80%赖氨酸硫酸盐与98%赖氨酸盐酸盐对生长中期草鱼生长性能、消化吸收能力和消化器官生长发育影响的比较研究
2017-12-16苏玥宁姜维丹周小秋
胡 凯 苏玥宁 冯 琳,3,4 刘 扬,3,4 姜维丹,3,4 吴 培,3,4 姜 俊,3,4 周小秋,3,4*
(1.四川农业大学动物营养研究所,成都 611130;2.成都农业科技职业学院畜牧兽医分院,成都 611130;3.鱼类营养与安全生产四川省高校重点实验室,成都 611130;4.动物抗病营养教育部重点实验室,成都 611130)
80%赖氨酸硫酸盐与98%赖氨酸盐酸盐对生长中期草鱼生长性能、消化吸收能力和消化器官生长发育影响的比较研究
胡 凯1,2苏玥宁1冯 琳1,3,4刘 扬1,3,4姜维丹1,3,4吴 培1,3,4姜 俊1,3,4周小秋1,3,4*
(1.四川农业大学动物营养研究所,成都 611130;2.成都农业科技职业学院畜牧兽医分院,成都 611130;3.鱼类营养与安全生产四川省高校重点实验室,成都 611130;4.动物抗病营养教育部重点实验室,成都 611130)
本试验通过比较80%赖氨酸硫酸盐[80%L-Lys·H2SO4,简称80赖氨酸(80-Lys)]与98%赖氨酸盐酸盐[98%L-Lys·HCl,简称98赖氨酸(98-Lys)]对生长中期草鱼生长性能、消化吸收能力和消化器官生长发育的影响,探讨80-Lys和98-Lys在草鱼上的生物效价并确定以80-Lys为赖氨酸(Lys)添加形式时饲料中Lys的适宜含量。选择初始体重为275.80 g左右的健康草鱼540尾,随机分成6组(每组3个重复,每个重复30尾鱼),分别饲喂Lys含量为0.8%(基础饲料)、1.0%、1.2%、1.4%和1.6%的添加80-Lys的饲料及Lys含量为1.2%的添加98-Lys的饲料60 d。结果表明:与基础饲料相比,饲料中添加适宜水平的80-Lys使饲料Lys含量达到1.2%时可显著提高生长中期草鱼的增重率(WGR),特定生长率(SGR),采食量(FI),全肠脂肪酶、淀粉酶活力,肝胰脏谷草转氨酶(GOT)和谷丙转氨酶(GPT)活力,前、中、后肠碱性磷酸酶(AKP)、肌酸激酶(CK)活力,肝体指数与肠体指数以及前、后肠皱襞高度(P<0.05),显著降低血清GOT和GPT活力(P<0.05),且80-Lys对上述指标的作用效果显著优于98-Lys(P<0.05);此外,还可显著提高生长中期草鱼的饲料效率(FE),全肠胰蛋白酶活力,前、中、后肠Na+,K+-ATP酶(Na+,K+-ATPase)和γ-谷胺酰转肽酶(γ-GT)活力,肠长与肠长指数以及中肠皱襞高度(P<0.05),但80-Lys对上述指标的作用效果与98-Lys差异不显著(P>0.05)。由此得出,与98-Lys相比,80-Lys能更有效地提高生长中期草鱼的消化吸收能力,进而促进其生长。以80-Lys为Lys添加形式,以SGR和FE为标识,生长中期草鱼(276~667 g)饲料中Lys的最适含量分别为1.31%(占饲料蛋白质的4.68%)和1.27%(占饲料蛋白质的4.54%)。
80%赖氨酸硫酸盐;98%赖氨酸盐酸盐;草鱼;生长性能;消化吸收能力

1 材料与方法
1.1 试验设计与试验饲料
试验用80-Lys和98-Lys均由长春大成实业集团有限公司提供。以大米蛋白粉、豆粕、棉籽粕、菜籽粕为主要蛋白质源配制基础饲料,向基础饲料中添加80-Lys使试验饲料中Lys含量分别为0.8%(基础饲料)、1.0%、1.2%、1.4%和1.6%,向基础饲料中添加98-Lys使试验饲料中Lys含量为1.2%(满足草鱼生长需要[17])。所有饲料通过添加甘氨酸(Gly)平衡蛋白质水平,试验饲料组成及营养水平见表1。参考Feng等[18]的方法分析试验饲料氨基酸组成,列于表2。
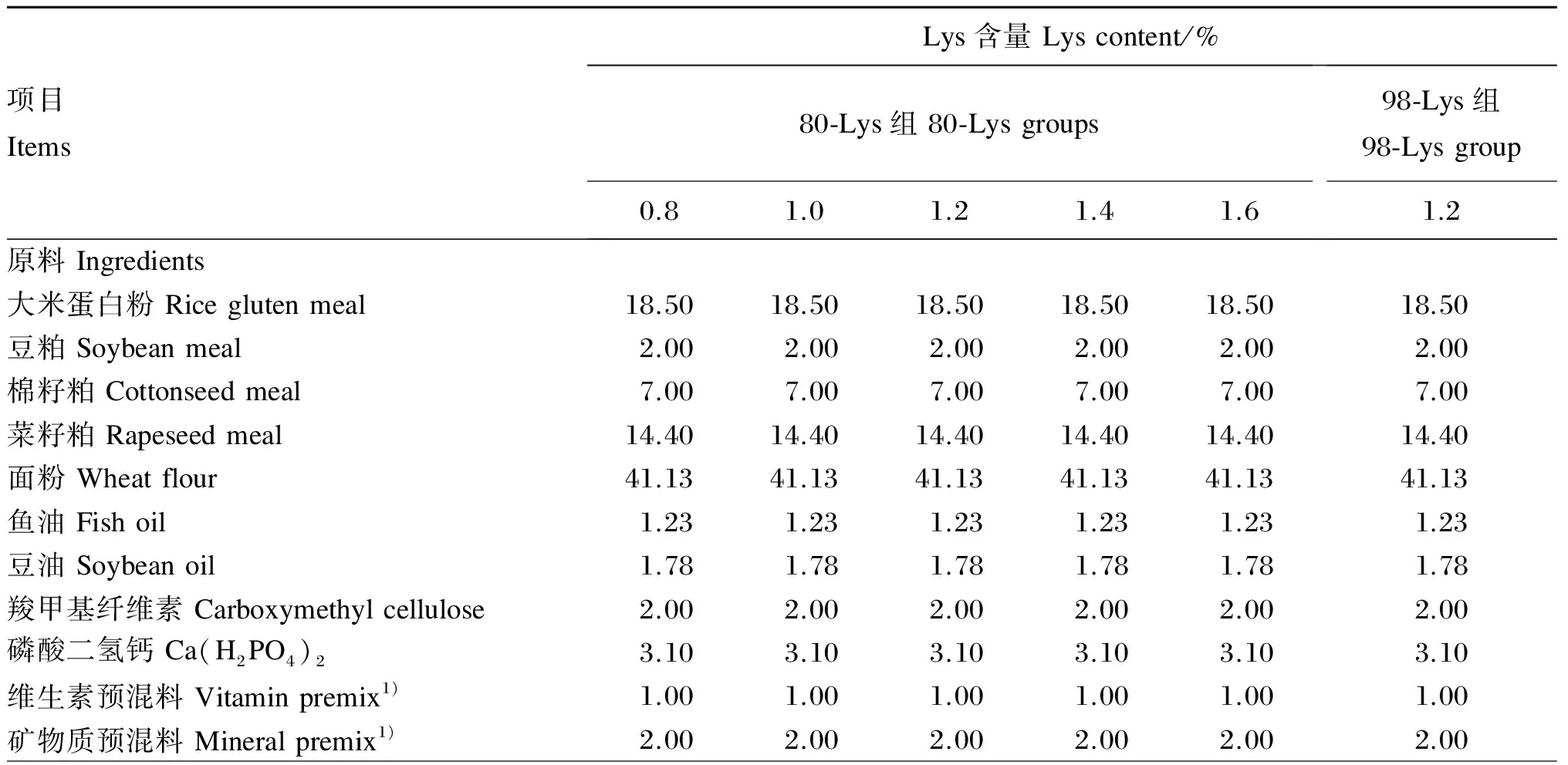
表1 试验饲料组成及营养水平(风干基础)
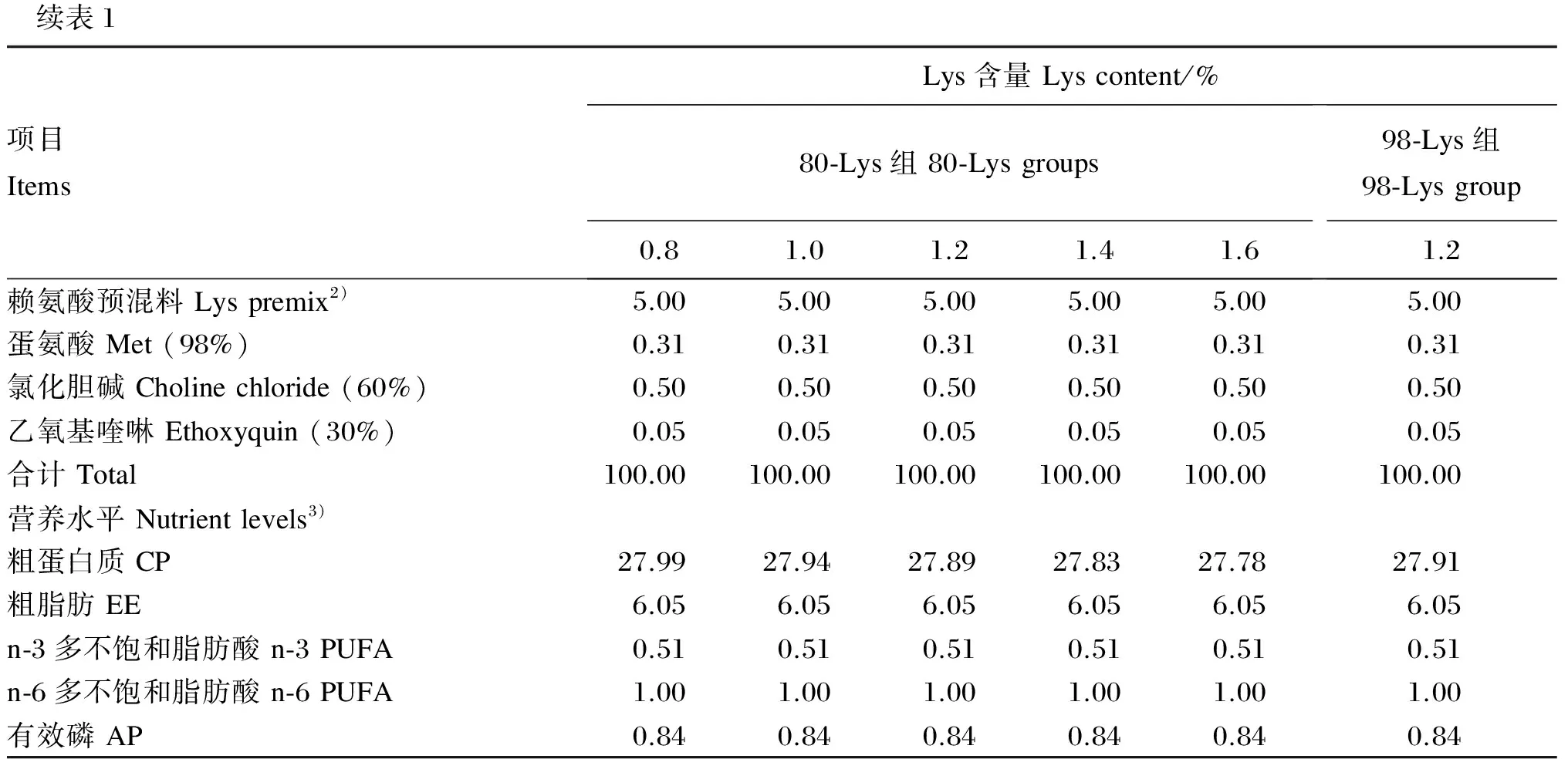
续表1项目ItemsLys含量Lyscontent/%80⁃Lys组80⁃Lysgroups0.81.01.21.41.698⁃Lys组98⁃Lysgroup1.2赖氨酸预混料Lyspremix2)5.005.005.005.005.005.00蛋氨酸Met(98%)0.310.310.310.310.310.31氯化胆碱Cholinechloride(60%)0.500.500.500.500.500.50乙氧基喹啉Ethoxyquin(30%)0.050.050.050.050.050.05合计Total100.00100.00100.00100.00100.00100.00营养水平Nutrientlevels3)粗蛋白质CP27.9927.9427.8927.8327.7827.91粗脂肪EE6.056.056.056.056.056.05n⁃3多不饱和脂肪酸n⁃3PUFA0.510.510.510.510.510.51n⁃6多不饱和脂肪酸n⁃6PUFA1.001.001.001.001.001.00有效磷AP0.840.840.840.840.840.84
1)维生素预混料和矿物质预混料均参照Xu等[19]配制。Vitamin premix and mineral premix were prepared according to Xu, et al[19].
2)每千克赖氨酸预混料中含有Contained the following per kg of lysine premix:80赖氨酸 80-Lys,0(0.8% 80-Lys组 0.8% 80-Lys group)、59.55(1.0% 80-Lys组 1.0% 80-Lys group)、119.10(1.2% 80-Lys组 1.2% 80-Lys group)、178.65(1.4% 80-Lys组 1.4% 80-Lys group)、238.20(1.6% 80-Lys组 1.6% 80-Lys group)、0 g(1.2% 98-Lys组 1.2% 98-Lys group);98赖氨酸 98-Lys,0(0.8% 80-Lys组0.8% 80-Lys group)、0(1.0% 80-Lys组 1.0% 80-Lys group)、0(1.2% 80-Lys组 1.2% 80-Lys group)、0(1.4% 80-Lys组 1.4% 80-Lys group)、0(1.6% 80-Lys组 1.6% 80-Lys group)、101.88 g(1.2% 98-Lys组 1.2% 98-Lys group);甘氨酸 Gly, 165.13(0.8% 80-Lys组 0.8% 80-Lys group)、123.85(1.0% 80-Lys组 1.0% 80-Lys group)、82.57(1.2% 80-Lys组 1.2% 80-Lys group)、41.28(1.4% 80-Lys组 1.4% 80-Lys group)、0(1.6% 80-Lys组 1.6% 80-Lys group)、82.56 g(1.2% 98-Lys组 1.2% 98-Lys group);其余用玉米淀粉补足 the rest was corn gluten meal complement。
3)粗蛋白质为测定值,其他营养水平根据NRC(2011)[17]饲料分析值计算得出。CP was a measured value, while the other nutrient levels were calculated according to feed analysis values of NRC (2011)[17].

表2 试验饲料氨基酸组成
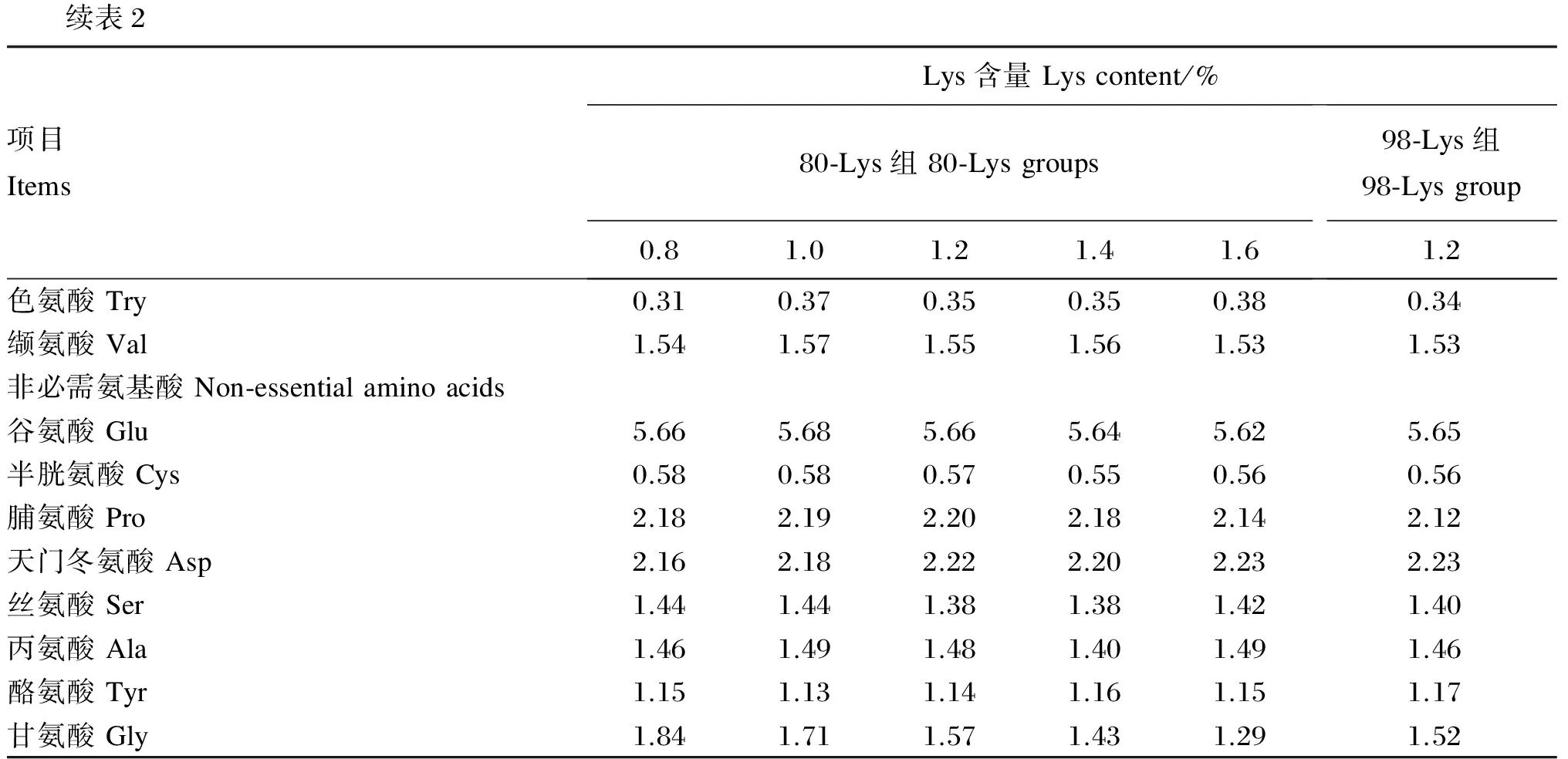
续表2项目ItemsLys含量Lyscontent/%80⁃Lys组80⁃Lysgroups0.81.01.21.41.698⁃Lys组98⁃Lysgroup1.2色氨酸Try0.310.370.350.350.380.34缬氨酸Val1.541.571.551.561.531.53非必需氨基酸Non⁃essentialaminoacids谷氨酸Glu5.665.685.665.645.625.65半胱氨酸Cys0.580.580.570.550.560.56脯氨酸Pro2.182.192.202.182.142.12天门冬氨酸Asp2.162.182.222.202.232.23丝氨酸Ser1.441.441.381.381.421.40丙氨酸Ala1.461.491.481.401.491.46酪氨酸Tyr1.151.131.141.161.151.17甘氨酸Gly1.841.711.571.431.291.52
1.2 饲养试验
饲养试验在四川农业大学水生动物营养研究室大邑研究基地进行,采用网箱养殖。草鱼购回经4周驯养后开始正式饲养试验。选择初始体重为(275.80±0.64) g的健康草鱼540尾,随机分成6组,每组3个重复,每个重复30尾鱼,以重复为单位放入1.4 m×1.4 m×1.4 m的网箱中,组间初始体重差异不显著(P>0.05)。各组分别饲喂添加80-Lys和98-Lys的饲料60 d。饲养管理参照本实验室前期研究建立的管理模式[20]进行,即每天定点投喂4次,每次投喂30 min后收集剩料并烘干称重。定期观察草鱼健康状况、换水及常规杀虫杀菌。试验期间水温为(28±2) ℃,pH维持在7.2±0.2,养殖水体中溶解氧浓度大于6 mg/L。
1.3 样品采集与指标测定
在生长试验起始和结束时分别以网箱为单位称量试验鱼体重,用以计算体增重(weight gain,WG)、增重率(weight gain rate,WGR)、特定生长率(specific growth rate,SGR);记录每日投饵量,收集残饵并称重,用以计算采食量(feed intake,FI)、饲料效率(feed efficiency,FE)。正式试验期间记录各组试验鱼死亡数,用以计算成活率(survival rate,SR)。饲养试验结束后,每组随机选择15尾生长中期草鱼,参照Geraylou等[21]描述的方法用对氨基苯甲酸对试验鱼进行麻醉,随后参考Huang等[22]描述的方法对试验鱼称重、测量体长以及采血,迅速分离肝胰脏和肠道并称重,测量肠长后定位分离前、中、后肠,再用液氮速冻后及时送超低温冰箱(-80 ℃)保存,用于实验室分析。每组另随机选择6尾试验鱼,参照Lin等[23]描述的方法测量其肠道皱襞高度。全肠胰蛋白酶、糜蛋白酶、脂肪酶、淀粉酶活力,前、中、后肠碱性磷酸酶(alkaline phosphatase,AKP)、肌酸激酶(creatine kinase,CK)、Na+,K+-ATP酶(Na+,K+-ATPase)、γ-谷胺酰转肽酶(γ-glutamyltransferase,γ-GT)活力的测定均参照Li等[24]描述的方法进行。血清、肝胰脏谷草转氨酶(glutamic oxalacetic transaminase,GOT)和谷丙转氨酶(glutamate pyruvate transaminase,GPT)活力的测定参照唐凌等[25]描述的方法进行。
1.4 计算公式
WGR=[WG(g)/初始体重(g)]×100;SGR={[ln终末体重(g)-ln初始体重(g)]/试验天数(d)}×100;FI=总投饵量(g)-总残饵量(g);FE=[WG(g)/摄食量(g)]×100;SR={[初始鱼总数(尾)-死亡鱼数(尾)]/初始鱼总数(尾)}×100;肝体指数(hepatosomatic index,HSI)=[肝胰脏重(g)/体重(g)]×100;肠体指数(intestosomatic index,ISI)=[肠重(g)/体重(g)]×100;肠长指数(relative gut length,RGL)=[肠长(cm)/体长(cm)]×100。
1.5 数据处理与统计分析
试验数据用平均值±标准差表示,采用SPSS 18.0统计软件进行统计分析。对不同Lys含量的80-Lys组的试验数据进行单因素方差分析,并结合Duncan氏法进行多重比较,以P<0.05作为差异显著水平,对统计学差异显著的指标进行回归分析。对饲料中Lys含量相同的80-Lys(1.2% Lys)组与98-Lys(1.2% Lys)组试验数据进行t检验,以检验不同处理之间的差异显著性,差异显著水平为P<0.05。
2 结 果
2.1 80-Lys和98-Lys对生长中期草鱼生长性能的影响
由表3可知,饲喂添加80-Lys和98-Lys的饲料的生长中期草鱼的SR均为100%。生长中期草鱼的终末体重、WG、WGR、SGR、FI、FE先随着饲料中80-Lys添加水平的增加而显著升高(P<0.05),在饲料中Lys含量为1.2%时均达到最大值,而后均随着饲料中80-Lys添加水平的进一步增加而显著降低(P<0.05)。在饲料中Lys含量均为1.2%的条件下,80-Lys组与98-Lys组相比,生长中期草鱼的FBW、WG、WGR、SGR、FI均显著升高(P<0.05),而FE和成活率则差异不显著(P>0.05)。
以80-Lys作为Lys的添加形式,分别对饲料中Lys含量(x)和生长中期草鱼的WG(yWG)、WGR(yWGR)、SGR(ySGR)、FI(yFI)、FE(yFE)进行回归分析,得到的回归方程分别为yWG=-431.56x2+1 132.06x-367.88(R2=0.869,P<0.01)、yWGR=-157.13x2+412.08x-134.38(R2=0.867,P<0.01)、ySGR=-1.20x2+3.15x-0.64(R2=0.884,P<0.01)、yFI=-370.60x2+1 009.99x-33.30(R2=0.895,P<0.01)、yFE=-0.38x2+0.97x-0.05(R2=0.795,P<0.01),分别以SGR和FE为标识,通过二次曲线分析确定的生长中期草鱼(276~667 g)饲料的Lys最适含量分别为1.31%和1.27%(图1)。
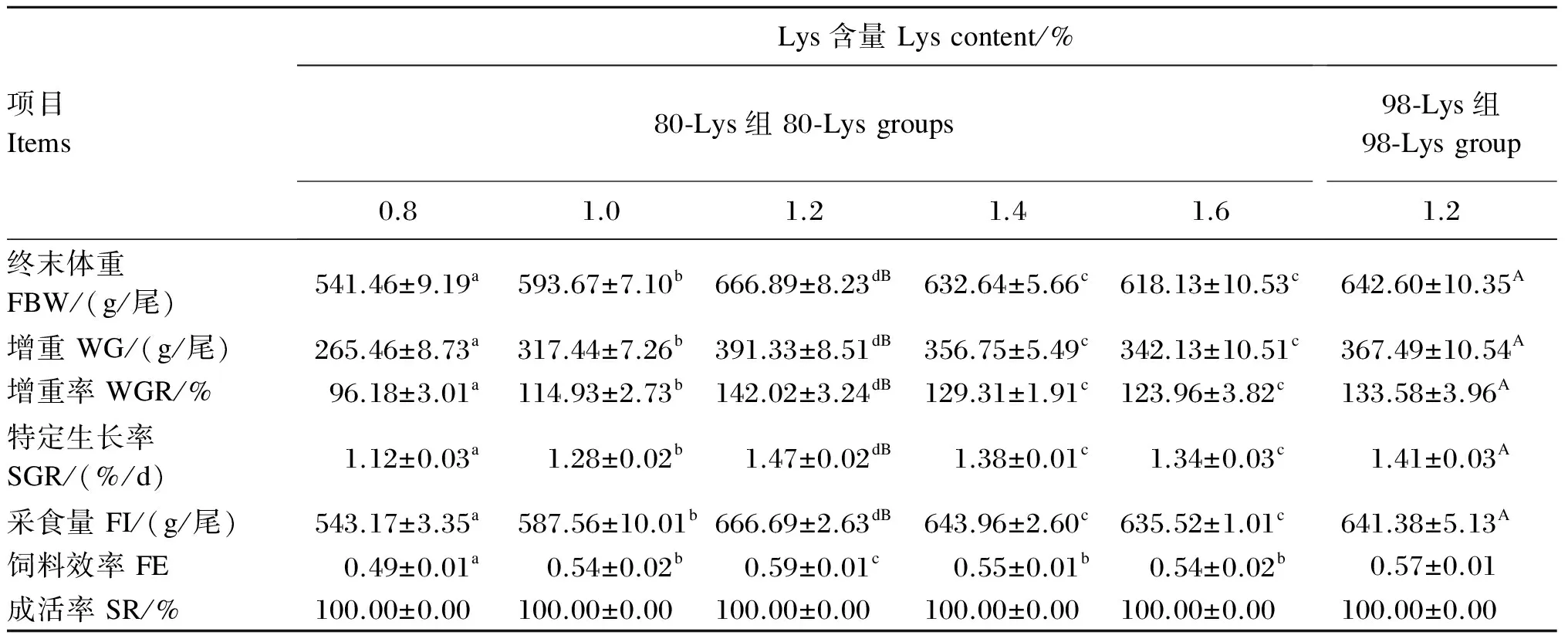
表3 80-Lys和98-Lys对生长中期草鱼生长性能的影响
同行数据肩标不同小写字母表示80-Lys组间差异显著(P<0.05),肩标不同大写字母表示80-Lys(1.2% Lys)组与98-Lys(1.2% Lys)组差异显著(P<0.05)。下表同。
In the same row, values with different small letter superscripts are significantly different among 80-Lys groups (P<0.05), and with different capital letter superscripts are significantly different between the 80-Lys (1.2% Lys) group and 98-Lys (1.2% Lys) group (P<0.05). The same as below.
2.2 80-Lys和98-Lys对生长中期草鱼全肠消化酶活力的影响
由表4可知,饲料中添加不同水平的80-Lys对生长中期草鱼全肠糜蛋白酶活力的影响不显著(P>0.05)。随饲料中80-Lys添加水平的增加,生长中期草鱼全肠胰蛋白酶活力先显著升高(P<0.05),在饲料中Lys含量为1.2%时达到最大值,而后随着饲料中80-Lys添加水平的进一步增加而显著降低(P<0.05)。不同添加水平的80-Lys对生长中期草鱼全肠脂肪酶和淀粉酶活力的影响与胰蛋白酶相似。在饲料中Lys含量均为1.2%的条件下,80-Lys组与98-Lys组相比,生长中期草鱼全肠脂肪酶和淀粉酶活力显著升高(P<0.05),而糜蛋白酶活力则显著降低(P<0.05),胰蛋白酶活力差异不显著(P>0.05)。
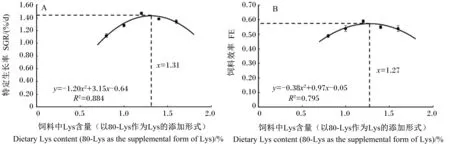
图1 饲料中Lys含量(以80-Lys作为Lys的添加形式)与生长中期草鱼特定生长率(A)或饲料效率(B)的二次曲线分析
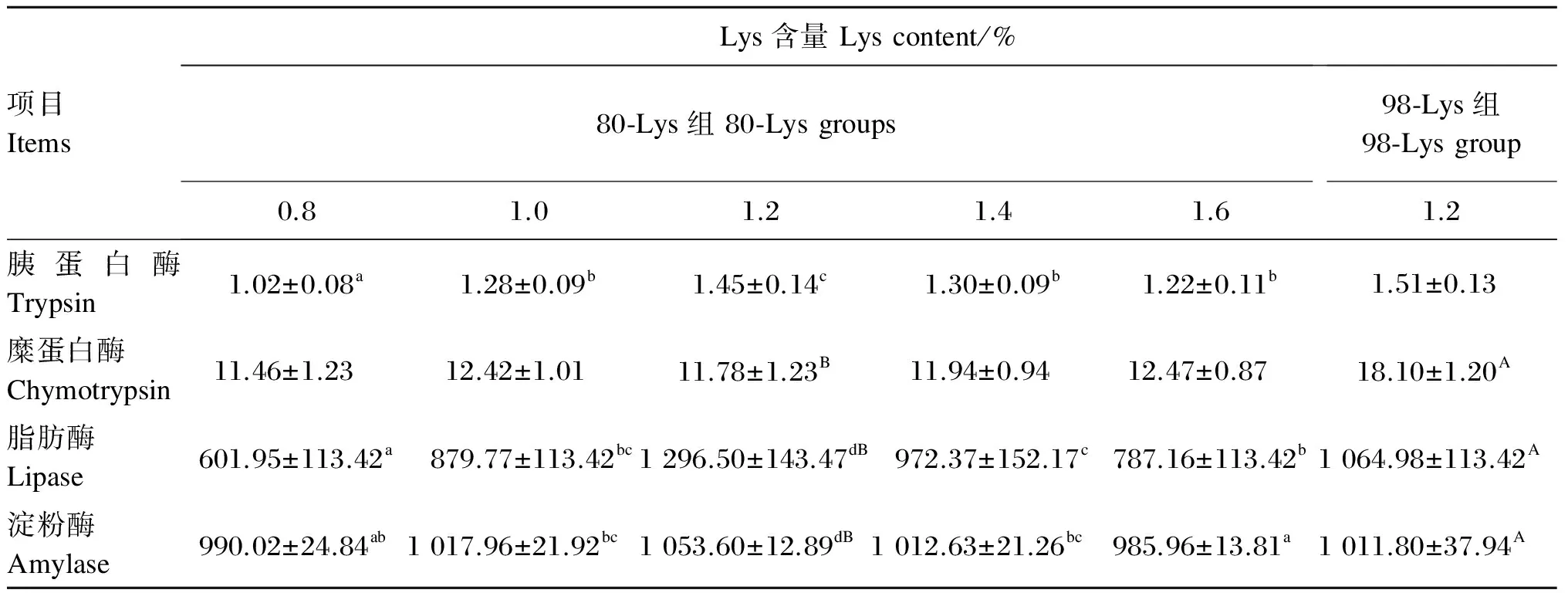
表4 80-Lys和98-Lys对生长中期草鱼肠道消化酶活力的影响
2.3 80-Lys和98-Lys对生长中期草鱼肠道刷状缘酶活力的影响
由表5可知,生长中期草鱼前、中、后肠AKP活力先随饲料中80-Lys添加水平的增加而显著升高(P<0.05),均在饲料中Lys含量为1.2%时达到最大值,而后随着饲料中80-Lys添加水平的进一步增加而显著降低(P<0.05)。饲料中添加不同水平的80-Lys对生长中期草鱼前、中、后肠CK活力,中、后肠γ-GT活力影响的变化趋势与前、中、后肠AKP相似。1.2% 80-Lys组生长中期草鱼前、中肠Na+,K+-ATPase活力均显著高于0.8%(基础饲料)、1.4%和1.6% 80-Lys组(P<0.05),但与1.0% 80-Lys组差异不显著(P>0.05)。1.2% 80-Lys组生长中期草鱼后肠Na+,K+-ATPase活力与前肠γ-GT活力显著高于0.8%、1.0%和1.6% 80-Lys组(P<0.05),但与1.4% 80-Lys组差异不显著(P>0.05)。在饲料中Lys含量均为1.2%的条件下,80-Lys组与98-Lys组相比,生长中期草鱼前、中、后肠AKP和CK活力均显著升高(P<0.05),而前、中、后肠Na+,K+-ATPase和γ-GT活力则差异不显著(P>0.05)。
以80-Lys作为Lys的添加形式,分别对饲料中Lys含量(x)和生长中期草鱼的前(yAKPPI)、中(yAKPMI)、后肠AKP活力(yAKPDI),后肠CK活力(yCKDI)以及中(yγ-GTMI)、后肠γ-GT活力(yγ-GTDI)进行回归分析,得到的回归方程分别为yAKPPI=-453.81x2+1 077.45x-487.36(R2=0.910,P<0.01)、yAKPMI=-612.86x2+1 499.27x-765.68(R2=0.971,P<0.01)、yAKPDI=-285.55x2+692.07x-352.23(R2=0.740,P<0.01)、yCKDI=-154.54x2+353.64x-96.88(R2=0.709,P<0.01)、yγ-GTMI=-185.1x2+469.02x-201.25(R2=0.748,P<0.01)、yγ-GTDI=-225.14x2+574.93x-275.62(R2=0.716,P<0.01)。
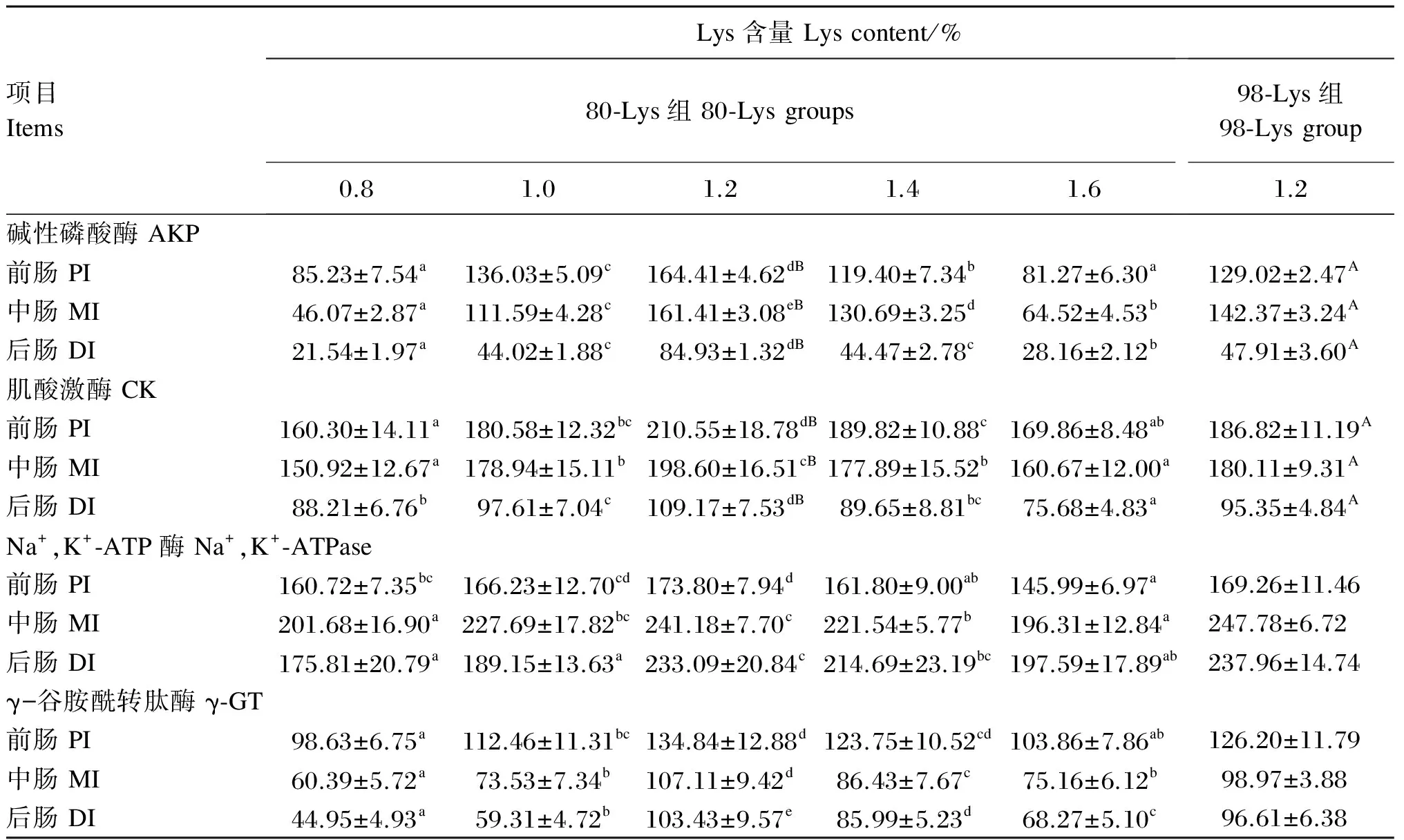
表5 80-Lys和98-Lys对生长中期草鱼肠道刷状缘酶活力的影响
2.4 80-Lys和98-Lys对生长中期草鱼血清、肝胰脏GOT和GPT活力的影响
由表6可知,生长中期草鱼血清GOT和GPT活力随饲料中80-Lys添加水平的增加先降低后升高,在饲料中Lys含量为1.2%时其血清GOT和GPT活力最低,其中GOT活力显著低于其他各组(P<0.05),GPT活力显著低于除1.0% 80-Lys组外的其他各组(P<0.05)。饲料中添加不同水平的80-Lys对生长中期草鱼肝胰脏GPT活力影响的变化趋势与血清GOT和GPT活力的变化趋势相反,以1.2% 80-Lys组肝胰脏GPT活力最高,显著高于其他各组(P<0.05)。1.2%和1.4% 80-Lys组生长中期草鱼肝胰脏GOT活力显著高于0.8%、1.0%和1.6% 80-Lys组(P<0.05),且1.2%和1.4% 80-Lys组间差异不显著(P>0.05)。在饲料中Lys含量均为1.2%的条件下,80-Lys组与98-Lys组相比,生长中期草鱼血清GOT和GPT活力均显著降低(P<0.05),而肝胰脏GOT和GPT活力则显著升高(P<0.05)。
以80-Lys作为Lys的添加形式,分别对饲料中Lys含量(x)和生长中期草鱼的肝胰脏(y肝胰脏GOT)和血清GOT活力(y血清GOT)进行回归分析,得到的回归方程分别为y肝胰脏GOT=-9.077x2+22.851x-7.002(R2=0.751,P<0.01)、y血清GOT=5.119x2-12.069x+8.289(R2=0.786,P<0.01)。
表680-Lys和98-Lys对生长中期草鱼血清、肝胰脏GOT和GPT活力的影响
Table 6 Effects of 80-Lys and 98-Lys on GOT and GPT activities in serum and hepatopancreas of young grass carp (Ctenopharyngodonidella)

项目ItemsLys含量Lyscontent/%80⁃Lys组80⁃Lysgroups0.81.01.21.41.698⁃Lys组98⁃Lysgroup1.2血清Serum/(U/mL)谷草转氨酶GOT1.85±1.50c1.45±1.15b0.96±0.65aA1.56±1.36b2.03±1.70d1.33±0.71B谷丙转氨酶GPT0.79±0.56b0.67±0.63a0.63±0.62aA0.83±0.67bc0.89±0.70c0.88±0.80B肝胰脏Hepatopancreas/(U/mg)谷草转氨酶GOT5.61±2.42a6.45±5.10b7.48±1.98cB8.49±6.91c6.22±4.19b6.41±4.04A谷丙转氨酶GPT6.04±2.04a6.19±4.42a7.03±5.51bB5.77±4.51a5.59±2.45a6.06±3.07A
2.5 80-Lys和98-Lys对生长中期草鱼肝胰脏和肠道生长发育的影响
由表7可知,生长中期草鱼肝胰脏重随饲料中80-Lys添加水平的增加先升高后降低,在饲料中Lys含量为1.2%时达到最高值,显著高于其他各组(P<0.05)。饲料中添加不同水平的80-Lys对生长中期草鱼肠长、肠长指数、肠重、肠体指数以及前、中、后肠皱襞高度影响的变化趋势与肝胰脏重相似。1.2% 80-Lys组生长中期草鱼肝体指数显著高于0.8%、1.4%和1.6% 80-Lys组(P<0.05),但与1.0% 80-Lys组差异不显著(P>0.05)。在饲料中Lys含量均为1.2%的条件下,80-Lys组与98-Lys组相比,生长中期草鱼肝胰脏重、肝体重指数、肠重、肠体指数以及前、中、后肠皱襞高度均显著升高(P<0.05),而肠长、肠长指数则差异不显著(P>0.05)。
以80-Lys作为Lys的添加形式,分别对饲料中Lys含量(x)和生长中期草鱼的肝胰脏重(y肝胰脏重)、肠重(y肠重)以及前(y前肠皱襞高度)、中(y中肠皱襞高度)、后肠皱襞高度(y后肠皱襞高度)进行回归分析,得到的回归方程分别为y肝胰重=-33.67x2+86.87x-35.32(R2=0.769,P<0.01)、y肠重=-40.92x2+101.02x-45.91(R2=0.795,P<0.01)、y前肠皱襞高度=-2 041.28x2+4 946.26x-1 691.77(R2=0.771,P<0.01)、y中肠皱襞高度=-1 070.57x2+2 616.35x-462.67(R2=0.790,P<0.01)、y后肠皱襞高度=-1 451.31x2+3 558.72x-1 128.55(R2=0.753,P<0.01)。
3 讨 论
3.1 80-Lys对生长中期草鱼生长性能的影响及其与98-Lys的比较研究
Lys是鱼类重要的必需氨基酸[26]。本试验结果表明:饲料中80-Lys的添加显著提高了生长中期草鱼的终末体重、WG、WGR和SGR,且饲料中Lys含量与生长中期草鱼的生长指标呈显著二次相关,说明添加适宜水平的80-Lys可以促进生长中期草鱼的生长。此外,与98-Lys比较,在饲料Lys含量均为1.2%的条件下,80-Lys对生长中期草鱼的促生长作用优于98-Lys。虽然目前未见80-Lys在鱼类上的研究报道,但本试验结果与其他形式Lys在其他生长阶段草鱼或其他鱼上的研究结果类似,如生长后期草鱼(L-Lys·H2SO4,Lys含量=51%)[1]、草鱼幼鱼(晶体L-Lys)[27-28]、建鲤(Cyprinuscarpiovar. Jian,包被Lys,Lys含量=70.04%)[2]、尼罗罗非鱼(L-Lys)[5]、太平洋鲅(Polydactylussexfilis,L-Lys)[3]、石斑鱼(Epinepheluscoioides,L-Lys·HCl)[29]、大菱鲆(Scophthalmusmaximus,L-Lys·HCl)[30]、大黄鱼(PseudosciaenacroceaR.,L-Lys·HCl)[4]等。然而,关于饲料中添加晶体L-Lys对草鱼生长性能的影响也存在不一致的报道,有研究则显示饲料中添加晶体L-Lys对草鱼幼鱼终末体重及WGR没有显著影响[31]。其不一致的原因可能是由于本试验增加了投喂频率,提高了氨基酸吸收的同步性,进而提高了生长中期草鱼对80-Lys及98-Lys的利用效率。鱼类的生长依赖于营养物质的摄入,而饲料中必需氨基酸的含量与动物的FI密切相关[2,32],因此,饲料中添加80-Lys的促生长作用可能与其调节鱼类FI有关。本研究结果表明:随着饲料中80-Lys添加水平的增加,生长中期草鱼的FI和FE先升高后降低,在饲料中Lys含量均为1.2%时达到最大值。在饲料中Lys含量均为1.2%的条件下,与98-Lys组比较,80-Lys组生长中期草鱼的FI显著提高。Wang等[27]在草鱼幼鱼上的研究结果与本试验有相似,即在饲料中添加适宜水平的晶体L-Lys,草鱼幼鱼的FE显著提高。这些结果说明,80-Lys可通过提高鱼类的FI和FE促进生长,且作用效果优于98-Lys。以80-Lys为Lys添加形式,分别以SGR和FE为标识,生长中期草鱼(276~667 g)饲料中Lys的最适含量分别为1.31%(占饲料蛋白质的4.68%)和1.27%(占饲料蛋白质的4.54%)。
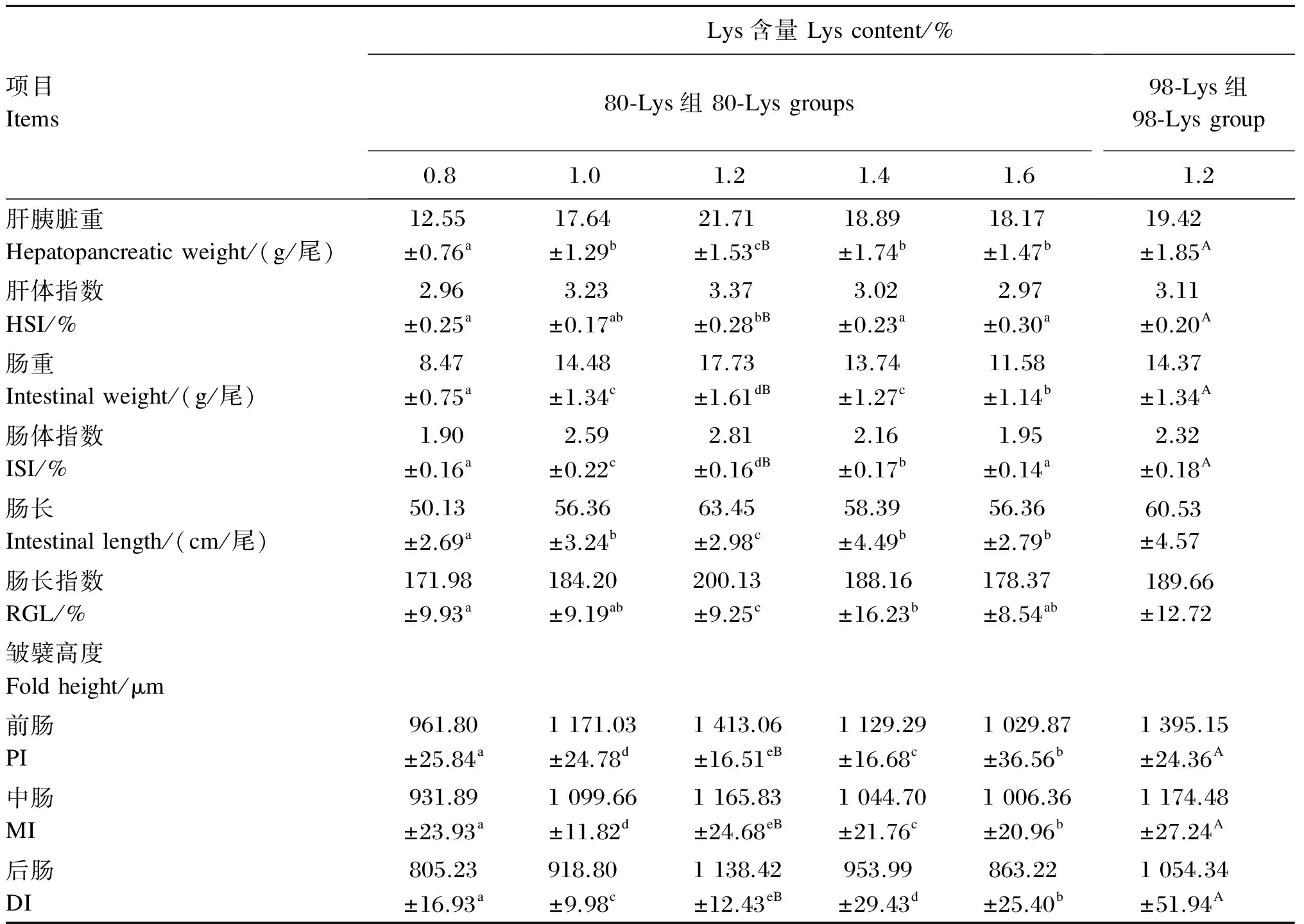
表7 80-Lys和98-Lys对生长中期草鱼肝胰脏和肠道生长发育的影响
3.2 80-Lys对生长中期草鱼消化吸收能力及机体和肝胰脏氨基酸代谢相关指标的影响及其与98-Lys的比较研究
鱼类消化吸收能力的强弱是影响鱼类FI和FE的重要因素[24]。鱼类肠道内消化酶的活力是反映鱼类消化能力的重要指标[33]。本试验结果表明:饲料中添加适宜水平的80-Lys可显著提高生长中期草鱼全肠胰蛋白酶、脂肪酶和淀粉酶的活力。在饲料中Lys含量均为1.2%的条件下,与98-Lys比较,80-Lys可显著提高生长中期草鱼全肠脂肪酶和淀粉酶活力,显著降低糜蛋白酶活力,胰蛋白酶活力则无显著变化。目前,关于80-Lys对鱼类消化能力影响的研究未见报道,但在其他形式的Lys上有相关报道。本实验室前期在L-Lys·H2SO4(Lys含量=51%)对生长后期草鱼肠道消化酶活力上的研究结果[1]与本试验结果相似。研究发现,饲料中添加适宜水平的L-Lys·HCl可显著提高鳡鱼(Elopichthysbambusa)幼鱼肠道蛋白酶活力[34]。但是,本试验中饲料中80-Lys添加水平对生长中期草鱼胰蛋白酶活力没有产生显著影响,与L-Lys·H2SO4(Lys含量=51%)在生长后期草鱼上的研究结果[1]存在差异。其可能原因为,本试验饲料基于商业饲料,其基础饲料中Lys含量(0.8%)可能已满足生长中期草鱼糜蛋白酶合成、分泌和酶原激活的需要。Lys影响鱼类消化酶活力可能与消化酶的分泌和释放有关。在金黄色石斑鱼上的研究发现Lys可作为鱼类胰腺释放胰蛋白酶原的刺激物[35]。这些结果说明,80-Lys可能比98-Lys能更有效地提高鱼类消化脂肪和淀粉的能力,进而比98-Lys能更有效地提高鱼类的FI,促进生长。
鱼类肠道也是其营养物质吸收的主要场所,其对营养物质的吸收与肠道刷状缘酶活力密切相关[24]。AKP是一种膜结合蛋白,可以分解正磷酸单酯为机体的磷代谢和核酸代谢提供磷酸基团,在生物体内分布广泛。分布于动物肠道上皮刷状缘细胞表面的肠型碱性磷酸酶(IAKP)与维生素D、钙、氨基酸、胆固醇、脂类和葡萄糖等多种营养物质吸收有关[36-37]。CK为催化肌酸和ATP偶联的促ATP生成酶,可为动物肠道内为营养物质的吸收提供能量[38]。Na+,K+-ATPase直接影响动物肠道营养物质主动转运过程的能量供给,参与大多数氨基酸和葡萄糖的吸收[39]。γ-GT是谷氨酸循环的关键酶,能促进氨基酸向细胞内转运,为蛋白质生物合成提供原料[40]。因此,常用肠道AKP、CK、Na+,K+-ATPase和γ-GT活力反映动物对营养物质的吸收能力。本试验结果表明,饲料中添加适宜水平的80-Lys可显著提高生长中期草鱼前、中、后肠AKP、CK、Na+,K+-ATPase和γ-GT活力,与本实验室前期以L-Lys·H2SO4(Lys含量=51%)作为Lys添加形式在生长后期草鱼上的研究结果[1]类似。然而,在饲料中Lys含量均为1.2%的条件下,与98-Lys相比,80-Lys仅显著提高了生长中期草鱼前、中、后肠AKP和CK活力。这些结果说明,80-Lys可能通过提高鱼类肠道刷状缘酶AKP和CK活力增强其肠道对营养物质的吸收,且与98-Lys相比,80-Lys更有效。
GOT和GPT是鱼体内氨基酸代谢的重要酶类,鱼类肝脏GOT和GPT活力是反映鱼类肝脏氨基酸代谢的重要指标[41]。本试验结果表明,随着饲料中80-Lys添加水平的增加,生长中期草鱼肝胰脏GOT和GPT活力也随之提高,当饲料Lys含量分别为1.4%和1.2%时,生长中期草鱼肝胰脏GOT和GPT活力分别达到最高,说明饲料中适宜添加水平的80-Lys增强了生长中期草鱼肝脏氨基酸的代谢作用。此外,在饲料中Lys含量均为1.2%的条件下,与98-Lys比较,80-Lys提高了生长中期草鱼肝胰脏氨基酸的代谢作用。关于Lys对水生动物肝脏GOT和GPT活力影响的研究有少量报道。研究显示,饲料中添加适宜水平的L-Lys·H2SO4(Lys含量=51%)可显著提高生长后期草鱼肝胰脏GOT活力[1],饲料中添加适宜水平的L-Lys可显著提高三疣梭子蟹(Portunustrituberculatus)肝胰腺GOT和GPT活力[42]。在幼建鲤上的研究发现,肝胰脏GOT活力与鱼体蛋白质沉积率呈显著正相关[43]。徐静[44]研究表明,适宜水平的蛋白质可能通过增强生长中期草鱼肝胰脏氨基酸的代谢,提高其对饲料蛋白质的利用率。因此,80-Lys可能比98-Lys能更有效地提高氨基酸的代谢,增强饲料蛋白质的利用率,进而促进鱼体生长,但其具体作用机制需要进一步研究。
3.3 80-Lys对生长中期草鱼消化器官生长发育的影响及其与98-Lys的比较研究
肝胰脏和肠道是鱼类最重要的消化吸收及营养物质代谢器官,鱼类消化吸收能力及氨基酸代谢与其消化器官的生长发育密切相关。本试验结果表明,饲料中添加适宜水平的80-Lys能显著增加生长中期草鱼肝胰重、肝体指数、肠重、肠体脂数和肠长指数。此外,在饲料中Lys含量均为1.2%的条件下,与98-Lys组相比,80-Lys组生长中期草鱼肝胰重、肝体指数、肠重和肠体指数显著提高。在草鱼幼鱼上的研究发现,饲料添加适宜水平的晶体L-Lys后草鱼幼鱼的肝体指数显著提高[27],与本试验结果相似。肠道皱襞高度是反映鱼类肠道生长发育及其肠道吸收面积的另一重要指标,与鱼类的吸收能力密切相关。本试验结果表明,饲料中添加适宜水平的80-Lys可显著提高生长中期草鱼前、中、后肠的皱襞高度,且在饲料中Lys含量均为1.2%的条件下80-Lys组生长中期草鱼前、后肠的皱襞高度显著高于98-Lys组。此外,肝脏细胞结构功能正常是鱼类消化和营养物质代谢的重要保障[45]。细胞通透性变化是细胞损伤的标志之一,胞浆酶GOT和GPT释放量可以反映出胞内酶的渗漏性,为肝细胞受损的特征性酶谱[46]。本试验结果表明,饲料中添加适宜水平的80-Lys可显著降低生长中期草鱼血清GOT和GPT活力,且在饲料中Lys含量均为1.2%的条件下,与98-Lys组相比,80-Lys组生长中期草鱼血清GOT和GPT活力显著降低。相似的研究表明,饲料中添加适宜水平的L-Lys·HCl显著降低鳡鱼幼鱼血清GOT和GPT活力[34]。这些结果说明,80-Lys可能比98-Lys能更有效地促进鱼类消化器官的生长发育,增加鱼类肠道吸收面积,保障鱼类肝脏细胞的结构功能正常,但其具体作用方式有待研究。
4 结 论
① 与98-Lys相比,80-Lys能更有效地提高生长中期草鱼的生长性能。
② 与98-Lys相比,80-Lys能更有效地保护生长中期草鱼肝胰脏结构完整性,增加肠道吸收面积,促进消化器官生长发育,提高消化吸收能力,进而增加FI,促进其生长。
③ 以80-Lys作为Lys的添加形式,以SGR和FE为标识,生长中期草鱼(276~667 g)饲料中赖氨酸的最适含量分别为1.31%(占饲料蛋白质的4.68%)和1.27%(占饲料蛋白质的4.54%)。
[1] LI X Y,TANG L,HU K,et al.Effect of dietary lysine on growth,intestinal enzymes activities and antioxidant status of sub-adult grass carp (Ctenopharyngodonidella)[J].Fish Physiology and Biochemistry,2014,40(3):659-671.
[2] ZHOU X Q,ZHAO C R,JIANG J,et al.Dietary lysine requirement of juvenile Jian carp (Cyprinuscarpiovar. Jian)[J].Aquaculture Nutrition,2008,14(5):381-386.
[3] DENG D F,DOMINY W,JU Z Y,et al.Dietary lysine requirement of juvenile Pacific threadfin (Polydactylussexfilis)[J].Aquaculture,2010,308(1/2):44-48.
[4] ZHANG C X,AI Q H,MAI K S,et al.Dietary lysine requirement of large yellow croaker,PseudosciaenacroceaR[J].Aquaculture,2008,283(1/2/3/4):123-127.
[5] MICHELATO M,DE OLIVEIRA VIDAL L,XAVIER T O,et al.Dietary lysine requirement to enhance muscle development and fillet yield of finishing Nile tilapia[J].Aquaculture,2016,457:124-130.
[6] ZHOU F,SHAO Q J,XIAO J X,et al.Effects of dietary arginine and lysine levels on growth performance,nutrient utilization and tissue biochemical profile of black sea bream,Acanthopagrusschlegelii,fingerlings[J].Aquaculture,2011,319(1/2):72-80.
[7] ZHOU X Q,ZHAO C R,LIN Y.Compare the effect of diet supplementation with uncoated or coated lysine on juvenile Jian carp (Cyprinuscarpiovar. Jian)[J].Aquaculture Nutrition,2007,13(6):457-461.
[8] 朱进龙,臧建军,曾祥芳,等.80赖氨酸与70赖氨酸和98赖氨酸对生长肥育猪饲喂效果的比较研究[J].中国畜牧杂志,2014,50(21):27-31.
[9] NIU J,CHEN X,LIN H Z,et al.Comparison ofL-lysine·HCl andL-lysine sulphate in the feed ofPenaeusmonodonand re-evaluation of dietary lysine requirement forP.monodon[J].Aquaculture Research,2017,48(1):134-148.
[10] 赵金鑫,李小勤,彭松,等.斑点叉尾鲖对不同形式赖氨酸利用的比较研究[J].水生生物学报,2016,40(1):19-26.
[11] RODEHUTSCORD M,BORCHERT F,GREGUS Z,et al.Availability and utilisation of free lysine in rainbow trout (Oncorhynchusmykiss):2.Comparison ofL-lysine·HCl and L-lysine sulphate[J].Aquaculture,2000,187(1/2):177-183.
[12] SMIRICKY-TJARDES M R,MAVROMICHALIS I,ALBIN D M,et al.Bioefficacy ofL-lysine sulfate compared with feed-gradeL-lysine·HCl in young pigs[J].Journal of Animal Science,2004,82(9):2610-2614.
[13] AHMAD G,MUSHTAQ T,MIRZA M A,et al.Comparative bioefficacy of lysine fromL-lysine hydrochloride orL-lysine sulfate in basal diets containing graded levels of canola meal for female broiler chickens[J].Poultry Science,2007,86(3):525-530.
[14] ANDERSON L C,LEWIS A J,PEO E R,Jr.,et al.Effect of various dietary arginine:lysine ratios on performance,carcass composition and plasma amino acid concentrations of growing-finishing swine[J].Journal of Animal Science,1984,58(2):362-368.
[15] ALAM M S,TESHIMA S I,ISHIKAWA M,et al.Effects of dietary arginine and lysine levels on growth performance and biochemical parameters of juvenile Japanese flounderParalichthysolivaceus[J].Fisheries Science,2002,68(3):509-516.
[16] 农业部渔业渔政管理局.中国渔业统计年鉴2016[M].北京:中国农业出版社,2016.
[17] NRC.Nutrient requirements of fish and shrimp[S].Washington,D.C.:National Academies Press,2011.
[18] FENG L,LUO J B,JIANG W D,et al.Changes in barrier health status of the gill for grass carp (Ctenopharyngodonidella) during valine deficiency:regulation of tight junction protein transcript,antioxidant status and apoptosis-related gene expression[J].Fish & Shellfish Immunology,2015,45(2):239-249.
[19] XU H J,JIANG W D,FENG L,et al.Dietary vitamin C deficiency depressed the gill physical barriers and immune barriers referring to Nrf2,apoptosis,MLCK,NF-κB and TOR signaling in grass carp (Ctenopharyngodonidella) under infection ofFlavobacteriumcolumnare[J].Fish & Shellfish Immunology,2016,58:177-192.
[20] XU H J,JIANG W D,FENG L,et al.Dietary vitamin C deficiency depresses the growth,head kidney and spleen immunity and structural integrity by regulating NF-κB,TOR,Nrf2,apoptosis and MLCK signaling in young grass carp (Ctenopharyngodonidella)[J].Fish & Shellfish Immunology,2016,52:111-138.
[21] GERAYLOU Z,SOUFFREAU C,RURANGWA E,et al.Effects of dietary arabinoxylan-oligosaccharides (AXOS) and endogenous probiotics on the growth performance,non-specific immunity and gut microbiota of juvenile Siberian sturgeon (Acipenserbaerii)[J].Fish & Shellfish Immunology,2013,35(3):766-775.
[22] HUANG S S Y,STRATHE A B,WANG W F,et al.Selenocompounds in juvenile white sturgeon:evaluating blood,tissue,and urine selenium concentrations after a single oral dose[J].Aquatic Toxicology,2012,109:158-165.
[23] LIN Y,ZHOU, X Q.Dietary glutamine supplementation improves structure and function of intestine of juvenile Jian carp (Cyprinuscarpiovar. Jian)[J].Aquaculture,2006,256(1/2/3/4):389-394.
[24] LI S Q,FENG L,JIANG W D,et al.Deficiency of dietary niacin decreases digestion and absorption capacities via declining the digestive and brush border enzyme activities and downregulating those enzyme gene transcription related to TOR pathway of the hepatopancreas and intestine in young grass carp (Ctenopharyngodonidella)[J].Aquaculture Nutrition,2016,22(6):1267-1282.
[25] 唐凌,孙崇岩,邝声耀,等.晶体色氨酸和包膜色氨酸对幼建鲤生长性能、蛋白质代谢及消化吸收能力影响的比较[J].动物营养学报,2014,26(2):411-419.
[26] NGUYEN L,DAVIS D A.Comparison of crystalline lysine and intact lysine used as a supplement in practical diets of channel catfish (Ictaluruspunctatus) and Nile tilapia (Oreochromisniloticus)[J].Aquaculture,2016,464:331-339.
[27] WANG S,LIU Y J,TIAN L X,et al.Quantitative dietary lysine requirement of juvenile grass carpCtenopharyngodonidella[J].Aquaculture,2005,249(1/2/3/4):419-429.
[28] GAN L,LIU Y J,TIAN L X,et al.Effects of dissolved oxygen and dietary lysine levels on growth performance,feed conversion ratio and body composition of grass carp,Ctenopharyngodonidella[J].Aquaculture Nutrition,2013,19(6):860-869.
[29] LUO Z,LIU Y J,MAI K S,et al.QuantitativeL-lysine requirement of juvenile grouperEpinepheluscoioides[J].Aquaculture Nutrition,2006,12(3):165-172.
[30] PERES H,OLIVA-TELES A.Lysine requirement and efficiency of lysine utilization in turbot (Scophthalmusmaximus) juveniles[J].Aquaculture,2008,275(1/2/3/4):283-290.
[31] 刘永坚,田丽霞,刘栋辉,等.实用饲料补充结晶或包膜赖氨酸对草鱼生长、血清游离氨基酸和肌肉蛋白质合成率的影响[J].水产学报,2002,26(3):252-258.
[32] TOME D.Protein,amino acids and the control of food intake[J].British Journal of Nutrition,2004,92(S1):S27-S30.
[33] RAY A K,GHOSH K,RINGØ E.Enzyme-producing bacteria isolated from fish gut:a review[J].Aquaculture Nutrition,2012,18(5):465-492.
[34] 杨威,樊启学,宗克金,等.鳡幼鱼对晶体氨基酸的利用效果及赖氨酸需求量的研究[J].动物营养学报,2012,24(7):1255-1263.
[35] NAZ M,TÜRKMEN M.Changes in the digestive enzymes and hormones of gilthead seabream larvae (Sparusaurata,L.1758) fed onArtemianauplii enriched with free lysine[J].Aquaculture International,2009,17(6):523-535.
[37] 张继平,林建成,谢进金,等.草鱼碱性磷酸酶的分离纯化与部分性质研究[J].厦门大学学报:自然科学版,2005,44(5):684-687.
[38] YUAN J,FENG L,JIANG W D,et al.Effects of dietary vitamin K levels on growth performance,enzyme activities and antioxidant status in the hepatopancreas and intestine of juvenile Jian carp (Cyprinuscarpiovar. Jian)[J].Aquaculture Nutrition,2016,22(2):352-366.
[39] ALMANSA E,SANCHEZ J,COZZI S,et al.Segmental heterogeneity in the biochemical properties of the Na+-K+-ATPase along the intestine of the gilthead seabream (SparusaurataL.)[J].Journal of Comparative Physiology B,2001,171(7):557-567.
[40] KOVACS-NOLAN J,RUPA P,MATSUI T,et al.InVitroandexvivouptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH afterinvivosupplementation[J].Journal of Agricultural and Food Chemistry,2014,62(39):9499-9506.
[41] ABDEL-TAWWAB M,AHMAD M H,KHATTAB Y A E,et al.Effect of dietary protein level,initial body weight,and their interaction on the growth,feed utilization,and physiological alterations of Nile tilapia,Oreochromisniloticus(L.)[J].Aquaculture,2010,298(3/4):267-274.
[42] JIN M,WANG M Q,HUO Y W,et al.Dietary lysine requirement of juvenile swimming crab,Portunustrituberculatus[J].Aquaculture,2015,448:1-7.
[43] 何伟.吡哆醇对幼建鲤消化吸收能力和免疫能力的影响[D].硕士学位论文.雅安:四川农业大学,2008.
[44] 徐静.蛋白对生长中期草鱼生产性能、肠道、机体和鳃健康及肌肉品质的作用及其作用机制[D].硕士学位论文.雅安:四川农业大学,2016.
[45] WU P,LIU Y,JIANG W D,et al.A Comparative study on antioxidant system in fish hepatopancreas and intestine affected by choline deficiency:different change patterns of varied antioxidant enzyme genes and Nrf2 signaling factors[J].PLoS One,2017,12(1):e169888.
[46] 姚仕彬,叶元土,蔡春芳,等.酵母培养物水溶物对离体草鱼肠道黏膜细胞生长及细胞膜完整性的影响[J].动物营养学报,2014,26(11):3478-3484.
AComparativeStudy:Effectsof80%L-lysine·H2SO4and98%L-lysine·HClonGrowthPerformance,DigestionandAbsorptionCapacitiesandGrowthDevelopmentofDigestiveOrgansofYoungGrassCarp(Ctenopharyngodonidella)
HU Kai1,2SU Yuening1FENG Lin1,3,4LIU Yang1,3,4JIANG Weidan1,3,4WU Pei1,3,4JIANG Jun1,3,4ZHOU Xiaoqiu1,3,4*
(1.AnimalNutritionInstitute,SichuanAgriculturalUniversity,Chengdu611130,China; 2.DepartmentofAnimalandVeterinaryScience,ChengduAgriculturalCollege,Chengdu611130,China; 3.FishNutritionandSafetyProductionUniversityKeyLaboratoryofSichuanProvince,SichuanAgriculturalUniversity,Chengdu611130,China; 4.KeyLaboratoryforAnimalDisease-ResistanceNutritionofChinaMinistryofEducation,SichuanAgriculturalUniversity,Chengdu611130,China)
This study aimed to compare the effects of 80%L-lysine·H2SO4(80-lysine) and 98%L-lysine·HCl (98-lysine) on growth performance, digestion and absorption capacities and growth development of digestive organs of young grass carp (Ctenopharyngodonidella), to explore the biological values of 98-lysine and 98-lysine, and to determine the optimum dietary lysine content using 80-lysine as the supplemental form of lysine. A total of 540 grass carp with an initial body weight about 275.80 g were randomly divided into 6 groups with 3 replicates each and 30 fish per replicate. Fish in 6 groups were fed adding 80-lysine diets containing 0.8% (basal diet), 1.0%, 1.2%, 1.4% and 1.6% lysine and adding 98-lysine diet containing 1.2% lysine for 60 d, respectively. The results showed as follows: compared with the basal diet, dietary lysine content reached 1.2% by adding suitable level of 80-lysine could significantly increase the weight gain rate (WGR), specific growth rate (SGR), feed intake (FI), the activities of lipase, amylase in total intestine, the activities of glutamic oxalacetic transaminase (GOT) and glutamate pyruvate transaminase (GPT) in hepatopancreas, the activities of alkaline phosphatase (AKP) and creatine kinase (CK)in proximal intestine (PI), mid intestine (MI) and distal intestine (DI), hepatosomatic index (HSI), intestosomatic index (ISI) and the fold height of PI and DI (P<0.05), and significantly decrease the activities of GOT and GPT in serum (P<0.05); the effects of 80-lysine on these parameters were better than 98-lysine (P<0.05). Moreover, compared with the basal diet, dietary lysine content reached 1.2% by adding suitable level of 80-lysine could significantly increase the feed efficiency (FE), total intestine trypsin activity, the activities of Na+, K+-ATPase and γ-glutamyltransferase (γ-GT) in PI, MI and DI, intestinal length, relative gut length (RGL) and fold height in MI (P<0.05); however, compared with 98-lysine, no significant effects of 80-lysine were observed on these parameters (P>0.05). In conclusion, 80-lysine is more effective than 98-lysine for improving growth, and digestion and absorption capacities of young grass carp. Using 80-lysine as the supplemental form of lysine, the optimum dietary lysine content for young grass carp (276 to 667 g) base on SGR and FE is 1.31% (4.68% of dietary protein) and 1.27% (4.54% of dietary protein), respectively.[ChineseJournalofAnimalNutrition,2017,29(12):4372-4385]
80%L-lysine·H2SO4; 98%L-lysine·HCl; grass carp (Ctenopharyngodonidella); growth performance; digestion and absorption capacities
10.3969/j.issn.1006-267x.2017.12.018
S963
A
1006-267X(2017)12-4372-14
2017-05-05
四川省重大科技成果转化示范(2015CC0011);四川省青年创新团队(2017TD0002)
胡 凯(1981—),男,四川雅安人,博士,从事动物营养与饲料科学研究。E-mail: linaturehu@126.com
*通信作者:周小秋,教授,博士生导师,E-mail: zhouxq@sicau.edu.cn
*Corresponding author, professor, E-mail: zhouxq@sicau.edu.cn
(责任编辑 菅景颖)
