The role of glycogen synthase kinase 3 beta in brain injury induced by myocardial ischemia/reperfusion injury in a rat model of diabetes mellitus
2017-11-08BoZhaowenweiGaoYajingLiuMengJiangLianLiuQuanYuanJiabaoJouZhongyuanXia
Bo Zhao, wen-wei Gao, Ya-jing Liu, Meng Jiang, Lian Liu, Quan Yuan, Jia-bao Jou , Zhong-yuan Xia,
1 Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Critical Care Medicine, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
How to cite this article: Zhao B, Gao WW, Liu YJ, Jiang M, Liu L, Yuan Q, Hou JB, Xia ZY (2017) e role of glycogen synthase kinase 3 beta in brain injury induced by myocardial ischemia/reperfusion injury in a rat model of diabetes mellitus. Neural Regen Res 12(10):1632-1637.
Funding: is study was supported by the National Natural Science Foundation of China, No. 81471844; the Natural Science Foundation of Hubei Province of China, No. 2016CFB167; the Basic Scientific Research Foundation of Central Universities, No. 2042017kf0147.
The role of glycogen synthase kinase 3 beta in brain injury induced by myocardial ischemia/reperfusion injury in a rat model of diabetes mellitus
Bo Zhao1, wen-wei Gao2, Ya-jing Liu1, Meng Jiang1, Lian Liu1, Quan Yuan1, Jia-bao Jou1, Zhong-yuan Xia1,*
1 Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Critical Care Medicine, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
How to cite this article: Zhao B, Gao WW, Liu YJ, Jiang M, Liu L, Yuan Q, Hou JB, Xia ZY (2017)e role of glycogen synthase kinase 3 beta in brain injury induced by myocardial ischemia/reperfusion injury in a rat model of diabetes mellitus. Neural Regen Res 12(10):1632-1637.
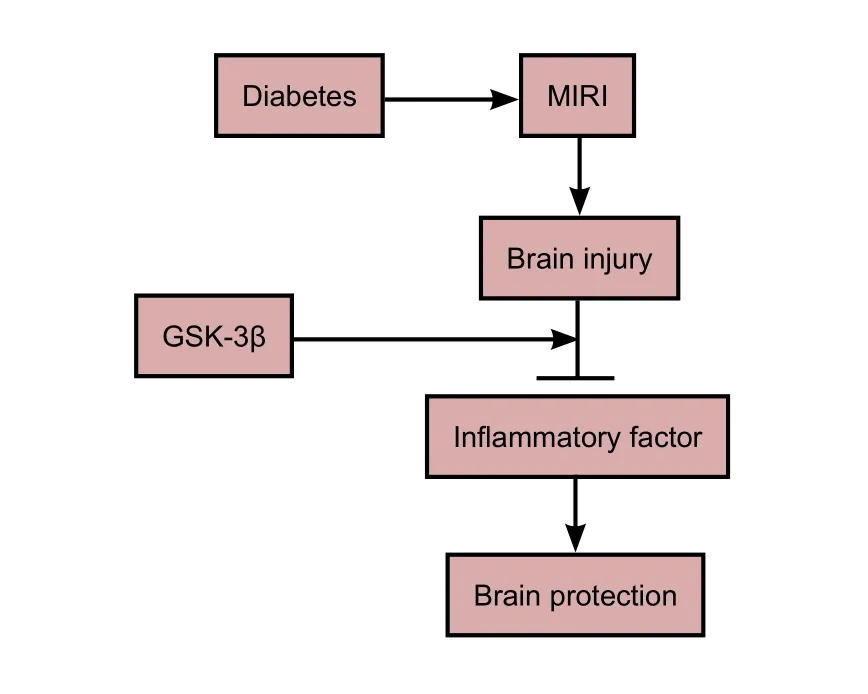
Myocardial ischemia/reperfusion injury can lead to severe brain injury. Glycogen synthase kinase 3 beta is known to be involved in myocardial ischemia/reperfusion injury and diabetes mellitus. However, the precise role of glycogen synthase kinase 3 beta in myocardial ischemia/reperfusion injury-induced brain injury is unclear. In this study, we observed the effects of glycogen synthase kinase 3 beta on brain injury induced by myocardial ischemia/reperfusion injury in diabetic rats. Rat models of diabetes mellitus were generated via intraperitoneal injection of streptozotocin. Models of myocardial ischemia/reperfusion injury were generated by occluding the anterior descending branch of the lecoronary artery. Post-conditioning comprised three cycles of ischemia/reperfusion. Immunohistochemical staining and western blot assays demonstrated that aer 48 hours of reperfusion, the structure of the brain was seriously damaged in the experimental rats compared with normal controls. Expression of Bax, interleukin-6, interleukin-8, terminal deoxynucleotidyl transferase dUTP nick end labeling, and cleaved caspase-3 in the brain was significantly increased, while expression of Bcl-2, interleukin-10, and phospho-glycogen synthase kinase 3 beta was decreased. Diabetes mellitus can aggravate inflammatory reactions and apoptosis. Ischemic post-conditioning with glycogen synthase kinase 3 beta inhibitor lithium chloride can effectively reverse these changes. Our results showed that myocardial ischemic post-conditioning attenuated myocardial ischemia/reperfusion injury-induced brain injury by activating glycogen synthase kinase 3 beta. According to these results, glycogen synthase kinase 3 beta appears to be an important factor in brain injury induced by myocardial ischemia/reperfusion injury.
nerve regeneration; myocardial ischemia/reperfusion injury; brain injury; glycogen synthase kinase 3 beta; ischemic post-conditioning;diabetes mellitus; neural regeneration
Introduction
Myocardial ischemia/reperfusion injury (MIRI) prompts a release of oxygen free radicals, intracellular calcium overload, accumulation of inflammatory reaction, and over-expression of apoptosis-inducing factor (Shang et al., 2010;Gao et al., 2013). MIRI not only leads to cardiac dysfunction but may also cause distant organ dysfunction, which can increase the morbidity and mortality of patients (Tapuria et al., 2008; Ren et al., 2011).e brain is highly susceptible to ischemia/reperfusion injury, and severe brain damage can substantially lower quality of life (Dai et al., 2013; Las Hayas et al., 2015). However, the effects of MIRI on the brain are unknown.
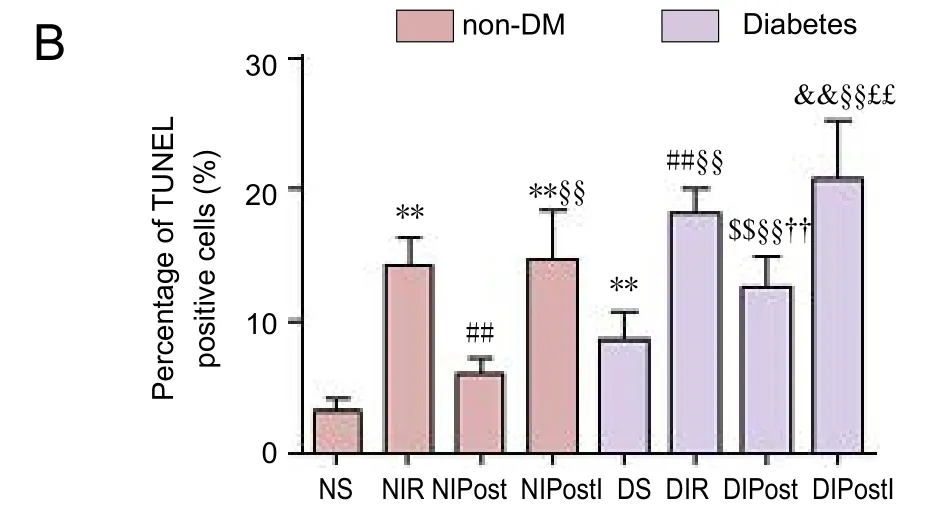
Figure 3 Effect of glycogen synthase kinase 3 beta (GSK-3β) on cell apoptosis in the brain of diabetic rats with myocardial ischemia/reperfusion injury (TUNEL assay).
Diabetes mellitus is an endocrine disorder that affects most population and is a causative factor for other chronic health problems including heart disease (Tanne, 2008; Collino et al., 2009). Diabetes mellitus can increase oxidative stress, reduce the tolerance of the organism, and increase morbidity and mortality (Rizk et al., 2006; Laursen et al.,2017). Diabetes is also a high risk factor for other diseases,and may increase susceptibility to MIRI (Settergren et al.,2009).
Glycogen synthase kinase 3 beta (GSK-3β) is highly expressed in the brain, lungs, and kidneys (Ilouz et al., 2006;Petit-Paitel et al., 2009). In recent years, GSK-3β has been found to play a crucial role in many conditions, such as tumors, Alzheimer’s disease, and diabetes mellitus (Amar et al., 2011; Bian et al., 2016; Hu et al., 2016). GSK-3β is also closely linked with oxidative stress, cell apoptosis, and inflammatory reactions (Dugo et al., 2007; Zhao et al., 2012;Wang et al., 2017). However, the differences in the role of GSK-3β in brain injury induced by myocardial ischemia/reperfusion in individuals with or without diabetes mellitus remain unclear.
In the present study, we explored whether inflammatory and apoptotic factors produced by MIRI could trigger brain injury, with a focus on the possible role of GSK-3β.
Materials and Methods
Animals
Sixty-four 8-week-old male Sprague-Dawley rats weighing 250–300 g were maintained at 25°C and 50% humidity.e rats had free access to chow and water and lived in individual ventilated cages under specific-pathogen-free conditions in the Animal Facility of the Experimental Research Center of Wuhan University of China (license No. SCXK(Jing) 2014-0004).e study protocol was approved by the Animal Ethics Committee of Wuhan University of China(WDRY2016-K145). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No.85-23, revised 1986).
Experimental protocols
(2) Normal myocardial ischemia reperfusion group (NIR):myocardial ischemia reperfusion;
(3) Normal ischemic post-conditioning group (NIPost):myocardial ischemia+ post-conditioning;
(4) Normal ischemic post-conditioning + GSK-3β inhibitor group (NIPostI): GSK-3β inhibitor + myocardial ischemia +post-conditioning;
(5) Diabetic sham group (DS): diabetes mellitus model +sham operation;
(6) Diabetic myocardial ischemia reperfusion group (DIR):diabetes mellitus model + myocardial ischemia reperfusion;
(7) Diabetic ischemic post-conditioning group (DIPost):diabetes mellitus model + myocardial ischemia + post-conditioning;
(8) Diabetic ischemic post-conditioning + GSK-3β inhibitor group (DIPostI): diabetes mellitus model + GSK-3β inhibitor + myocardial ischemia + post-conditioning.
The NS and DS groups were subjected to thoracotomy.We induced MIRI in the NIR and DIR groups by blocking the left anterior descending coronary artery. The NIPost group and DIPost group underwent three cycles of 10-second reperfusion followed by a 10-second ischemia treatment immediately at the onset of reperfusion.e NIPostI group and DIPostI group were intraperitoneally injected with GSK-3β inhibitor 10 minutes before receiving MIRI and received three cycles of 10-second reperfusion followed by a 10-second ischemia treatment immediately at the onset of reperfusion.
Diabetes mellitus model and MIRI model
Histopathology of brain tissue
After reperfusion, the rats were sacrificed and the whole brains harvested, except the cerebellum and stem.e tissue was fixed in 10% formaldehyde solution for 48 hours, embedded in paraffin, cut into 4 μm pieces using a microtome(Leica, Germany), and stained with hematoxylin-eosin.e staining was quantified under a BX51 microscope (× 400;Olympus, Tokyo, Japan).
Immunohistochemical staining and terminal deoxynucleotidyl transferase dUTP nick end labeling(TUNEL) assay
We performed immunohistochemical staining and a TUNEL assay on four rats in each group for Bax, Bcl-2,interleukin (IL)-6, IL-8, and IL-10 in the brain. Paraffin-embedded sections were dewaxed and rehydrated. After immersing the sections in equilibration buffer three times, they were incubated with 3% (v/v) H2O2and 10% (v/v) methanol in phosphate buffered saline (pH 7.4) at room temperature in a humidified chamber for 10 minutes to block endogenous peroxidase activity. Sections were then incubated in stop/wash buffer and subjected to antigen retrieval by boiling sections in 10 mM sodium citrate buffer (pH 6.0) in a microwave for 20 minutes. After cooling sections to room temperature, slides were washed with PBS (pH 6.5). After being blocked in normal goat serum (1:10; Boster Biotech Inc., Wuhan, China) for 10 minutes at room temperature,sections were incubated overnight at 4°C with rabbit anti-IL-6, IL-8, IL-10 (1:200; Boster Biotech Inc.), Bax, and Bcl-2 (1:100; ZSGB Biotech Inc.) polyclonal antibodies.After washing the sections with PBS, they were incubated for 10 minutes at room temperature with biotinylated goat anti-rabbit IgG (1:10; Maixin Biotech Inc.) and washed with PBS, followed by incubation in a streptavidin-biotin-peroxidase complex (Maixin Biotech Inc.) for 10 minutes at room temperature. Staining was visualized using diaminobenzidine (Maixin Biotech Inc.) as a substrate. Negative control sections were not treated with primary antibodies. The percentage of positive cells was quantified by the number of positive cells/the number of total cells × 100% under BX51 microscope (400×; Olympus).
Paraffin-embedded sections were dewaxed and rehydrated,then incubated in 20 μL/mL proteinase K for 15 minutes.TUNEL was accomplished using an in situ cell death detection kit (Roche Inc., Germany). After immersion in equilibration buffer for 10 minutes, sections were incubated with TdT and dUTP-digoxigenin in a humidified chamber and then incubated in the stop/wash buffer. Sections were washed before incubation in anti-digoxigenin-peroxidase solution(1:500 in PBS), and visualized with diaminobenzidine-H2O2solution. TUNEL-positive cells were identified by the presence of a brown color in the nucleus of dead cells.e ratio of TUNEL-positive cells in the brain was quantified by the number of TUNEL-positive cells/the number of total cells × 100%,under the BX51 microscope (400×; Olympus).
western blot assay
We used four rats in each group to determine the expression of phosphorylated-GSK-3β (p-GSK-3β), total GSK-3β, caspase-3, and cleaved caspase-3 in brain tissue via a western blot assay. Aer 2 hours of reperfusion, brain tissue was sampled (100 mg) and homogenized with 1,000 μL lysis buffer. The homogenates were centrifuged at 12,000 × g at 4°C for 15 minutes. Equivalent amounts (50 μg/lane) of total protein extracts were loaded into each lane, which were separated by 10% sodium dodecyl sulphate gels, and then transferred onto a polyvinylidene fluoride membrane (current:200 mA, time: 40–70 minutes).e membrane was blocked with 5% bovine serum albumin for 1 hour and then incubated with the following primary rabbit monoclonal antibodies diluted in 5% w/v bovine serum albumin overnight at 4°C:p-GSK-3β (at Ser9), total GSK-3β, caspase-3, and cleaved caspase-3 (1:1,000; Cell Signaling Technology, USA). After three washes with Tris-Buffered Saline and Tween 20,the membrane was incubated with fluorescent-tagged goat anti-rabbit polyclonal IgG (1:10,000; LI-COR, USA) for 1 hour at room temperature followed by additional washing.GADPH was chosen as a loading control to further assure that all samples had the same volume. The measurement grayscale of each band was as follows: the protein in the NS group was taken as 100%, and we compared the target protein to the protein in NS group as the relative expression.
Statistical analysis
Data are presented as the mean ± SD and were analyzed using SPSS 17.0 soware (SPSS, Chicago, IL, USA).e means of different groups were compared using one-way analysis of variance and the Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
Results
Cerebral hematoxylin and eosin staining
In the NS group, the cortical neurons were arranged in neat rows with abundant cytoplasm, and the nuclei were round and basophilic. In the NIR group, the cortical neurons had a damaged structure. The cytoplasm was stained light red with uneven distribution and vacuoles, and the nuclei were condensed. In the NIPost group, however, the cell structure was normal. Most neurons had complete membrane integrity and the nuclei were clear. In the NIPostI group, the GSK-3β inhibitor eliminated the protective effect of ischemic post-conditioning. Histopathological changes were more severe in diabetic vs. non-diabetic rats (Figure 1).
Expression of Bax, Bcl-2, IL-6, IL-8, and IL-10 in the brainBax and Bcl-2 belong to the Bcl-2 gene family, which is an essential factor in cell death. IL-6, IL-8, and IL-10 are important indicators of inflammatory response. Our results indicated significantly increased Bax, IL-6, and IL-8, and significantly decreased Bcl-2 and IL-10 expression in the brain in the NIR group (P < 0.01). In contrast, post-conditioning reversed these changes in protein expression following NIR(P < 0.01). In the NIPostI group, the consequence was no better than those in the NIR group.ese data indicate that diabetes mellitus can aggravate the expression of inflammatory reaction and apoptosis-inducing factor (Figure 2).
Expression of apoptosis in the brain as detected by a TUNEL assay
Effect of post-conditioning on GSK-3β and caspase-3
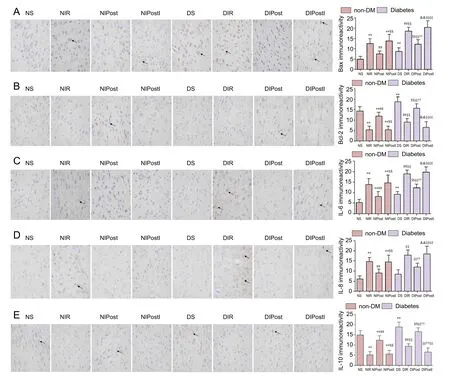
Figure 2 Effect of glycogen synthase kinase 3 beta (GSK-3β) on Bax, Bcl-2, interleukin (IL)-6, IL-8, and IL-10 immunoreactivity in the brain of diabetic rats with myocardial ischemia/reperfusion injury (%).
We examined cerebral GSK-3β, p-GSK-3β, caspase-3, and cleaved-caspase-3 via western blot assay. As shown, we found no noticeable differences in the expression of total GSK-3β and caspase-3 in the eight groups. p-GSK-3β levels were markedly decreased in the NIR and NIPostI groups (P < 0.01).Post-conditioning increased the level of p-GSK-3β (P < 0.01).The expression of cleaved-caspase-3 was conserved when compared with the expression of p-GSK-3β.ese results indicate that diabetes may impair GSK-3β function (Figure 4).
Discussion

Figure 4 Effect of post-conditioning with glycogen synthase kinase 3 beta (GSK-3β) on the protein expression of GSK-3β and caspase-3 in the brain of diabetic rats with myocardial ischemia/reperfusion injury.
Myocardial infarction is one of the leading causes of death globally. Ischemia/reperfusion injury is the dominant event associated the abovementioned mortality. Numerous factors contribute to the pathogenesis of ischemia/reperfusion injury, such as oxygen free radicals, intracellular calcium overload, accumulation of inflammatory molecules, and over-expression of pro-apoptosis factor (Meng et al., 2015;Bagheri et al., 2016; del Zoppo, 2016). However, the detailed contributions of these different factors are unknown.
MIRI may not only lead to cardiac dysfunction but also may cause distant organ dysfunction. In this study, we found that after MIRI, the structures of cortical neurons were damaged. Specifically, the cytoplasm was stained light red with uneven distribution and vacuoles, and nuclei were condensed, indicating ischemic brain injury. Little is known about this phenomenon, but catecholamine toxicity, elevated pro-inflammatory mediators, and immune cell activation be implicated (Koga et al., 2010; Wybraniec et al., 2014).
Diabetes mellitus is the most common endocrine disease in the world. It is well characterized by chronic hyperglycemia and abnormal glucose metabolism. Diabetes mellitus is oen concurrent with hypertension and dyslipidemia (Song et al., 2016). Diabetic patients have a higher incidence of myocardial infarction and a poorer prognosis afterwards,indicating that diabetes mellitus may increase the susceptibility to ischemia/reperfusion injury. The increased oxidative stress and inflammatory response following ischemia/reperfusion injury under diabetic conditions may explain this phenomenon (Tang et al., 2012; Luo et al., 2013).
Post-conditioning can alleviate the effects of myocardial ischemia/reperfusion injury. We demonstrated that posy-conditioning can also effectively attenuate cerebral inflammatory responses and reduce brain injury. However,the beneficial effect was partly compromised by diabetes mellitus (Zhang et al., 2012; Ramagiri et al., 2017).
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that has recently emerged as a key regulatory factor in the modulation of inflammatory responses. GSK-3 comprises two similar subunits named GSK-3α and GSK-3β(Woodgett, 1990; Garcia-Herreros et al., 2012).e precise difference between these two molecules is unclear. Recent research has focused on GSK-3α.e activation of GSK-3β,which may be regulated by the mPTP channel, has been reported to play a pivotal role in the pathophysiology of organ injury/dysfunction associated with MIRI (Das et al., 2008;Miura et al., 2012). Gross et al. (2004) showed that in an in vivo model of ischemia and reperfusion, GSK inhibitors,added either before ischemia or at the start of reperfusion,could decrease necrosis (Gross et al., 2004).
In the present study, we found that during ischemia/reperfusion injury, GSK-3β was unchanged while p-GSK-3β expression was significantly modulated. This phenomenon indicates that at the early stage of ischemia/reperfusion, a large number of inflammatory cytokines regulate the concentration of Ca2+and K+via GSK-3β (Gomez et al., 2008; Xi et al., 2010). When mPTP channels open, organs are dam-aged by the active oxygen free radicals (Zhu et al., 2010). Diabetes mellitus can stimulate an organism to produce more oxygen free radicals, and decrease the speed of oxygen free radical removal (Khullar et al., 2010; Zhao et al., 2017).
GSK-3β inhibition appeared to attenuate the protective effect of post-conditioning on ischemia/reperfusion injury.The most commonly used GSK-3β inhibitors are 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione and SB216763.Here, we used LiCl as a GSK-3β inhibitor, like several other researchers (Kim et al., 2014; Tatsumoto et al, 2016).Post-conditioning can induce GSK-3β auto-phosphorylation, thus lessening the degree of reperfusion injury (Nayak et al., 2012; Wu et al., 2012), this can be partly counteracted by the diabetic state. GSK-3β inhibition has been found to attenuate the activation of the two stress-activated MAPKs:p38 and JNK1/2 in transient cerebral ischemia/reperfusion injury (Collino et al., 2008; Sharma et al., 2012). Our results supported this finding.
In conclusion, activation of GSK-3β is involved in brain injury induced by MIRI in both normal and diabetic subjects. Inhibition of GSK-3β activation alleviates the protective effects of post-conditioning, although the particular mechanisms underlying this effect have yet to be defined.Further investigations are warranted to delineate the various signaling pathways that involve GSK-3β.
Limitations
Author contributions:BZ and ZYX designed this study. BZ, WWG and LL performed experiments. YJL, QY and JBH analyzed data. BZ and MJ wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:e study protocol was approved by the Animal Ethics Committee of Wuhan University of China (WDRY2016-K145). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986). All efforts were made to minimize animal suffering and the number of animals necessary to produce reliable results.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Amar S, Belmaker RH, Agam G (2011) The possible involvement of glycogen synthase kinase-3 (GSK-3) in diabetes, cancer and central nervous system diseases. Curr Pharm Des 17:2264-2277.
Bagheri F, Khori V, Alizadeh AM, Khalighfard S, Khodayari S, Khodayari H (2016) Reactive oxygen species-mediated cardiac-reperfusion injury: Mechanisms and therapies. Life Sci 165:43-55.
Bian H, Bian W, Lin X, Ma Z, Chen W, Pu Y. (2016). RNA Interference Silencing of Glycogen Synthase Kinase 3β Inhibites Tau Phosphorylation in Mice with Alzheimer Disease. Neurochem Res 41:2470-2480.
Collino M, Aragno M, Castiglia S, Tomasinelli C, Thiemermann C,Boccuzzi G, Fantozzi R (2009) Insulin reduces cerebral ischemia/reperfusion injury in the hippocampus of diabetic rats a role for glycogen synthase kinase-3β. Diabetes 58:235-242.
Collino M, Thiemermann C, Mastrocola R, Gallicchio M, Benetti E,Miglio G, Castiglia S, Danni O, Murch O, Dianzani C, Aragno M,Fantozzi R (2008) Treatment with the glycogen synthase kinase-3 beta inhibitor, TDZD-8, affects transient cerebral ischemia/reperfusion injury in the rat hippocampus. Shocke 30:299-307.
Dai SS, Wang H, Yang N, An JH, Li W, Ning YL, Zhu PF, Chen JF,Zhou YG (2013) Plasma glutamate-modulated interaction of A2AR and mGluR5 on BMDCs aggravates traumaticbrain injury-induced acute lung injury. J Exp Med 210:839-851.
Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C (2008) Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res 103:983-991.
del Zoppo GJ (2006). Stroke and neurovascular protection. N Engl J Med 354:553-555.
Gao D, Kawai N, Nakamura T, Lu F, Fei Z, Tamiya T (2013) Anti-inflammatory effect of D-allose in cerebral ischemia/reperfusion injury in rats. Neurol Med Chir (Tokyo) 53:365-374.
Garcia-Herreros M, Aparicio IM, Rath D, Fair T, Lonergan P (2012)Differential glycolytic and glycogenogenic transduction pathways in male and female bovine embryos produced in vitro. Reprod Fertil Dev 24:344-352.
Gomez L, Paillard M,ibault H, Derumeaux G, Ovize M (2008) Inhibition of GSK-3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation 117:2761-2768.
Gross ER, Hsu AK, Gross GJ (2004) Opioid-induced cardioprotection occurs viaglycogen synthase kinase beta inhibition during reperfusion in intact rathearts. Circ Res 94:960-966.
Hu B, Wu Y, Liu J, Shen X, Tong F, Xu G, Shen R (2016) GSK-3beta inhibitor induces expression of Nrf2/TrxR2 signaling pathway to protect against renal ischemia/reperfusion injury in diabetic rats.Kidney Blood Press Res 41:937-946.
Ilouz R, Kowalsman N, Eisenstein M, Eldar-Finkelman H (2006) Identification of novel glycogen synthase kinase-3beta substrate-interacting residues suggests a common mechanism for substrate recognition. J Biol Chem 281:30621-30630.
Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK (2010)Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol 88:233-240.
Kim EY, Kim A, Kim SK, Kim HJ, Chang J, Ahn CM, Chang YS (2014)Inhibition of mTORC1 induces loss of E-cadherin through AKT/GSK-3β signaling-mediated upregulation of E-cadherin repressor complexes in non-small cell lung cancer cells. Respir Res 15:26.
Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, Yuasa M, Maekawa T (2010) Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res 32:925-932.
Las Hayas C, López de Arroyabe E, Calvete E (2015) Resilience in family caregivers of persons with acquired brain injury. Rehabil Psychol 60:295-302.
Laursen DH, Christensen KB, Christensen U, Frølich A (2017) Assessment of short and long-term outcomes of diabetes patient education using the health education impact questionnaire (HeiQ). BMC Res Notes 10:213.
Liu M, Zhou B, Xia ZY, Zhao B, Lei SQ, Yang QJ, Xue R, Leng Y, Xu JJ,Xia Z (2013) Hyperglycemia induced inhibition of DJ-1 expression compromised the effectiveness of ischemic postconditioning cardioprotection in rats. Oxid Med Cell Longev 2013:564902.
Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J,Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME (2013) Diabetes increases mortality aer myocardial infarction by oxidizing CaMKII.J Clin Invest 123:1262-1274.
Meng S, Su Z, Liu Z, Wang N, Wang Z (2015) Rac1 contributes to cerebral ischemia reperfusion-induced injury in mice by regulation of Notch2. Neuroscience 306:100-114.
Miura T, Tanno M (2012)e mPTP and its regulatory proteins: final common targets of signalling pathways for protection against necrosis. Cardiovasc Res 94:181-189.
Nayak G, Cooper GM (2012) p53 is a major component of the transcriptional and apoptotic program regulated by PI 3-kinase/Akt/GSK3 signaling. Cell Death Dis 3:e400.
Petit-Paitel A, Brau F, Cazareth J, Chabry J (2009) Involvment of cytosolic and mitoehondrial GSK-3β in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. PLoS One 4:e5491.
Ramagiri S, Taliyan R (2017) Remote limb ischemic post conditioning during early reperfusion alleviates cerebral ischemic reperfusion injury via GSK-3β/CREB/ BDNF pathway. Eur J Pharmacol 803:84-93.
Ren C, Gao M, Dornbos D 3rd, Ding Y, Zeng X, Luo Y, Ji X (2011)Remote ischemic postconditioning reduced brain damage in experimental ischemia/ reperfusion injury. Neurol Res 33:514-519.
Rizk NN, Rafols JA, Dunbar JC (2006) Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res 1096:204-212.
Settergren M, Böhm F, Malmström RE, Channon KM, Pernow J (2009)L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis 204:73-78.
Shang L, Ananthakrishnan R, Li Q, Quadri N, Abdillahi M, Zhu Z, Qu W, Rosario R, Touré F, Yan SF, Schmidt AM, Ramasamy R (2010)RAGE modulates hypoxia reoxygenation injury in adult murine cardiomyocytes via jnk and GSK-3β signaling pathways. PLoS One 5:e10092.
Sharma N, Bhat AD, Kassa AD, Xiao Y, Arias EB, Cartee GD (2012)Improved insulin sensitivity with calorie restriction does not require reduced JNK1/2, p38, or ERK1/2 phosphorylation in skeletal muscle of 9-month-old rats. Am J Physiol Regul Integr Comp Physiol.302:R126-136.
Song JQ, Teng X, Cai Y, Tang CS, Qi YF (2009) Activation of Akt/GSK-3β signaling pathway is involved in intermedin1-53 protection against myocardial apoptosis induced by ischemia/reperfusion.Apoptosis 14:1299-1307.
Song QQ, Zhou HL, Zhen HT, Wang N, Deng J, Wang JX, Pan XH(2016) Establishment and evaluation of a rhesus monkey model of experimental type 2 diabetes mellitus. Zhongguo Zuzhi Gongcheng Yanjiu 20:6048-6053.
Tang WH, Martin KA, Hwa J (2012) Aldose reductase, oxidative stress,and diabetic mellitus. Front Pharmacol 3:87.
Tanne D (2008) Impaired glucose metabolism and cerebrovascular diseases. Adv Cardiol 45:107-113.
Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR (2008) Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury-a review. J Surg Res 150:304-330.
Tatsumoto N, Arioka M, Yamada S, Takahashi-Yanaga F, Tokumoto M, Tsuruya K, Kitazono T, Sasaguri T (2016) Inhibition of GSK-3β increases trabecular bone volume but not cortical bone volume in adenine-induced uremic mice with severe hyperparathyroidism.Physiol Rep 4:e13010.
Wang D, Tian Y, Feng W, Zhao L, Zhao M, Liu J, Wang Q (2017)Pseudolaric acid B induces endometrial cancer Ishikawa cell apoptosis and inhibits metastasis through AKT-GSK-3β and ERK1/2 signaling pathways. Anticancer Drugs 28:603-612.
Woodgett JR (1990) Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 9:2431-2438.
Wu QL, Shen T, Shao LL, Ma H, Wang JK (2012) Ischemic postconditioning mediates cardioprotection via PI3K/GSK-3β/β-catenin signaling pathway in ischemic rat myocardium. Shock 38:165-169.
Wu Y, Xia ZY, Dou J, Zhang L, Xu JJ, Zhao B, Lei S, Liu HM (2011)Protective effect of ginsenoside Rb1 against myocardial ischemia reperfusion injury in streptozotocin induced diabetic rats. Mol Biol Rep 38:4327-4335.
Wybraniec MT, Mizia-Stec K, Krzych Ł (2014) Neurocardiogenic injury in subarachnoid hemorrhage: a wide spectrum of catecholamine-mediated brain- heart interactions. Cardiol J 21:220-228.
Xi J, Tian W, Zhang L, Jin Y, Xu Z (2010) Morphine prevents the mitochondrial permeability transition pore opening through NO/cGMP/PKG/Zn2+/GSK-3beta signal pathway in cardiomyocytes. Am J Physiol Heart Circ Physiol 298:H601-607.
Xia R, Zhao B, Wu Y, Hou JB, Zhang L, Xu JJ, Xia ZY (2011) Ginsenoside Rb1 preconditioning enhances enos expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J Biomed Biotechnol. 2011:767930.
Zhang C, Lu X, Tan Y, Li B, Miao X, Jin L, Shi X, Zhang X, Miao L, Li X,Cai L (2012) Diabetes-induced hepatic pathogenic damage, inflammation, oxidative stress, and insulin resistance was exacerbated in zinc deficient mouse model. PLoS One 7:e49257.
Zhao B, Gao W, Hou J, Wu Y, Xia Z (2012). Ischemic postconditioning enhances glycogen synthase kinase-3β expression and alleviates cerebral ischemia reperfusion injury. Neural Regen Res 7:1507-1512.
Zhao D, Yang J, Yang L (2017) Insights for Oxidative Stress and mTOR Signaling in Myocardial Ischemia/Reperfusion Injury underDiabetes. Oxid Med Cell Longev doi: 10.1155/2017/6437467.
Zhao S, Fu J, Liu X, Wang T, Zhang J, Zhao Y (2012) Activation of Akt/GSK-3beta/ beta-catenin signaling pathway is involved in survival of neurons aer traumatic brain injury in rats. Neurol Res 34:400-407.
Zhu J, Rebecchi MJ, Tan M, Glass PS, Brink PR, Liu L (2010) Age-Associated Differences in Activation of Akt/GsK-3β signaling Pathways and inhibition of Mitochondrial Permeability transition Pore Opening in the Rat Heart. J Gerontol A Biol Sci Med Sci 65:611-619.
Zhu YM, Wang CC, Chen L, Qian LB, Ma LL, Yu J, Zhu MH, Wen CY,Yu LN, Yan M (2013) Both PI3K/Akt and ERK1/2 pathways participate in the protection by dexmedetomidine against transient focal cerebral ischemia/reperfusion injury in rats. Brain Res 1494:1-8.
Graphical Abstract
Glycogen synthase kinase 3 beta (GSK-3β) attenuates brain injury induced by myocardial ischemia/reperfusion injury (MIRI)
*Correspondence to:Zhong-yuan Xia, M.D.,xiazhongyuan2005@aliyun.com.
orcid:
0000-0002-5807-9554
(Zhong-yuan Xia)
10.4103/1673-5374.217337
Accepted: 2017-09-22
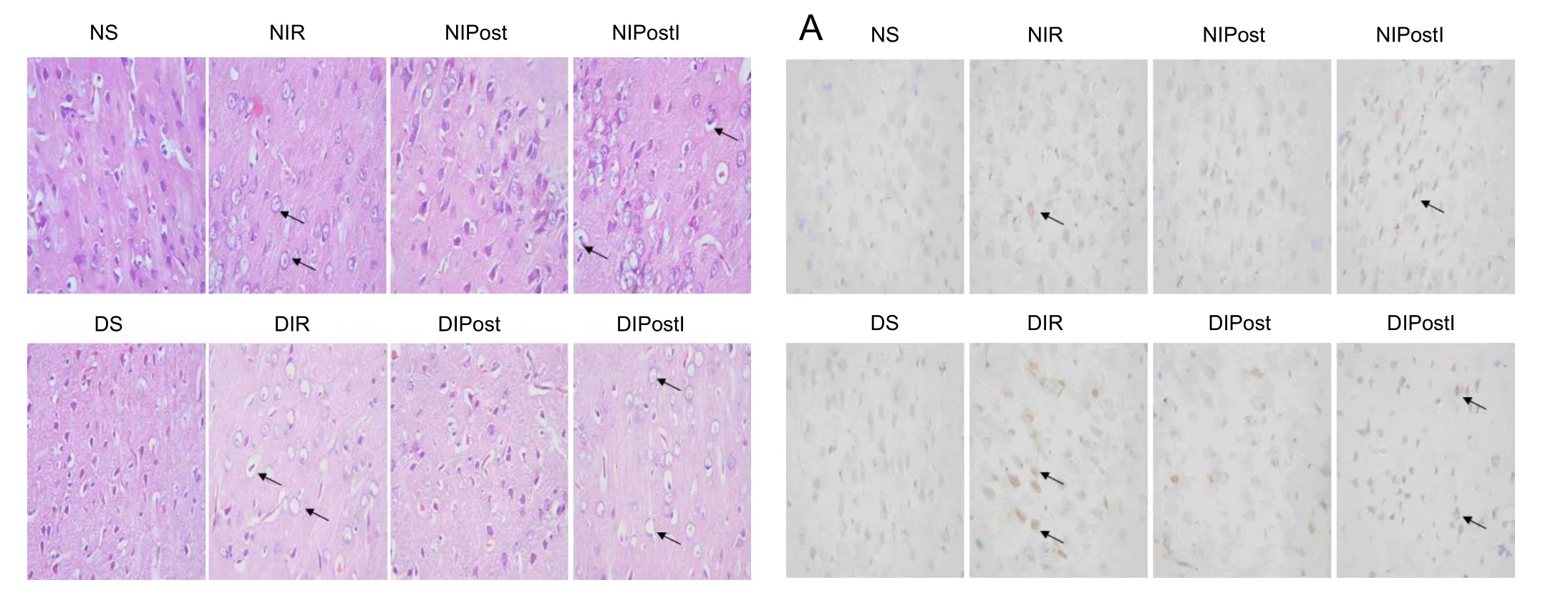
Figure 1 Effects of glycogen synthase kinase 3 beta (GSK-3β) on the morphology of brain tissue in diabetic rats with myocardial ischemia/reperfusion injury (hematoxylin-eosin staining, original magnification, 400×).
Microscopic observation showed that the cortical neurons were arranged in neat rows with abundant cytoplasm, and the nuclei were round and basophilic in the NS group. Arrows point to the damaged cells. The structures of the cortical neurons were damaged: the cytoplasm was stained light red with uneven distribution and vacuoles, and nuclei were condensed in the NIR group. In the NIPost group, however,the cell structure was normal. Most of the neurons exhibited complete membrane integrity and the nuclei were clear.e tissue in the NIPostI group resembled that in the NIR group. Histopathological changes in diabetic rats were more severe than in non-diabetic rats. Only the NS and DS groups received a thoracotomy. Myocardial ischemia/reperfusion was conducted in the NIR and DIR groups by blocking the leanterior descending coronary artery. The NIPost and DIPost groups were subjected to three cycles of 10-second reperfusion followed by a 10-second ischemia treatment immediately at the onset of reperfusion.e NIPostI and DIPostI groups were intraperitoneally injected with GSK-3β inhibitor 10 minutes before receiving MIRI, and received three cycles of 10-second reperfusion followed by a 10-second ischemia treatment immediately at the onset of reperfusion. NS group: Normal sham group; NIR group: normal myocardial ischemia reperfusion group; NIPost group: normal ischemic post-conditioning group; NIPostI group:normal ischemic post-conditioning + GSK-3β inhibitor group; DS group: diabetic sham group; DIR group: diabetic myocardial ischemia reperfusion group; DIPost group: diabetic ischemic post-conditioning group; DIPostI group: diabetic ischemic post-conditioning + GSK-3β inhibitor group.
an intraperitoneal streptozotocin injection (Sigma-Aldrich (Shanghai)Trading Co., Ltd., Shanghai, China) 65 mg/kg. Three days aer the streptozotocin injection, the rats were fasted for 5 hours before collection of tail blood samples. We measured fasting blood glucose using a SureStrep glucometer (Johnson& Johnson Company, USA). Rats with blood glucose level ≥16.7 mM were considered diabetic models (Wu et al., 2011).The rats were maintained for 8 weeks (until they were 16 weeks old), and then brain tissue specimens were harvested from all rats at the end of reperfusion. Rats were intraperitoneally anesthetized with pentobarbital sodium (50 mg/kg)followed by tracheotomy and artificial ventilation (Tidal volume: 6 mL/kg; frequency: 80 beats/min; DW-2000, Yilian,Shanghai, China). A fourth-intercostal space thoracotomy was performed, and the pericardium was excised to expose the heart.e leanterior descending coronary artery was ligated 2 mm above the left auricle by a 6-0 silk suture. A small polypropylene tube was placed between the ligature and the leanterior descending coronary artery.e artery was occluded for 30 minutes by tightening the ligature.After 30 minutes of ischemia, the ligature was loosened to allow reperfusion for 2 hours. The sham group underwent the same surgical procedures, apart from tying the 6-0 silk suture. Ischemic post-conditioning was achieved via three cycles of 10-second reperfusion followed by a 10-second ischemia treatment immediately at the onset of reperfusion.A GSK-3β inhibitor; 0.5% lithium chloride (LiCl, 3 mmol/kg, Sigma-Aldrich (Shanghai) Trading Co., Ltd.), was injected intraperitoneally 10 minutes before MIRI in the DIPostI and NIPostI groups (Xia et al., 2011; Liu et al., 2013).
Copyedited by Koke S, Wysong S, Wang J, Li CH, Qiu Y, Song LP, Zhao M
杂志排行
中国神经再生研究(英文版)的其它文章
- Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Using 3D bioprinting to produce mini-brain
- Beta secretase activity in peripheral nerve regeneration
- Embracing oligodendrocyte diversity in the context of perinatal injury
- On the road towards the global analysis of human synapses