Antioxidant Activity of Methanol Extract and Its Fractions from Spine Grape (Vitis davidii Foëx.) Peel
2017-12-11LUJunZHANGJiaqiLUODanWANGJunRENYanyanLIZhonghai
LU Jun, ZHANG Jiaqi, LUO Dan, WANG Jun, REN Yanyan, LI Zhonghai,*
(1. College of Food Science and Engineering, Central South University of Forestry and Technology, Changsha 410004, China;2. National Engineering Laboratory of Rice and By-Product Deep Processing, Changsha 410004, China;3. Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, Beijing 100193, China)
Antioxidant Activity of Methanol Extract and Its Fractions from Spine Grape (Vitis davidii Foëx.) Peel
LU Jun1,2, ZHANG Jiaqi1, LUO Dan1, WANG Jun3, REN Yanyan1, LI Zhonghai1,2,*
(1. College of Food Science and Engineering, Central South University of Forestry and Technology, Changsha 410004, China;2. National Engineering Laboratory of Rice and By-Product Deep Processing, Changsha 410004, China;3. Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, Beijing 100193, China)
In order to evaluate the antioxidant activity and components of spine grape peel, crude extract from spine grape peel was prepared by ultrasonic assisted extraction with acidified methanol as the extraction solvent and separated by macroporous resin adsorption to obtain five main fractions (I–V). Spine grape peel showed high contents of total phenolics(1.836 mg/g mf), total flavonoids (0.874 mg/g mf), VC (3.567 mg/g mf), total condensed tannins (3.578 mg/g mf) and total anthocyanins (2.970 mg/g mf) . The antioxidant activity of the methanol extract as evaluated by means of 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid (ABTS) assay and ferric reducing antioxidant power (FRAP) was 22.325, 17.595 and 26.487 μmol TE/g mf, respectively. Among 5 fractions, the watereluted fraction I contained the highest contents of phenolics, flavonoids, VC, tannin and anthocyanins (0.876, 0.116, 1.577,1.576 and 1.330 mg/g mf, respectively) and presented the best antioxidant activity (3.636, 10.109 and 13.415 μmol TE/g mffor DPPH radical scavenging activity, ABTS+· scavenging activity and FRAP, respectively). Signif i cant correlations were observed between antioxidant activity and anthocyanins, tannin and VC contents, which suggested that these compounds might be the major antioxidants contributing to the observed activity. Therefore, spine grape peel is a promising source of antioxidants and can be used as a dietary supplement for health promotion.
spine grape peel; antioxidant activity; active components
In recent years, there are cumulative evidence that oxidative stress related to aging and aging associated diseases, such as cancer[1-2], inflammation, cardiovascular diseases[3-5]and diabetes[6]. A lot of studies indicate that dietary supplement could prevent these oxidative stress related diseases from happening, including cancer[7],neurodegeneration[8], and cardiovascular disease[9-10],inflammation, diabetes[11], etc. by scavenging the free radicals,reducing agents, chelating agents, and so on. Thus, efforts have been made to search for natural antioxidants from fruits,vegetables, herbs, and spices as potential food supplements to deal with these diseases. Thence, there is a great interest in evaluating the bioactive properties of these natural compounds obtained from fruits, vegetables and other plants.
Spine grape (Vitis davidii Foëx.), a famous mountain grape genera, is widely distributed in China, especially in the west of Hunan province[12]. Spine grape has high nutritional value and variety of bioactive compounds such as proanthocyanidins, resveratrol, anthocyanin[13], oleanolic acid, superoxide dismutase, ascorbic acid and other active ingredients[14], especially that the procyanidins content of spine (22.76 mg/mL) was much higher than that of Kyoho(2.06 mg/mL)[15]. The spine grape peel is rich in many nutrient elements including soluble solids (11.86%), total sugar (9.76%),protein (0.342%) and tannin (0.786%), etc. The extraction quantity of total phenolic compounds was 16.823 mg/g mdpeel[16], four times of that of apple peel (4.53 mg/g md)[17].There are 6 kinds of anthocyanins (598.71 mg/kg) and 15 kinds of non-anthocyanin phenolic compounds in spine grape peel, and its major anthocyanins are malvidin-3,5-O-diglucoside, malvidin-3,5-O-diglucoside-coumary,delphinidin-3-rutinoside and malvidin-3-rutinoside[18-19]. It also contains high amount of anthocyanins (2.522 7 mg/g mf)[20], two times over that of strawberry[21], the colour-value ranges from 20 to 134, 49 times over that of Europe and American grape, and 2.43, 2.87 and 21.75 times over that of Chixiazhu, Hongti and Jufeng grape respectively[20]. So far,the researches about the spine grape were mostly focused on the fresh fruit quality and spine grape wine[22-23]. Rarely study was focused on the spine grape peel, especially about the antioxidant components and antioxidant capacity, but as far as we know, the spine grape peel has a huge trading potential, since it can be consumed in natural form and also used by industry as ingredient to produce cosmetics and food products. Therefore, there is a need in the detailed information about antioxidant properties and antioxidant compounds of spine grape peel which can be used to develop nutraceutical as a reference material.
In order to investigate the antioxidant characteristics of the spine grape peels, the goals of this study were: 1) to separate the phenolic substances in the crude spine grape peel extract by adsorption-desorption, and macro resin column chromatograph to fractionate substances into different groups;2) to determine the antioxidant potential of spine grape peels extracts and the isolated fractions; 3) to characterize the phenolic compositions of fractions, as well as to fi nd out the relationships between the activity and the composition of the grape peel. This study could help improving the processing and utilization of grape peel, reducing the waste of resources,and providing scientif i c basis for large scale production and comprehensive utilization of the grape peel.
1 Materials and Methods
1.1 Materials and reagents
The spine grape were purchased from Mayang county,Hunan province. They were washed with distilled water and manually peeled. The peels were air-dried at room temperature to remove excess of water, ground into pulp with a mill, and the peel pulp was stored at -20 ℃ prior to use.
vanillin, 1,1-diphenyl-2-picrylhydrazyl (DPPH)Sigma (USA); 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ)Nanjing Doulai Biotechnology Co. Ltd. (China); 2,2’-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS)Hefei Bomei Biological Co. Ltd.; rutin National Institutes for Food and Drug Control (China); gallic acid National Medicine Group Chemical Reagent Co. Ltd. (China); Folin-Ciocalteu reagent (FCR) Shanghai Yuanye Co. Ltd. (China);all other chemicals used in the study were in analytical grade.
1.2 Instruments and equipments
UV1800 ultraviolet-visible spectrophotometer Shimazu (Japan); JY92-Ⅲ ultrasonic cell disruptor Ningbo Shizhi Biotechnology Co. Ltd. (China); R-3 rotary evaporators Buchi Labortecknic AG (Switzerland).
1.3 Methods
1.3.1 Preparation of extract and fraction from spine grape peel
The crude extract was prepared by ultrasonic assisted extract (UAE) as described by Meng Jiangfei et al.[22]with slight modif i cations. Brief l y, 10 g of peel pulp were accurately weighted into 200 mL flask, then 100 mL of 1% (V/V) of acidified methanol (including 1 mol/L hydrochloric acid)was added, and the UAE was performed at 200 W power and 50 ℃ for 1 h. After extraction, the extracts solution was filtrated by Buchner funnel and crude extract of spine grape (CE) was obtained after the fi ltrate was evaporated to remove the solvent using a rotary evaporator at 40 ℃. The dried residue was redissolved in water to obtain the 15 mL solution and store at – 4 ℃ for analysis or further separation.For fraction preparation, the 15 mL of redissolved solution was poured into a column prepacked LS-46D macroporous resin (column height 23 cm, diameter 2.4 cm, bed volume 33.5 mL) and washed at the flow rate of 2.9 mL/min.The wash solutions were 100 mL of each of distilled water,20%, 50%, 70% and 90% ethanol elution. Filtrate was collected at 20 mL/tube and scanned with ultraviolet-visible spectrophotometer (200–760 nm), and the same absorption peak of eluent were combined to get 5 different fractions.
1.3.2 Determination of total phenolic content
Total phenolic content (TPC) was estimated by Folin-Ciocalteu assay based on the procedure described by Bursal et al[24]. Briefly, 1.0 mL of test sample was pipetted into a test tube, and then distilled water was added to fi nal volume of 23.0 mL. Afterwards, 0.5 mL of Folin-Ciocalteu reagent and 0.3 mL of Ca2CO3(10%, m/m) were added. The mixture was vortexed and kept at room temperature for 30 min. The absorbance at 760 nm was recorded. Gallic acid was served as the reference standard and the total phenolic content was expressed as garlic equivalents (mg GAE/g mf).
1.3.3 Determination of total fl avonoids content
Total flavonoids content (TFC) was determined according to the aluminum chloride method described by Pei Yongping et al[25]. Briefly, 1.0 mL of test sample and 1 mL 0.1 mol/L of aluminum chloride were added into a test tube then replenished with 70% ethanol to the volume of 25 mL.The mixture was vortexed and left at room temperature for 15 min. The absorbance at 416 nm was measured. Rutin was served as the reference standard and total fl avonoids content was expressed as rutin equivalents (mg RE/g mf).
1.3.4 Determination of total tannins content
Total tannins content (TTC) was analyzed as described by Zhao Wenjie et al[26]. Brifely, 1.0 mL of test sample and 30 mL distilled water were added into a 50 mL volumetric flask, followed by addition of 2.5 mL of FD reagent (50 g of Na2WO4, 10 g of phosphomolybdic acid and 25 mL of phosphoric acid in 375 mL water,then refluxed for 2 h to room temperature and diluted to 500 mL) and 5 mL of 20%(m/m) Na2CO3solution, then distilled water was added to reach 30 mL. The mixture was vortexed and left at room temperature for 30 min. The absorbance at 650 nm was measured. Tannic acid was used as the reference standard and TTC was expressed as tannin equivalents (mg TAE/g mf).
1.3.5 Determination of VC content
Determination of VC content (VCC) in the extract followed the method of Ma Hongfei et al[27]. 1 mL of test sample was pipetted into 50 mL volumetric flask and the volume was adjusted to 50 mL by distilled water. The absorbance at 243 nm was measured. Ascorbic acid content was expressed as mg VC/g mf.
1.3.6 Determination of total anthocyanin contents
Determination of total anthocyanins content (TAC) in the extract followed method of Xu Honggao et al[28]. The absorbance values of the samples were measured at 520 and 700 nm in the buffers at pH 1.0 and 4.5, respectively, and the TAC was calculated by the following equation:
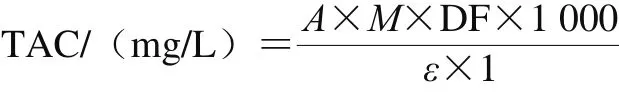
Where A = (A520nm- A700nm)pH1.0- (A520nm- A700nm)pH4.5;M was molecular weight of cyanidin-3-glucoside (493.2 g/mol);DF was dilution factor; 1 was optical path length/cm; ε was cyanidin-3-glucoside molar extinction coefficient (28 000 L/(mol·cm));1 000 was the conversion factor from g to mg.
1.3.7 DPPH radical scavenging capacity assay
The antioxidant capacity of the sample was determined using the DPPH radical scavenging activity assay as described by Gorjanović et al.[29]with some modif i cations. In a nutshell, 100 μL of a serial of Trolox standard solution (2.5-15.0 μmol/L) or test sample was added to 1.9 mL of 0.094 mmol/L DPPH in methanol. The mixture was kept in the dark for 30 min at room temperature and the absorbance at 517 nm was measured. Trolox was used as the reference standard and antioxidant capacity was expressed as micromole Trolox equivalents per gram fresh material(μmol TE/g mf). The regression equation was Y = 10.877X +0.715 8 (R2= 0.999 5).
1.3.8 ABTS+· scavenging capacity assay
ABTS+· scavenging capacity was determined according to the method described by Oh et al.[30]with minor modif i cation. ABTS+· were generated by reacting 7.0 mmol/L ABTS solution with 2.45 mmol/L potassium persulfate(1:1, V/V). The mixture was kept for 12-16 h in the dark at room temperature. In order to give an absorbance of 0.70 ± 0.02 at 734 nm, the ABTS solution was then diluted with phosphate buffer (10 mmol/L). 1 mL test sample or Trolox solution was mixed with diluted ABTS solution (4.0 mL). The mixture was vortexed and kept at 30 ℃ water bath for 6 min.The absorbance of the reaction mixture was measured at 734 nm with a spectrophotometer. Trolox was used as the reference standard and antioxidant capacity was expressed as micromole Trolox equivalents per gram fresh material(μmol TE/g mf). The regression equation was Y = 6.194 3X +0.565 8 (R2= 0.999 5).
1.3.9 FRAP assay
The assay was performed according to the method described by LU Xiaonan et al[31]. Briefly, the ferric reducing antioxidant power (FRAP) reagent was prepared by mixing 2.5 mL of 10 mmol/L TPTZ in 40 mmol/L HCL with 2.5 mL of 20 mmol/L FeCl3and 25 mL of 0.30 mol/L acetate buffer (pH 3.6), then vortexed and the mixture was heated to 37 ℃ until using. Sample solution (90 μL) was added to 270 μL of distilled water and 2.7 mL of FRAP reagent then incubated at 37 ℃ for 30 min. The absorbance of the resulting solution was measured at 595 nm using a spectrophotometer. A standard was included for each plate with a series of Trolox concentrations (0.000, 0.018, 0.036,0.045, 0.054, 0.063, 0.072, 0.090 μmol/mL). Trolox was used as the reference standard and antioxidant capacity was expressed as micromole Trolox equivalents per gram fresh material (μmol TE/g mf). The regression equation was Y =10.033X + 0.124 7 (R2= 0.999 9).
1.4 Statistical analysis
All experiments were repeated three times and the data were presented as ± s. All data was checked the normality and homogeneity of variance by SPSS 17.0 software. Kruskal-Wallis test was used when the data did not follow normal distribution or homogeneity of variance. The relationship between the antioxidant content and antioxidant activity were investigated by Spearman’s correlation analysis.The signi fi cance was calculated for P < 0.05.
2 Results and Analyses
2.1 Separation of CE
Twenty five tubes were used to collect the eluent and each tube collected 25 mL. One hundred of each eluent(distilled water, 20%, 50%, 70% and 90% ethanol) was run through the column stepwise and the collected eluent was checked by UV1800 ultraviolet-visible spectrophotometer from 200 to 760 nm. The tubes having high absorbance in each eluent were 1-4, 5-8, 9-11, 12-17 and 18-22. Therefore,the tubes of 1-4, 5-8, 9-11, 12-17, 18-22 were combined to FrⅠ, FrⅡ, FrⅢ, FrⅣ and FrⅤ, respectively.
2.2 Antioxidant components content of the CE and fractions

Table 1 Antioxidant constituents of CE and its fractions from spine grape peel mg/g mf
It can be seen from the Table 1 that the contents of the different fractions were different. The highest TPC content was found in FrⅠ, followed by FrⅣ, Ⅴ, Ⅲ and Ⅱ. The highest content of flavonoids was found in FrⅣ, followed by FrⅠ, Ⅲ, Ⅴ, Ⅱ. The content of VC in these 5 fractions followed the same sequence as that of TPC. And the TTC was mainly found in the Fr Ⅰ, followed by Fr Ⅳ, Ⅲ, Ⅴ and Ⅱ. The FrⅡ and Ⅴ did not contain TAC. The TAC was found to be highest in FrⅠ and lowest in FrⅢ. It was obvious that the main active components were enriched in FrⅠ, and so the antioxidant components of the crude extract can be eluted by distilled water.
2.3 Antioxidant activity of the CE and fractions
The antioxidant activity could not be attributed solely to the complex nature of phytochemicals, and the antioxidant activity depended on the reaction mechanism. Therefore, it is important to use manifold methods for the determination of the antioxidant activity of plant extracts or phytochemicals.Many methods have been developed to determine antioxidant activity and to explain the mechanism of antioxidant activity.Among them, DPPH radical scavenging capacity, ABTS+·scavenging capacity and FRAP are the most commonly accepted methods for the determination of antioxidant activity of the food matrix[32]. Therefore, a series of assays, included DPPH radical scavenging capacity, ABTS+· scavenging capacity, and FRAP was used to evaluate the antioxidant activity in this experiment.
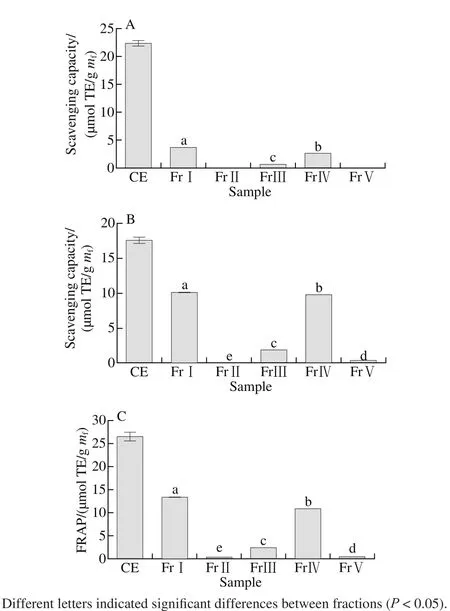
Fig. 1 DPPH radical (A), ABTS+· (B) scavenging capacity and FRAP (C) of the CE and its fractions
DPPH test has been widely used to evaluate the effect of natural antioxidants on scavenging free radicals. DPPH radical is a stable organic free radical. When accepting an electron or hydrogen in the presence of a hydrogen-donating antioxidant, the lone pair electrons were paired, the deep purple DPPH radical can be reduced to a yellow non-radical form, DPPH-H, from modena to yellow. And the decoloring degree of DPPH radical has a quantitative relation with the number of the recipient electron. Therefore, it is possible to make quantitative analysis[26]by measuring the change of absorbance. The DPPH radical scavenging activity of all extracts and fractions was expressed by Trolox equivalents and the results was shown in Fig. 1A. Except for FrⅡ andⅤ, CE and other fractions showed good DPPH radical scavenging capacity. FrⅠ showed the highest DPPH radical scavenging capacity (3.636 μmol TE/g mf), followed by FrⅣ (2.571 μmol TE/g mf) and FrⅢ (0.626 μmol TE/g mf).ABTS+· scavenging capacity test is the method based on the ability of antioxidant compounds to quench the ABTS+·and to reduce the radical to the colorless neutral form. As shown in Fig. 1B, the antioxidant activity of samples were in the following order: FrⅠ (10.109 μmol TE/g mf) > FrⅣ(9.781 μmol TE/g mf) > FrⅢ (1.852 μmol TE/g mf) > FrⅤ(0.360 μmol TE/g mf) > FrⅡ (0.167 μmol TE/g mf). The results of the FRAP assays for antioxidant activity was shown in Fig. 1C in the following order: FrⅠ (13.415 μmol TE/g mf) >FrⅣ (10.886 μmol TE/g mf) > FrⅢ (2.405 μmol TE/g mf) >FrⅤ (0.403 μmol TE/g mf)> FrⅡ (0.316 μmol TE/g mf). This order was closely similar to the orders in DPPH and ABTS assay. These antioxidant activity maybe correlated well with TTC, VCC and TAC, as their content in different fractions was similar in the following order: VCC ≥ TTC > TAC >TPC > TFC in FrⅠ, VCC > TTC > TAC > TPC > TFC in the FrⅣ and TTC > VCC > TAC > TPC > TFC in the FrⅢ. In all,FrⅠ demonstrated the highest antioxidant activity, followed by FrⅣ and FrⅢ. The content of phenolics, fl avonoids, VC,tannin and anthocyanins in FrⅠ were 0.876, 0.116, 1.577,1.576 and 1.330 mg/g mf, respectively, and the antioxidant activity were 3.636, 10.109 and 13.415 mg TE/g mffor DPPH radical scavenging capacity, ABTS+· scavenging capacity and FRAP, respectively. The relative high yield of three components is considered, and the antioxidant activity of spine grape peels may be mainly attributed to the condensed tannins, VC and TAC. There are reported 6 kinds of anthocyanins and 15 kinds of non-anthocyanin phenolic compounds in spine grape peel[19], the major anthocyanins were elphinidin-3,5-O-diglucoside and malvidin-3,5-O-diglucoside, and in Vitis ficifolia Bge. malvidin-3,5-O-diglucoside and petunidin-3,5-O-diglucoside. Feruloytartaric acid and its derivatives,ferulic acid hexose ester and quercetin-O-rhamnoside were the major nonanthocyanin phenolic compounds in spine grape peel. As shown in Fig.1, all 5 fractions exhibited lower antioxidant activity than CE, it was due to that the antioxidant components of CE were concentrated, and the antioxidant components were dispersed in each fraction by separation(volume of each combined fraction ranged from 60 to 120 mL).As a result, the concentration of TPC, TFC, VCC, TTC and TAC in CE was significantly higher than that of fractions(Table 1), so CE showed a strong antioxidant activity.
2.4 Antioxidant components and antioxidant activity relationships
To analyze the correlative relationships among total antioxidant activity and phytochemical contents (TPC, TFC,VCC, TTC, TAC), a Spearman correlation coefficients was conducted, and the results are shown in Table 2.
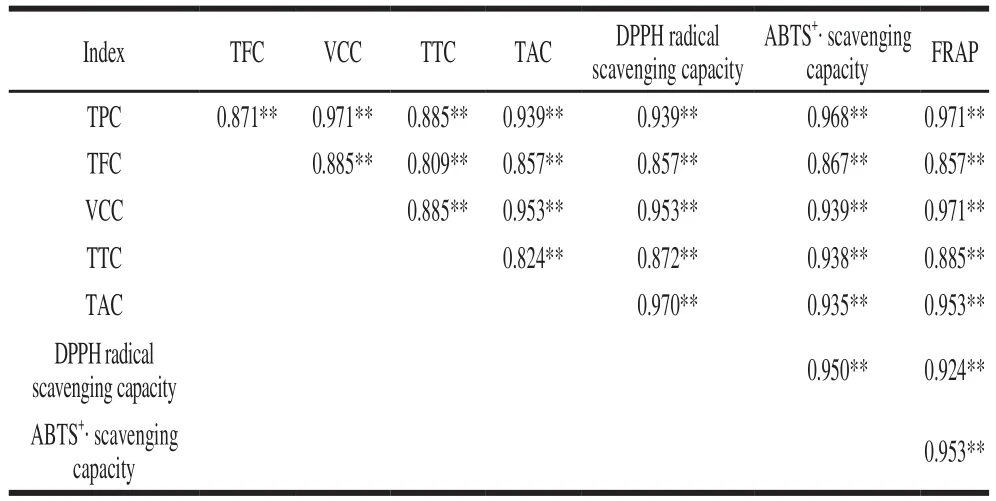
Table 2 Spearman correlation coeff i cients between antioxidant activity and phytochemical contents
The correlation coefficient r between the antioxidant activity and phytochemical compound contents was compared, which showed that there was a significant correlation (P < 0.01) existed between various parameters.That is, with regard to DPPH radical scavenging capacity, a good correlation was found with TAC (r = 0.970), an inferior one with VCC (r = 0.953) and TPC (r = 0.939). ABTS+·scavenging activity has a significant correlation with TPC(r = 0.968), and a lower one with VCC (r = 0.939), TTC(r = 0.938) and TAC (r = 0.935). For FRAP, the correlation coeff i cient was 0.971, 0.971 and 0.953 with TPC, VCC and TAC, respectively. All coeff i cients showed strong relationship(r > 0.8). In addition, the antioxidant activity assay (DPPH radical scavenging capacity, ABTS+· scavenging capacity and FRAP) showed significant correlation (P < 0.01),indicating that all of the antioxidant activity assays were reliable and could be interchangeable. For example, DPPH radical scavenging capacity has a good correlation with ABTS+·scavenging activity (r = 0.950) and FRAP (r = 0.924),whereas ABTS correlated well with FRAP (r = 0.953).The similar research results was reported by Zou Yanping et al.[33]have also shown TPC, TFC correlated well with ABTS+· scavenging capacity, FRAP, whereas DPPH radical scavenging capacity correlated well with ABTS+· scavenging capacity and FRAP.
3 Conclusion
In this study, the bioactive components including phenolic, flavonoid, anthocyanin, total tannic and VC from CE and fractions have been evaluated, and their antioxidant activity was tested by DPPH radical scavenging capacity,ABTS+· scavenging capacity and FRAP. The results showed that TPC, TFC, VCC, TTC and TAC were 1.836, 0.874, 3.567,3.578, 2.970 mg/g mf, respectively in CE, and their DPPH redical scavenging capacity, ABTS+· scavenging capacity and FRAP were 22.325, 17.595 and 26.487 μmol TE/g mf,respectively. Except flavonoids, the highest levels of polyphenols, tannins, VC and anthocyanin in were well desorbed with water was and founded in FrⅠ, High correlations were found among total antioxidant activity(DPPH radical, ABTS+· scavenging capacity and FRAP)and antioxidant constituents (TPC, TFC, VCC, TTC, TAC),which indicating that the antioxidant activity of spine grape peels maybe mainly attributed to the condensed tannins and VC and anthocyanin. The present study also suggested that the spine grape peel extract and its antioxidant enriched fractions could be developed for the food industry as a dietary supplement for health promotion. Further research is needed to determine the antioxidant activity of spine grape peel extract in vivo and its antioxidant mechanisms.
[1] SCIBIOR-BENTKOWSKA D, CZECZOT H. Cancer cells and oxidative stress[J]. Postępy Higieny I Medycyny Doświadczalnej,2009, 63(9): 58-72.
[2] SUNG H J, MA W, STAROST M F, et al. Ambient oxygen promotes tumorigenesis[J]. PLoS ONE, 2011, 6(5): 167-173. DOI:10.1371/journal.pone.0019785.
[3] LAKKUR S, JUDD S, BOSTICK R M, et al. Oxidative stress,inf l ammation, and markers of cardiovascular health[J]. Atherosclerosis,2015, 243(1): 38-43. DOI:10.1016/j.atherosclerosis.2015.08.032.
[4] DHALLA N S, TEMSAH R M, NETTICADAN T. Role of oxidative stress in cardiovascular diseases[J]. Journal of Hypertension, 2000,18(6): 655-673. DOI:10.1097/00004872-200018060-00002.
[5] GRIENDLING K K, FITZGERALD G A. Oxidative stress and cardiovascular injury: part I: basic mechanisms and in vivo monitoring of ROS[J]. Circulation, 2003, 108(16): 1912-1916. DOI:10.1161/01.cir.0000093660.86242.bb.
[6] CERIELLO A, MOTZ E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease?the common soil hypothesis revisited[J]. Arteriosclerosis Thrombosis and Vascular Biology, 2004, 24(5): 816-823. DOI:10.1161/01.atv.0000122852.22604.78.
[7] KEENAN K P, SOPER K A, SMITH P F, et al. Diet, overfeeding, and moderate dietary restriction in control Sprague-Dawley rats: I. effects on spontaneous neoplasms[J]. Toxicologic Pathology, 1995, 23(3):269-286. DOI:10.1177/019262339502300305.
[8] HALAGAPPA V K M, GUO Z H, PEARSON M, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral def i cits in the triple-transgenic mouse model of Alzheimer’s disease[J].Neurobiology of Disease, 2007, 26(1): 212-220. DOI:10.1016/j.nbd.2006.12.019.
[9] FONTANA L, MEYER T E, KLEIN S, et al. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans[J]. Proceedings of the National Academy of Sciences, 2004,101(17): 6659-6663. DOI:10.1073/pnas.0308291101.
[10] FONTANA L, VILLAREAL D T, WEISS E P, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors.a randomized, controlled trial[J]. American Journal of Physiology,Endocrinology and Metabolism, 2007, 293(1): 197-202. DOI:10.1152/ajpendo.00102.2007.
[11] GOKSIN N Y, YAVUZ D, ESAT A, et al. Grape seed extract has superior beneficial effects than vitamin E on oxidative stress and apoptosis in the hippocampus of streptozotocin induced diabetic rats[J].Gene, 2014, 555(2): 119-126. DOI:10.1016/j.gene.2014.10.052.
[12] OUYANG J E, XIONG X Y, LIU D B, et al. The comprehensive exploitation and utilization of spine grape[J]. Southern Horticulture,2007, 18(2): 29-31. DOI:10.3969/j.issn.1674-5868.2007.02.016.
[13] MENG J F, XU T F, QIN M Y, et al. Phenolic characterization of young wines made from spine grape (Vitis davidii Foëx.) grown in Chongyi County (China)[J]. Food Research International, 2012, 49(2):664-671. DOI:10.1016/j.foodres.2012.09.013.
[14] BAO R F. Study thorn thorns grapes and wine aroma components[D].Changsha: Hunan Agricultural University, 2010: 5.
[15] WANG R C. Extraction and pharmacological evaluation of functional compositions as well as juice processing from fruits of spine grape[D].Changsha: Hunan Agricultural University, 2006: 78.
[16] JIN X M, ZHU C Y, XIA D Z. Study on the extraction and antioxidant activity of total phenolic compounds from the spine grape [Vitis davidii(Rom. Caill.) Foëx.][J]. Journal of Yunnan University of Traditional Chinese Medicine, 2016, 39(1): 21-26. DOI:10.19288/j.cnki.issn.1000-2723.2016.01.006.
[17] LI Z. Research on extraction, purification and antioxidant effects of polyphenols in apple pomace[D]. Beijing: Chinese Academy of Agricultural Sciences, 2014: 33.
[18] WANG W Q, DENG J H, SHI X B, et al. Isolation, purif i cation and structure identif i cation of anthocyanins from Vitis davidii Foëx. skin[J].Transactions of the Chinese Society of Agricultural Engineering, 2016,32(4): 296-301. DOI:0.11975/j.issn.1002-6819.2016.04.042.
[19] ZHANG M X, LIU C H, ZHANG J. Composition analysis of phenols in Vitis ficifolia Bge. and Vitis davidii Foëx. fruit skins[J]. Food Science, 2011, 32(14): 264-267.
[20] DENG J H. Study on the brier grape (Vitis davidii Foëx.) skin pigment[D]. Changsha: Hunan Agricultural University, 2007: 12.
[21] SUN H Y. Optimization for extraction of anthocyanin from strawberry fruit using response surface methodology[J]. Science and Technology of Food Industry, 2013, 34(10): 243-250. DOI:10.13386/j.issn1002-0306.2013.10.057.
[22] MENG Jiangfei, FANG Yulin, QIN Yangmin, et al. Varietal differences among the phenolic prof i les and antioxidant properties of four cultivars of spine grape (Vitis davidii Foëx.) in Chongyi County(China)[J]. Food Chemistry, 2012, 134(4): 2049-2056. DOI:10.1016/j.foodchem.2012.04.005.
[23] MENG J F, XU T F, SONG C Z, et al. Characteristic free aromatic components of nine clones of spine grape (Vitis davidii Foëx.) from Zhongfang County (China)[J]. Food Research International, 2013,54(2): 1795-1800. DOI:10.1016/j.foodres.2013.09.039.
[24] BURSAL E, KÖKSAL E, GÜLÇIN İ, et al. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium, L.) determined by LC-MS/MS[J]. Food Research International, 2013, 51(1): 66-74.DOI:10.1016/j.foodres.2012.11.022.
[25] PEI Yongping, LI Weilin, ZHANG Hanqin. Improvement of AlCl3colorimetry for determination of total flavonoids[J]. Research and Practice on Chinese Medicines, 2009(4): 58-60.
[26] ZHAO Wenjie, XUE Bin, HU Minhua, et al. Extraction and purification technology of tannins from grape residue and determination of tannins contents[J]. China Brewing, 2010, 46(5): 721-745. DOI:10.3969/j.issn.0254-5071.2010.08.048.
[27] MA Hongfei, LU Shengju, HAN Qiuju, et al. Determination of vitamin C content in five kinds of fruits and vegetables by UV spectrophotometry[J]. Chemistry & Bioengineering, 2012, 29(8): 92-94. DOI:10.3969/j.issn.1672-5425.2012.08.026.
[28] XU Honggao, LIU Xuan, YAN Qiuli, et al. A novel copigment of quercetagetin for stabilization of grape skin anthocyanins[J]. Food Chemistry, 2015, 166: 50-55. DOI:10.1016/j.foodchem.2014.05.125.
[29] GORJANOVIĆ S, KOMES D, PASTOR F T, et al. Antioxidant capacity of teas and herbal infusions: polarographic assessment[J].Journal of Agricultural and Food Chemistry, 2012, 60(38): 9573-9580.DOI:10.1021/jf302375t.
[30] OH J, JO H, CHO A R, et al. Antioxidant and antimicrobial activities of various leafy herbal teas[J]. Food Control, 2013, 31(2): 403-409.DOI:10.1016/j.foodcont.2012.10.021.
[31] LU Xiaonan, ROSS C F, POWERS J R, et al. Determination of total phenolic content and antioxidant activity of garlic (Allium sativum) and elephant garlic (Allium ampeloprasum) by attenuated total reflectancefourier transformed infrared spectroscopy[J]. Journal of Agricultural and Food Chemistry, 2011, 59(10): 5215-5221. DOI:10.1021/jf201254f.
[32] LU H H, KAO SYLIU T Y, LIU S T, et al. Areca nut extract induced oxidative stress and upregulated hypoxia inducing factor leading to autophagy in oral cancer cells[J]. Autophagy, 2010, 6(6): 725-737.DOI:10.4161/auto.6.6.12423.
[33] ZOU Yanping, CHANG S K C, GU Yan, et al. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton)extract and its fraction[J]. Journal of Agricultural and Food Chemistry,2011, 59(6): 2268-2276. DOI:10.1021/jf104640k.
刺葡萄皮醇提物和不同洗脱组分抗氧化成分及活性
陆 俊1,2,张佳琦1,罗 丹1,王 珺3,任艳艳1,李忠海1,2,*
(1.中南林业科技大学食品科学与工程学院,湖南 长沙 410004;2.稻谷及副产物深加工国家工程实验室,湖南 长沙 410004;3.中国农业科学研究院农产品加工研究所,北京 100193)
以酸化甲醇为溶剂对刺葡萄皮进行超声波辅助提取,并用大孔树脂进行分离得到5 种不同洗脱组分,研究刺葡萄皮醇提物和不同洗脱组分的活性成分及抗氧化能力。结果表明,刺葡萄皮含有较高含量的多酚(1.836 mg/g mf)、黄酮(0.874 mg/ g mf)、VC(3.567 mg/g mf)、丹宁(3.578 mg/g mf)和花青素(2.970 mg/g mf),其1,1-二苯基-2-三硝基苯肼(1,1-diphenyl-2-picrylhydrazyl,DPPH)自由基清除能力、2,2’-二氮-双(3-乙基苯并噻唑-6-磺酸)二铵盐自由基(2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) radical,ABTS+·)清除能力、铁离子还原能力(ferric reducing antioxidant power,FRAP)分别为22.325、17.595、26.487 μmol TE/g mf。在5 种组分中以水解吸组分活性成分含量最高,其多酚、黄酮、VC、丹宁和花青素含量分别为0.876、0.116、1.577、1.576 mg/g mf和1.330 mg/g mf,其DPPH自由基、ABTS+·清除能力和FRAP分别为3.636、10.109、13.415 μmol TE/g mf,相关性分析表明抗氧化活性与活性成分花青素、丹宁、VC含量呈强相关关系,表明抗氧化活性可能主要由这3 种活性物质贡献。本研究结果表明,刺葡萄皮有潜力作为膳食补充抗氧化物质的主要来源。
刺葡萄皮;抗氧化活性;活性成分
2016-07-24
湖南省科技支撑计划项目(2015NK3022)
陆俊(1978—),男,副教授,博士,研究方向为天然产物开发与利用。E-mail:690056167@qq.com
TS255.2
A
1002-6630(2017)23-0087-07
*通信作者:李忠海(1960—),男,教授,博士,研究方向为天然产物开发与利用。E-mail:lizh11@163.com
10.7506/spkx1002-6630-201723015
LU Jun, ZHANG Jiaqi, LUO Dan, et al. Antioxidant activity of methanol extract and its fractions from spine grape (Vitis davidii Foëx.) peel[J]. 食品科学, 2017, 38(23): 87-93.
10.7506/spkx1002-6630-201723015. http://www.spkx.net.cn LU Jun, ZHANG Jiaqi, LUO Dan, et al. Antioxidant activity of methanol extract and its fractions from spine grape (Vitis davidii Foëx.) peel[J]. Food Science, 2017, 38(23): 87-93. (in English with Chinese abstract) DOI:10.7506/spkx1002-6630-201723015. http://www.spkx.net.cn
