Transepithelial Transport of Milk-Derived Peptide QEPV in Vitro and in Vivo
2017-12-11LIWanruCHENJingCHENGZhicaiSHENPengGAOYangLIXianZHANGShaohui
LI Wanru, CHEN Jing, CHENG Zhicai, SHEN Peng, GAO Yang, LI Xi’an, ZHANG Shaohui,3,*
(1. School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China;2. Zhejiang Panda Dairy Group Co. Ltd., Wenzhou 325800, China;3. SJTU-Bor S. Luh Food Safety Research Center, Shanghai Jiao Tong University, Shanghai 200240, China)
Transepithelial Transport of Milk-Derived Peptide QEPV in Vitro and in Vivo
LI Wanru1, CHEN Jing1, CHENG Zhicai1, SHEN Peng1, GAO Yang1, LI Xi’an2, ZHANG Shaohui1,3,*
(1. School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China;2. Zhejiang Panda Dairy Group Co. Ltd., Wenzhou 325800, China;3. SJTU-Bor S. Luh Food Safety Research Center, Shanghai Jiao Tong University, Shanghai 200240, China)
Fermented milk benef i ts human health in many respects. Gln-Glu-Pro-Val (QEPV) is a peptide produced through enzymatic hydrolysis of the milk-derived peptide Gln-Glu-Pro-Val-Leu. QEPV elicits immunomodulatory effects on lymphocytes as a bioactive peptide. In this research, the transepithelial transport of QEPV was investigated in vitro and in vivo by using the Caco-2 cell monolayer model and mouse models. Results showed that QEPV exhibited good stability and it could integrally cross the Caco-2 cell monolayer in vitro. A limited transepithelial transport rate was observed at the concentration of 3.00 mg/mL in the Caco-2 cell monolayer model. Besides, QEPV was not detected in Caco-2 cells by both flow cytometry (FCM) and ultra performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry (UPLC-Q-TOF-MS). Therefore, QEPV could be absorbed as a bioactive peptide via the paracellular pathway in vitro. The in vivo experiments demonstrated that QEPV could be absorbed by mice via gastrointestinal and peritoneal routes. QEPV was less eff i ciently absorbed from the intestine than from the peritoneal cavity.
bioactive peptide; Caco-2 cell monolayer model; mouse model; transepithelial transport
Milk proteins serve as important sources of bioactive peptides that provide beneficial effects on human health when they are released during gastrointestinal digestion or food processing; milk proteins also play basic macro- and micro-nutritional roles[1-2]. Milk protein-derived bioactive peptides can help regulate nervous, gastrointestinal,cardiovascular, and immune systems[3-5]. These peptides also exhibit antihypertensive, antidiabetic, antioxidant,immunomodulatory and mineral-binding properties[6-8].Thus, these bioactive peptides have been investigated in terms of scientif i c research and applications, and recently the mechanism research of their functions becomes more and more attractive[9-10].
Oral delivery is the preferred mode of bioactive peptide administration. The absorption and transport of bioactive peptides across the intestinal epithelium in vivo are essential processes for their proper function. Human colonic adenocarcinoma (Caco-2) cell lines are widely used as in vitro models of small intestinal epithelial cells because these cell lines are functionally similar to fully differentiated enterocytes[11-12]. Differentiated Caco-2 cells are broadly applied to investigate the intestinal absorption and transport of bioactive peptides and peptide drugs. Augustijns et al.[13]used highly sensitive reversedphase high performance liquid chromatography (HPLC) to determine a mixture of fl uorescent marker compounds in the Caco-2 system with programmed wavelength fluorescence detection; they successfully applied this method to assure the quality of Caco-2 monolayers in terms of integrity and P-glycoprotein functionality. Osborne et al.[14]examined the transepithelial transport of β-casomorphin-7 (β-casein[60-66],YPFPGPI (Tyr-Pro-Phe-Pro-Gly-Pro-Ile) and β-CM7) and its metabolites (YP (Tyr-Pro), GPI (Gly-Pro-Ile) and FPGPI(Phe-Pro-Gly-Pro-Ile)) by Caco-2 cells. They found that YP,GPI, FPGFI and β-CM7 could transport across the Caco-2 monolayer. The transport rates of YP and GPI were higher than the other peptides.
Lactobacillus-fermented milk can promote human health by reducing the risk of chronic diseases or by enhancing natural immune protection[15-16]. In our previous work,one peptide, isolated from the fermented milk produced by Lactobacillus delbrueckii ssp. bulgaricus LB340, was identified as Gln-Glu-Pro-Val-Leu (QEPVL). QEPV, the digestion product of QEPVL, was demonstrated to be resistant to digestion. QEPVL and QEPV could signif i cantly activate lymphocytes and promote lymphocyte proliferation in vitro and in vivo[17]. In present work, the transepithelial transport of QEPV in vitro and in vivo was investigated by using the Caco-2 cell monolayer model and the mouse model.
1 Materials and Methods
1.1 Animal, materials and reagents
Six-week-old male BALB/c mice were purchased from Shanghai Laboratory Animal Center, Chinese Academy Sciences (Shanghai, China) and grown at (25 ± 1) ℃ with 60% relative humidity under controlled lighting from 8:30 to 20:30. Animal experiments were performed in compliance with the protocol approved by the Institutional Animal Care and Use Committee at Shanghai Jiao Tong University (Animal Use License NO. is SYXK (HU) 2013-0052) and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23).
Synthetic QEPV and fl uorescein isothiocyanate (FTIC)-QEPV peptides were purchased from Top Peptide Co. Ltd.(Shanghai, China). The Caco-2 cell lines were preserved by Cell Resource Center, Shanghai Institutes for Biological Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, KeyGEN BioTECH Co. Ltd. (Nanjing, China)) supplemented with 10% (V/V)fetal bovine serum (FBS, HyClone, Logan, UT, USA) and 1% (m/m) nonessential amino acids (NEAA) were incubated at(37 ± 1) ℃ with 5% CO2and 95% relative humidity in a Thermo 3000 Series incubator.
Sodium fluorescein China Peptides Corporation(Shanghai, China); trypsin solution KeyGEN BioTECH Co. Ltd. (Nanjing, China); red blood cell lysis buffer Sigma-Aldrich (St. Louis, MO, USA).
1.2 Instruments and equipments
3000 Series incubator Thermo Fisher Scientific(Waltham, MA, USA); 3422 Transwell chamber (12-well plate with polycarbonate membrane) Corning Costar Corp(NY, USA); ERS-2 Volt-Ohm meter Merck Millipore(Bedford, MA, USA); Infinite M200 Pro microplate reader Tecan Group Ltd. (Männedorf, Switzerland); TDL-40B centrifuge Shanghai Anting Scientif i c Instrument Factory(Shanghai, China); Accuri C6 flow cytometer with CFlow Plus Software BD Biosciences (San Jose, CA, USA); ultra performance liquid chromatography and quadrupole-timeof-flight mass spectrometer (UPLC-Q-TOF-MS) Waters Corporation (Milford, MA, USA).
1.3 Methods
1.3.1 The establishment of Caco-2 cell monolayer model
The Caco-2 cells were seeded into a transwell apparatus at a cell density of 1 × 105cells/mL and cultured in growth medium for 21 d with the medium changed every two days at the fi rst week and changed every day for the last two weeks.The integrity of Caco-2 cell monolayers was monitored by measuring the transepithelial electrical resistance (TEER)using Millicell ERS-2 Volt-Ohm Meter[18]. The control was placed in a transwell apparatus without being seeded with Caco-2 cells.
Sodium fl uorescein fl ux was measured as an additional control for the monolayer integrity[13]. Briefly, on the Day 21, 0.5 mL of sodium fl uorescein (2.0 mg/mL, diluted with Hank’s Balanced Salt Solution (HBSS)) was added to the apical (AP) side and 1.5 mL of pre-warmed HBSS was added to the basolateral (BL) side. After 90 min of incubation at 37 ℃, the samples from BL side were collected and detected OD value by the multifunctional microplate reader at 490 nm on the UV visible spectrum.
1.3.2 Transport of QEPV through a Caco-2 cell monolayer
The lyophilized powder of QEPV peptide was dissolved in double distilled water to the concentrations of 0.01, 0.05, 0.10,0.33 and 0.50 mg/mL for HPLC analysis. The relationship (a typical calibration graph) between the chromatographic peak area and the QEPV concentration was established.
HPLC analysis: Chromatographic separation was performed on an Agela Venusil MP-C18(2.1 mm × 150 mm,5 μm) at a column temperature of 45 ℃. The mobile phase consisted of solvent A (0.1% formic acid (FA) in water, V/V) and solvent B (0.1% FA in 100% acetonitrile,V/V). The optimized HPLC elution was analyzed in 25 min.The fl ow rate was set at 250 μL/min at 232 nm. The injection volume was 20 μL.
To the AP side, 0.5 mL of different concentrations of HBSS-diluted QEPV solution (0.2, 0.3, 0.5, 1.0, 2.0, 2.5 and 3.0 mg/mL) were separately added and 1.5 mL of prewarmed HBSS was separately added to the BL side. After 90 min of incubation, 500 μL of the samples from the BL side of the monolayer was collected and detected by HPLC as described above. The concentration of QEPV at the BL side was calculated according to the typical calibration graph previously investigated.
1.3.3 Flow cytometer analysis of investigating the transport route
FITC-QEPV was dissolved and diluted with HBSS to a concentration of 2.0 mg/mL. The Caco-2 cells at the logarithmic growth stage were cultured in the FITCQEPV solution for 90 min. The control groups were cultured in the QEPV or FITC solution at the same concentration. Then the Caco-2 cells were washed with phosphate buffered saline (PBS) for three times. The Caco-2 cells were digested by 0.25% (m/m) trypsin solution and immediately centrifuged at 3 000 r/min for 15 min with PBS by TDL-40B centrifuge. The collected Caco-2 cells were detected and analyzed by Accuri C6 flow cytometer with CFlow Plus Software.
1.3.4 UPLC-Q-TOF-MS analysis of investigating the transport route
QEPV was dissolved and diluted with HBSS to a concentration of 2.0 mg/mL. The Caco-2 cells at the logarithmic growth stage were cultured with or without QEPV peptide for 90 min. Then the Caco-2 cells were washed with PBS for three times. The Caco-2 cells were digested by 0.25% trypsin(m/m) solution and immediately centrifuged at 3 000 r/min for 15 min with PBS. Red blood cell lysis buffer was added to the Caco-2 cells and centrifuged at 3 000 r/min for 15 min. The supernatant and QEPV standard solution were detected through UPLC-Q-TOF-MS. UPLC-Q-TOF-MS was performed as described previously[17]. In addition, multiple reaction monitoring method was used to conform a highselective isolation of monoisotopic precursor ion.
UPLC analysis: Chromatographic separation was performed on a CSH C18column (2.1 mm × 100 mm, 1.7 μm)at a column temperature of 45 ℃. The mobile phase consisted of solvent A (0.1% formic acid in water, V/V) and solvent B(0.1% formic acid in acetonitrile, V/V). The optimized UPLC elution condition was set at 99% to 50% solvent A. The fl ow rate was set at 0.4 mL/min. The injection volume was 5 μL.
Q-TOF-MS analysis: The scan range was m/z 80-1 000.The MS was operated using electrospray ionization (ES+) for positive electrospray modes. The capillary and cone voltages were set at 3.0 and 35.0 kV, respectively. The desolvation gas was set to 600 L/h at 350 ℃.
1.3.5 Transport study in vivo
FITC or FITC-QEPV solution (3.0 mg/mL) was prepared with PBS before in vivo experiments. After 12 h of fasting, the six-week-old male BALB/c mice were randomly separated into 5 groups. The first group received the FITC solution through the oral administration of peptides by gavage. The second group received the FITC solution through intraperitoneal injection. The third group received the QEPV solution through the oral administration of peptides by gavage. The fourth group received the FITC-QEPV solution through intraperitoneal injection. The control group received the same volume of PBS without peptide. After 2 h, all blood samples were collected from eyes into the ethylene diamine tetraacetic acid (EDTA) tube. Red blood cell lysis buffer was added to the tube and centrifuged at 3 000 r/min for 15 min.The supernatant immediately detected by Accuri C6 flow cytometer with CFlow Plus Software.
1.4 Statistical analysis
All data were expressed as ± s for the three independent experiments performed with SPSS 19.0 software(IBM Corporation, Armonk, NY, USA).
2 Results and Analyses
2.1 Verif i cation of Caco-2 cell monolayer model
The results showed that the TEER on Day 21 was 440.9 Ω·cm2that was between 350 and 750 Ω·cm2, indicating that the model was successfully established and can be selected for permeability measurements[14,19]. On Day 21, the Caco-2 cell monolayers showed that tight junction formed between adjacent cells with the epithelial cells structure and without any phenomenon, such as cavity and apoptosis.Fig. 1 showed that the cells reached confluence with a columnar shape, established desmosomes (a tight junction between cells) and mature microvilli. The cells appeared morphologically differentiated with a polarized distribution of brush border enzymes[20-21].

Fig. 1 Microstructure of Caco-2 cells on Day 21 imaged by transmission electron microscopy
The sodium fl uorescein concentration at the BL side was calculated according to the calibration curve of standard sodium fl uorescein concentration and OD490nm. On the Day 21, after 90 min of incubation, the OD490nmof sodium fluorescein at the BL side was 0.066 7 ± 0.003 4 and the concentration of sodium fl uorescein was (0.003 4 ± 0.000 2) mg/mL. The sodium fluorescein flux value was 0.30%/(h·cm2). Augustijns[13]and Raub[22]et al.revealed that when the value was below 0.60%/(h·cm2),the Caco-2 cell monolayer model was successfully established. Other studies reported that the value should be under 0.50%/(h·cm2)[23-24]. The value in the current study was 0.30%/(h·cm2), which was smaller than 0.50%/(h·cm2);this result indicated that sodium fl uorescein could not transit through the Caco-2 cell monolayers. Therefore, Caco-2 cell monolayers were integrated. As such, they could be used for transport analysis.
2.2 Transprort of QEPV through a Caco-2 cell monolayer

Fig. 2 Concentrations of QEPV in BL side after 90 min incubation at different original concentrations in AP side
The relationship (a typical calibration graph) between chromatographic peak area (x) and QEPV concentration(y) was expressed as equation: y =1.331 7 × 10-7x-0.009 4(R2> 0.99). After 90 min of incubation, the QEPV concentration at the BL side was calculated using the chromatographic peak area according to the equation. We successfully quantified the QEPV at the BL side as shown in Fig. 2. The QEPV concentration at the BL side has a good linear relationship with the original concentration(ranging from 0 to 2 mg/mL) at the AP side, indicating that the transport rate was relatively constant. The transport rate increased unregularly with the original concentration (ranging from 2 to 3 mg/mL) of QEPV at the AP side.
The apparent permeability coefficients of Caco-2 cell monolayers could predict the passive particles transport in humans across the intestinal tract. The rate of peptide transport across the transwell membrane was determined by the apparent permeability coefficients (Papp) as previously described[25-26]. Pappwas calculated as following equation:
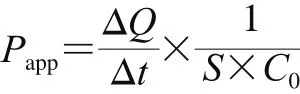
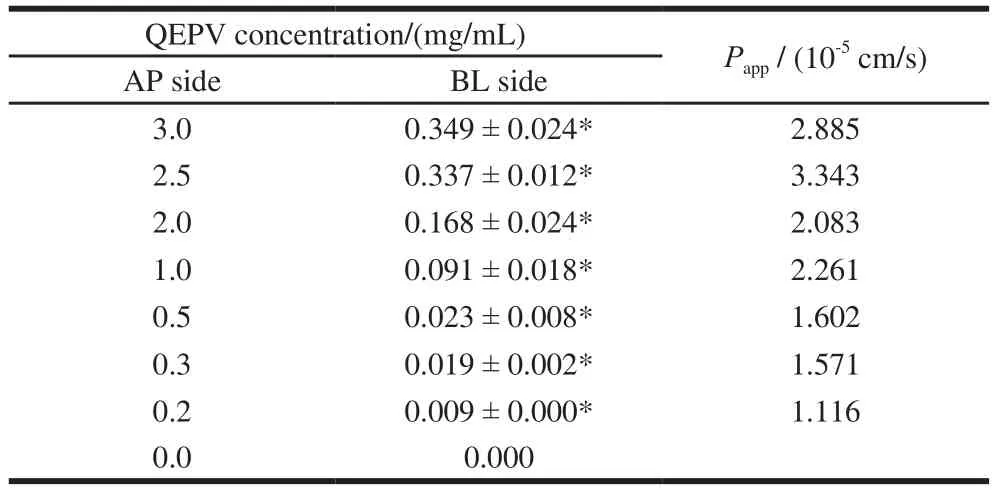
Table 1 Concentration and relative apparent permeability coeff i cients of QEPV at the BL side after 90 min incubation at different original concentrations at the AP side
The apparent permeability coefficients of QEPV at different original concentrations were calculated as above and the results were shown in Table 1. Grès et al.[27]studied the correlation between oral absorption in humans and the apparent permeability coeff i cients in the Caco-2 cell line and TC-7 clone, determining the threshold value for the apparent permeability coeff i cients as 2 × 10-6cm/s, indicating that the particles whose Pappvalues were greater than 2 × 10-6cm/s could be 100% absorbed by humans. Artursson et al.[21]reported that completely absorbed drugs were found to have high apparent permeability coefficients (> 1 × 10-6cm/s)in Caco-2 cell lines and incompletely absorbed drugs to have low apparent permeability coefficients (< 1 × 10-7cm/s).Table 1 showed that the Pappvalues at different original concentrations were above 1 × 10-6cm/s, predicting that peptide QEPV could be absorbed across the intestinal tract.
2.3 Transport route of QEPV in Caco-2 cell monolayers model
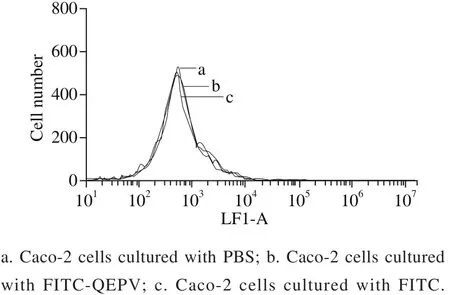
Fig. 3 Flow cytometric analysis of Caco-2 cells
The transport routes of QEPV across the intestinal epithelium include the passive transcellular, passive paracellular, active carrier-mediated transcellular and transcytosis routes. Rapidly and completely absorbed particles through the passive transcellular route are generally lipophilic and can be distributed into the cell membranes.The transcytosis route had been reported to transport certain peptides, but QEPV peptide might not be hydrophobic enough for this route. If QEPV peptide transit was by passive transcellular, active carrier-mediated transcellular or transcytosis route, we could detect QEPV in the Caco-2 cells after incubation with QEPV.

Fig. 4 UPLC-Q-TOF-MS mass spectra of Caco-2 cells cultured with or without QEPV
The fl ow cytometry results (Fig. 3) showed there was no differences between the three groups (Caco-2 cell separately incubation with PBS, FITC and FITC-QEPV), indicating that peptide QEPV was not absorbed into the cell or attached to the cell membrane surface. After 90 min of incubation with QEPV peptide, the cell was digested, centrifuged, cracked and detected by UPLC-Q-TOF-MS. As shown in Fig. 4, no signal in the corresponding QEPV time in the Caco-2 cell lines was detected, which indicated QEPV could not transport into the Caco-2 cells.
A limited transport rate existed according to Fig. 2.Only the passive paracellular and active carrier-mediated transcellular routes were saturable for the limited number of carriers in the Caco-2 cell membranes and micro pores between the tight junction. In another view, QEPV peptide distributed poorly into the cell membranes because of its hydrophilic physico-chemical properties. In conclusion, we could predict that the QEPV peptide might most probably be absorbed by the passive paracellular route.
Several similar studies had been reported in the recent years. Satake et al.[28]studied the transepithelial transport route of tripeptide Val-Pro-Pro (VPP) using the Caco-2 cell monolayer model. They found that larger numbers of intact VPP could transport through the Caco-2 cell monolayer with the existence of a competitive substrate for transporter PepT1, indicating that the passive paracellular route should be the intestinal absorption pathway and not the active carrier-mediated transcellular route. Quirós et al.[29]reported that the peptide His-Leu-Pro-Leu-Pro (HLPLP) produced by Enterococcus faecalis could be absorbed through the paracellular route using the Caco-2 cell monolayer model.According to the result, QEPV was not absorbed by Caco-2 cells and was predicted to transport by the passive paracellular route. Therefore it was very likely to transport through the intestine into blood circulation in vivo.
2.4 Transport in vivo
In our previous work, we found that QEPV peptide could not be broken down in the gastrointestinal environment and showed good stability in buffer[17].
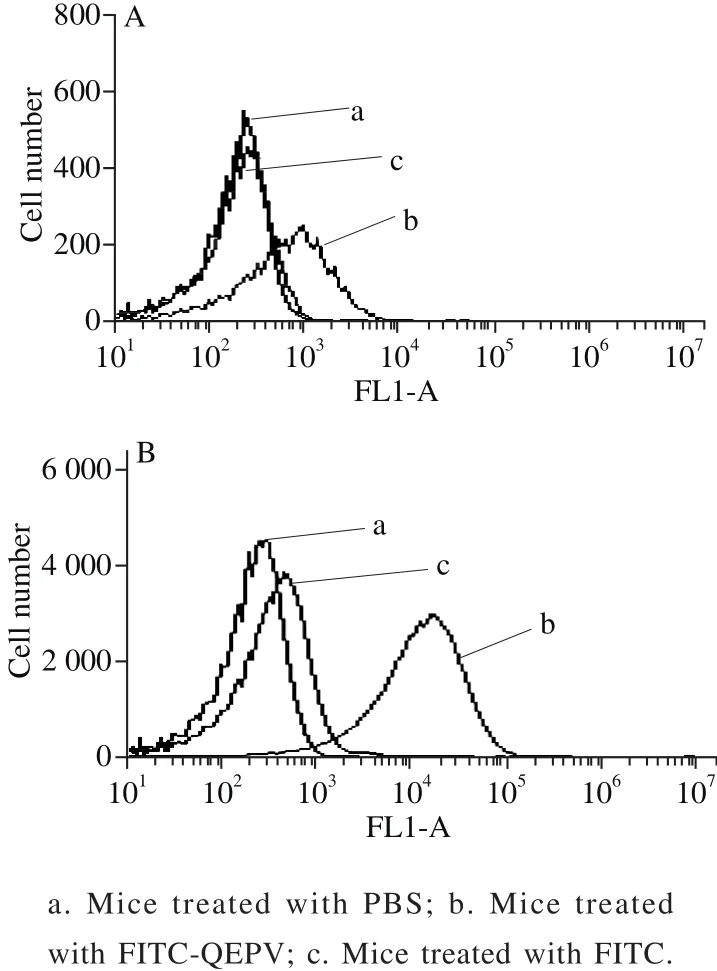
Fig. 5 Flow cytometric analysis of blood cells from six-week-old BALB/c mice treated by intraperitoneal injection (A) and gavage (B)
The absorption of QEPV in vivo experiment was carried out using male BALB/c mice, as shown in Fig.5.The QEPV-FITC treated mice by gavage and intraperitoneal injection demonstrated successful transductions into the whole blood cells compared with the untreated control mice.A significant increase was observed in the QEPV-FITC treated through intraperitoneal injection mice compared with by gavage, indicating that the absorption of QEPV through intraperitoneal injection is more effective. The FITC-treated mice via intraperitoneal injection showed an increase in the background fluorescence of the blood cell; this finding indicated that FITC was absorbed through the lymphatic system into the blood. Ho et al.[30]applied fl ow cytometry to examine the in vivo transport of the cell-penetrating peptide Thr-Ala-Thr (TAT) and obtained a similar result; thus, fl ow cytometry is quite useful in the research of peptide transport in vivo and in vitro.
3 Conclusion
In this report, Caco-2 cell monolayer and mouse models were successfully established to investigate the transepithelial transport of QEPV in vitro and in vivo. Morphological characteristics, TEER and sodium fluorescein flux were analyzed to ensure the integrity of the Caco-2 cell monolayer model. We also examined the absorption and transport route of QEPV across the intestinal tract in vitro and in vivo through UPLC-Q-TOF-MS and flow cytometry. The results indicated that QEPV could be absorbed as a bioactive peptide via the paracellular pathway in vitro and QEPV can be absorbed into the blood across the intestinal tract in vivo. The metabolism of QEPV in vivo will be explored in our future work.
[1] NAGPAL R, BEHARE P, RANA R, et al. Bioactive peptides derived from milk proteins and their health benef i cial potentials: an update[J].Food & Function, 2011, 2(1): 18-27. DOI:10.1039/C0FO00016G.
[2] MANZANARES P, SALOM J B, GARC TEJEDOR A, et al.Unraveling the mechanisms of action of lactoferrin-derived antihypertensive peptides: ACE inhibition and beyond[J]. Food &Function, 2015, 6(8): 2440-2452. DOI:10.1039/C5FO00580A.
[3] PATIL P, WADEHRA A, GARG V, et al. Biofunctional properties of milk protein derived bioactive peptides: a review[J]. Asian Journal of Dairy and Food Research, 2015, 34(4): 253-258. DOI:10.18805/ajdfr.v34i4.6873.
[4] HAQUE E, CHAND R, KAPILA S. Biofunctional properties of bioactive peptides of milk origin[J]. Food Reviews International, 2008,25(1): 28-43. DOI:10.1080/87559120802458198.
[5] MASOOD R, KHOSRAVIDARANI K. Biopeptides in milk: opiate and antithrombotic ef f ects[J]. Mini Reviews in Medicinal Chemistry,2015, 15(10): 872-877. DOI:10.2174/1389557515666150519104219.
[6] NONGONIERMA A B, FITZGERALD R J. The scientif i c evidence for the role of milk protein-derived bioactive peptides in humans:a review[J]. Journal of Functional Foods, 2015, 17: 640-656.DOI:10.1016/j.jf f.2015.06.021.
[7] NONGONIERMA A B, FITZGERALD R J. Strategies for the discovery, identification and validation of milk protein-derived bioactive peptides[J]. Trends in Food Science & Technology, 2016, 50:26-43. DOI:10.1016/j.tifs.2016.01.022.
[8] PHELAN M, KERINS D. The potential role of milk-derived peptides in cardiovascular disease[J]. Food & Function, 2011, 2(3/4): 153-167.DOI:10.1039/C1FO10017C.
2018年11月2日,AI《汽车制造业》技术服务“面对面”活动走进舍弗勒太仓工厂,受邀专家们围绕智能制造以及工业互联网应用等话题,与舍弗勒太仓工厂的工程技术人员们进行了深入的交流与探讨。
[9] SÁNCHEZ-RIVERA L, MARTÍNEZ-MAQUEDA D, CRUZHUERTA E, et al. Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides[J]. Food Research International,2014, 63: 170-181. DOI:10.1016/j.foodres.2014.01.069.
[10] KORHONEN H. Milk-derived bioactive peptides: from science to applications[J]. Journal of Functional Foods, 2009, 1(2): 177-187.DOI:10.1016/j.jf f.2009.01.007.
[11] MUKHOPADHYA A, NORONHA N, BAHAR B, et al. The antiinflammatory potential of a moderately hydrolysed casein and its 5 kDa fraction in vitro and ex vivo models of the gastrointestinal tract[J].Food & Function, 2015, 6(2): 612-621. DOI:10.1039/C4FO00689E.
[12] ARTURSSON P. Epithelial transport of drugs in cell culture. I: a model for studying the passive dif f usion of drugs over intestinal absorbtive(Caco-2) cells[J]. Journal of Pharmaceutical Sciences, 1990, 79 (6):476-482. DOI:10.1002/jps.2600790604.
[13] AUGUSTIJNS P, MOLS R. HPLC with programmed wavelength fl uorescence detection for the simultaneous determination of marker compounds of integrity and P-gp functionality in the Caco-2 intestinal absorption model[J]. Journal of Pharmaceutical and Biomedical Analysis, 2004, 34(5): 971-978. DOI:10.1016/j.jpba.2003.11.016.
[14] OSBORNE S, CHEN W, ADDEPALLI R, et al. In vitro transport and satiety of a beta-lactoglobulin dipeptide and beta-casomorphin-7 and its metabolites[J]. Food & Function, 2014, 5(11): 2706-2718.DOI:10.1039/C4FO00164H.
[15] QIAN B, XING M, CUI L, et al. Antioxidant, antihypertensive, and immunomodulatory activities of peptide fractions from fermented skim milk with Lactobacillus delbrueckii ssp. bulgaricus LB340[J].Journal of Dairy Research, 2011, 78(1): 72-79. DOI:10.1017/S0022029910000889.
[16] WAKAI T, YAMAMOTO N. Antihypertensive peptides specific to Lactobacillus helveticus fermented milk[J]. InTech, 2012: 159-172.DOI:10.5772/28695.
[17] JIEHUI Z, LIULIU M, HAIHONG X, et al. Immunomodulating ef f ects of casein-derived peptides QEPVL and QEPV on lymphocytes in vitro and in vivo[J]. Food & Function, 2014, 5(9): 2061-2069. DOI:10.1039/C3FO60657K.
[19] DEPREZ S, MILA I, HUNEAU J F, et al. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells[J]. Antioxidants and Redox Signaling, 2004, 3(6): 957-967. DOI:10.1089/152308601317203503.
[20] HILGERS A R, CONRADI R A, BURTON P S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa[J]. Pharmaceutical Research, 1990, 7(9): 902-910.DOI:10.1023/A:1015937605100.
[21] ARTURSSON P, PALM K, LUTHMAN K. Caco-2 monolayers in experimental and theoretical predictions of drug transport[J].Advanced Drug Delivery Reviews, 2012, 64: 280-289. DOI:10.1016/j.addr.2012.09.005.
[22] RAUB T J. Signal transduction and glial cell modulation of cultured brain microvessel endothelial cell tight junctions[J]. American Journal of Physiology, 1996, 271(2): C495-C503.
[23] INGELS F, DEFERME S, DESTEXHE E, et al. Simulated intestinal fluid as transport medium in the Caco-2 cell culture model[J].International journal of pharmaceutics, 2002, 232(1/2): 183-192.DOI:10.1016/S0378-5173(01)00897-3.
[24] AUGUSTIJNS P, ANNAERT P, HEYLEN P, et al. Drug absorption studies of prodrug esters using the Caco-2 model: evaluation of ester hydrolysis and transepithelial transport[J]. International Journal of Pharmaceutics, 1998, 166(1): 45-53. DOI:10.1016/S0378-5173(98)00013-1.
[25] MORENO F J, RUBIO L A, OLANO A, et al. Uptake of 2S albumin allergens, Ber e 1 and Ses i 1, across human intestinal epithelial Caco-2 cell monolayers[J]. Journal of Agricultural and Food Chemistry, 2006,54(22): 8631-8639. DOI:10.1021/jf061760h.
[26] ANTUNES F, ANDRADE F, FRANCISCA A, et al. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2013, 83(3): 427-435. DOI:10.1016/j.ejpb.2012.10.003.
[27] GRÈS M C, JULIAN B, BOURRI M, et al. Correlation between oral drug absorption in humans, and apparent drug permeability in TC-7 cells, a human epithelial intestinal cell line: comparison with the parental Caco-2 cell line[J]. Pharmaceutical Research, 1998, 15(5):726-733. DOI:10.1023/A:1011919003030.
[28] SATAKE M, ENJOH M, NAKAMURA Y, et al. Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers[J]. Bioscience, Biotechnology, and Biochemistry, 2002, 66(2): 378-384. DOI:10.1271/bbb.66.378.
[29] QUIRÓS A, DÁVALOS A, LASUNCIÓN M A, et al. Bioavailability of the antihypertensive peptide LHLPLP: transepithelial flux of HLPLP[J]. International Dairy Journal, 2008, 18(3): 279-286.DOI:10.1016/j.idairyj.2007.09.006.
[30] HO A, SCHWARZE S R, MERMELSTEIN S J, et al. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo[J]. Cancer Research, 2001, 61(2): 474-477.
乳源生物活性肽QEPV体内外的吸收转运
李婉如1,陈 静1,程志才1,沈 鹏1,高 扬1,李锡安2,张少辉1,3,*
(1.上海交通大学农业与生物学院,上海 200240;2.浙江熊猫乳业集团股份有限公司,浙江 温州 325800;3.上海交通大学陆伯勋食品安全研究中心,上海 200240)
发酵乳作为一种长寿食品备受关注。多肽Gln-Glu-Pro-Val(QEPV)是一种来源于发酵乳,具有免疫调节作用的生物活性肽。通过Caco-2细胞单层膜模型及小鼠模型,研究乳源性生物活性肽QEPV在体外和体内的转运吸收情况。结果表明:乳源性生物活性肽QEPV具有非常好的稳定性,且能够穿过由Caco-2细胞形成的转运模型,但当QEPV质量浓度高于3.00 mg/mL时穿膜速率趋于稳定。流式细胞术和超高效液相色谱-四极杆飞行时间质谱结果表明Caco-2细胞不能吸收QEPV,由于QEPV是不具有空间结构的小肽,可以初步推测出QEPV主要通过胞旁转运方式透过Caco-2细胞单层膜模型的结论。同时,QEPV可以经过腹腔注射和灌胃方式被小鼠吸收,但肠道吸收效率较差,腹腔吸收效率较好。
生物活性肽;Caco-2细胞单层膜模型;小鼠模型;转运吸收
Q516
A
1002-6630(2017)23-0224-07
2016-09-07
浙江辉肽生命健康科技有限公司支持项目
李婉如(1993—),女,硕士研究生,研究方向为乳品科学。E-mail:varo729@sjtu.edu.cn
10.7506/spkx1002-6630-201723036
LI Wanru, CHEN Jing, CHENG Zhicai, et al. Transepithelial transport of milk-derived peptide QEPV in vitro and in vivo[J].食品科学, 2017, 38(23): 224-230.
10.7506/spkx1002-6630-201723036. http://www.spkx.net.cn
LI Wanru, CHEN Jing, CHENG Zhicai, et al. Transepithelial transport of milk-derived peptide QEPV in vitro and in vivo[J]. Food Science,2017, 38(23): 224-230. (in English with Chinese abstract) DOI:10.7506/spkx1002-6630-201723036. http://www.spkx.net.cn
*通信作者:张少辉(1965—),男,研究员,博士,研究方向为乳品科学。E-mail:shaohuizhang@sjtu.edu.cn
