studies[J]. J Ethnopharmacol,2011,136(1):188-196.
[10] Zhao BS, Song XR, Hu PY,etal. Hyperbaric oxygen treatment at various stages following chronic constriction injury produces different antinociceptive effects via regulation of P2X4R expression and apoptosis[J]. PLoS One,2015,10(3):e120122.
[11] Shu S, Li CM, You YL,etal. Electroacupuncture ameliorates cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis[J]. Evid Based Complement Alternat Med,2016,2016:7297425.
[12] Yang Y, Gao K, Hu Z,etal. Autophagy upregulation and apoptosis downregulation in DAHP and triptolide treated cerebral ischemia[J]. Mediators Inflamm,2015,2015:120198.
Effectsofrapamycinonneuropathicpainandapoptosisofneuronsinspinalcordofratsafterspinalnerveligation
FENG Tao*,CHEN Gong,DING Jie,HUANG Fengzhen
(DepartmentofAnesthesiology,BaoanCentralHospital,Shenzhen518102,China;*Correspondingauthor,E-mail:374379335@qq.com)
ObjectiveTo investigate the effects of rapamycin on neuropathic pain and apoptosis of neurons in the spinal cord of rats after spinal nerve ligation.MethodsThirty male SD rats were randomly divided into three groups(n=10): sham group, spinal nerve ligation group(SNL group) and rapamycin group(Rap group). After intrathecal catheter implantation between L4-5 vertebrae, the rats in SNL group and Rap group underwent L5 spinal nerve ligation. The surgical procedure in sham group was identical except nerve ligation.The rats were injected with rapamycin(0.1 μg/10 μl) per day for 7 d via intrathecal catheter in Rap group after L5 spinal nerve ligation, while the rats were injected intrathecally with the same volume of vehicle(5%DMSO) in sham group and SNL group. Paw withdrawal threshold(PWT) and paw withdrawal latency(PWL) were measured at 1, 3, 5, and 7 d after surgery. At day 7 after surgery, the rats were sacrificed. Ipsilateral L5 spinal cord dorsal horn was examined using transmission electron microscope. Expression of LC3, NeuN and a-caspase3 in the L5 cord dorsal horn were measured by Western blot.ResultsCompared with sham group, PWT and PWL of rats were significantly decreased at 1, 3 day after SNL in SNL group, respectively, and lasted for 7 d(P<0.05). At day 7 after SNL, PWT[(12.5±1.0)g] and PWL[(7.8±0.4)s] were decreased by 67% and 44%, respectively(P<0.05), the levels of LC3 Ⅱ and a-caspase3 expression increased by 96% and 359%, respectively(P<0.05), while the levels of NeuN expression decreased by 21%. Compared to SNL group, PWT and PWL of rats were significantly increased in Rap group(P<0.05). At day 7 after SNL, PWT[(24.9±1.6)g]and PWL[(11.8±0.8)s]were increased by 99% and 51%, respectively(P<0.05), the levels of LC3 Ⅱ and NeuN expression increased by 44% and 19%, respectively(P<0.05), while the levels of a-caspase3 expression decreased by 27%(P<0.05). More autophagosomes were found in Rap group than in SNL group.ConclusionIntrathecal rapamycin may ameliorate SNL-induced neuropathic pain by increasing the autophagy and inhibiting the apoptosis of neurons.
rapamycin; neuropathic pain; autophagy; apoptosis
广东省深圳市卫生计生系统暨深圳市宝安区科技创新局科研项目(201507067)
冯涛,男,1979-09生,博士,副主任医师,E-mail:374379335@qq.com
2017-08-10
R365
A
1007-6611(2017)12-1236-05
10.13753/j.issn.1007-6611.2017.12.008
