On the road towards the global analysis of human synapses
2017-11-08AlephPrietoCarlCotman
G. Aleph Prieto, Carl w. Cotman
Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, CA, USA
How to cite this article: Prieto GA, Cotman CW (2017) On the road towards the global analysis of human synapses. Neural Regen Res 12(10):1586-1589.
Funding: is study was supported by National Institutes of Health Grants R21-AG048506, P01-AG000538 and RO1-AG34667 (to CWC), UC MEXUS-CONACYT Grant CN-16-170 (to GAP and CWC).
On the road towards the global analysis of human synapses
G. Aleph Prieto*, Carl w. Cotman
Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, CA, USA
How to cite this article: Prieto GA, Cotman CW (2017) On the road towards the global analysis of human synapses. Neural Regen Res 12(10):1586-1589.
Synapses are essential units for the flow of information in the brain. Over the last 70 years, synapses have been widely studied in multiple animal models including worms, fruit flies, and rodents. In comparison,the study of human synapses has evolved significantly slower, mainly because of technical limitations. However, three novel methods allowing the analysis of molecular, morphological, and functional properties of human synapses may expand our knowledge of the human brain. Here, we briefly describe these methods,and evaluate how the information provided by each unique approach may contribute to the functional and anatomical analysis of the synaptic component of human brain circuitries. In particular, using tissue from cryopreserved human brains, synaptic plasticity can be studied in isolated synaptosomes by fluorescence analysis of single-synapse long-term potentiation (FASS-LTP), and subpopulations of synapses can be thoroughly assessed in the ribbons of brain tissue by array tomography (AT). Currently, it is also possible to quantify synaptic density in the living human brain by positron emission tomography (PET), using a novel synaptic radio-ligand. Overall, data provided by FASS-LTP, AT, and PET may significantly contribute to the global understanding of synaptic structure and function in both healthy and diseased human brains, thus directly impacting translational research.
fluorescence analysis of single-synapse long-term potentiation; array tomography; positron emission tomography; synaptosomes; flow cytometry; microscopy; [11C]UCB-J[(R)-1-((3-(11C-methyl-11C)pyridin-4-yl)methyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one]
Introduction
Processing, storage and retrieval of information in the brain rely on circuits of neurons connected by synapses, the “mode of nexus between neurons”. Over the last 70 years, structural and functional properties of synapses have been studied in invertebrate animal models such as C. elegans and D. melanogaster, as well as in vertebrate models including mice, rats,cats, and non-human primates. In comparison, the study of human synapses has evolved significantly slower, mainly because of technical limitations. A better understanding of structural and functional dynamics of human synapses is both timely and critical, as synapse dysfunction is a major cause of most brain diseases, which are increasingly in prevalence in the fast-growing aged population (Selkoe, 2002; Grant, 2012;Morrison and Baxter, 2012).
Rapid technical progresses coupled with creative approaches developed by neuroscientists have opened up opportunities to study human synapses in vitro and in vivo. Here, we briefly describe three novel methods that allow the analysis of molecular, morphological, and functional properties of human synapses. First, we present a new method that allows the study of synaptic plasticity, specifically long term potentiation (LTP),from synaptosomes isolated from cryopreserved postmortem human brain tissue (Prieto et al., 2017). Second, we discuss how a detailed analysis of subpopulation of synapses can be derived by array tomography (AT), also in postmortem brains(Kay et al., 2013).ird, we discuss how in vivo quantification of synaptic density can now be studied by tracking a novel synaptic radio-ligand by positron emission tomography (PET)(Finnema et al., 2016). We conclude by evaluating of how the information provided by these methods may contribute to the global-functional and anatomical-analysis of synaptic populations in both healthy and diseased human brains.
Fluorescence Analysis of Single-Synapse Long-Term Potentiation (FASS-LTP)
A fundamental property of synapses is their ability to show long term change. In 1973, Bliss and Lomo first discovered that brief patterns of afferent activity can initiate a long lasting strengthening of synapse (Bliss and Lomo, 1973), a phenomena first called long-lasting potentiation and currently known as LTP (Bliss and Collingridge, 1993). LTP is commonly held to be a cellular mechanism serving learning and memory (Morris et al., 1986; Roman et al., 1987; Whitlock et al., 2006; Fedulov et al., 2007; Nabavi et al., 2014), and is a topic of intense study in many laboratories (Lynch et al., 2007).
LTP has been studied for decades, both in vivo and in vitro,using electrophysiological methods which deliver trains of electrical stimulation bursts to initiate LTP in intact neural circuitries (Huganir and Nicoll, 2013). A method to study LTP in human brain would extend studies on LTP from animal models to the human brain. Recently, we developed a method to study LTP in isolated synaptosomes, including synaptosomes isolated from cryopreserved postmortem human brains,which we refer to as FASS-LTP (Prieto et al., 2017).
LTP reflects the insertion of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors(AMPAR) into the post-synaptic surface (Muller et al., 1988),the essential event for the potentiation of synaptic transmission. FASS-LTP, first described using mouse hippocampus(Prieto et al., 2015), evaluates LTP in isolated synaptosomes by focusing on the insertion of AMPAR into the post-synaptic surface following chemically induced LTP (cLTP). Flow cytometry analysis identifies synaptosomes on the basis of size-related parameters, and evaluates the activity-dependent increase in levels of surface GluA1-containing AMPARs, using immunofluorescence labeling of extracellular epitopes (Figure 1A). Simultaneous labeling for the presynaptic marker neurexin-1β (Nrx1β), which is stabilized at the membrane surface by synaptic activity (Fu and Huang, 2010) and captures postsynaptic surface GluA1 via PSD95 (de Wit et al., 2009; Mondin et al., 2011), further allows to focus on “snowman-shaped”synaptosomes that contain both pre- and post-synaptic elements (GluA1 and Nrx1β double-labeling). FASS-LTP has several unique strengths: synapses are examined directly, multiple samples can be tested in parallel, and minimal amounts of tissue are needed for each assay (Table 1).
A crucial advantage of FASS-LTP over the classical electrophysiological recordings relies on the possibility of study LTP in the human brain. Indeed, using synaptosomes of Alzheimer’s disease (AD) and control cases, FASS-LTP-derived data have provided the first direct evidence to support the idea that AD-diseased synapses are intrinsically defective in LTP (Prieto et al., 2017), thereby answering a long standing question in neuroscience. In addition, FASS-LTP have been also used to screen a drug library from over 40 hippocampal samples derived from AD cases in a single day, a novel and relevant application for identifying therapeutics.us, FASSLTP could provide the basis for protocols to study LTP in humans, a previously unattainable goal.
AT
For decades, synapse structure analysis has been mostly based on electron microscopy (EM). Although EM is a powerful technique to visualize synapses, this method is time-consuming and has technical limitations for immunolabeling synaptic proteins, mainly due to high levels of non-specific staining, and because only a restricted number of markers can be simultaneously visualized. A similar disadvantage accompanies Golgi silver staining, a common method to estimate synaptic density but not suited for immunolabeling of synaptic proteins. In recent years, labeling of pre- and postsynaptic structures for counting and determining size have been significantly improved by AT, a fluorescence-based imaging method allowing single-synapse imaging analysis of diverse synapse populations (Micheva and Smith, 2007; Micheva et al., 2010). Originally used to study synapses in rodent brain,AT has been extended to study synapses in serial sections of human brain tissue (Koffie et al., 2009). One key advantage of the AT technique relies on imaging serial ribbons sections of 70-nm, since the axial (z)-resolution is adequate at this width for imaging synapses by light microscopy (Figure 1B), which is perfectly suited for multi-parameter fluorescence analysis.
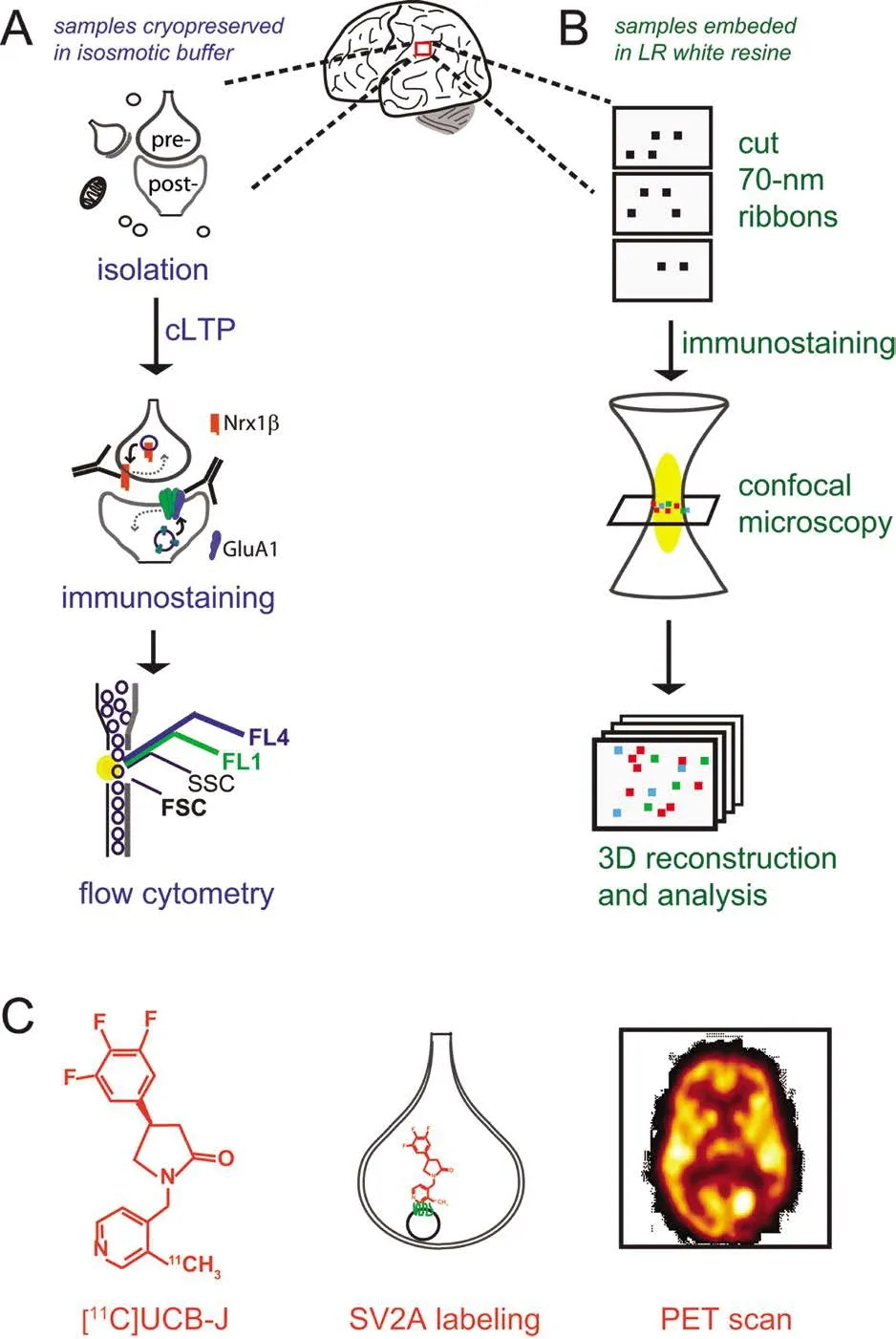
Figure 1 Novel approaches to study human synapses.
FASS-LTP and AT relies on availability and quality of human brain tissue. When using postmortem tissue, cryopreservation is crucial to the preservation of the functional response evaluated by FASS-LTP (Prieto et al., 2017). For AT,a detailed protocol for tissue collection has been described,where low temperatures and orientation of samples are crucial parameters (Kay et al., 2013). While there are a number of challenges with using postmortem human brain tissue in research, with one of the most significant being the postmortem interval (PMI), several reports indicate that basic biochemical reactions remain well-preserved in the postmortem human brain. For instance, protein-protein interactions in human PSD fractions (Hahn et al., 2009), glycine-dependent NMDAR activation (Hahn et al., 2006), and insulin signaling (Talbot et al., 2012) are relatively insensitive to variation in PMI (< 15 hours) or age (relatively constant from 70-90 years), suggesting that dynamic and anatomical properties evaluated by FASS-LTP and AT, respectively, are resistant to some variables associated with a postmortem approach.
Synapse Density Analysis by PET
FASS-LTP and AT are powerful techniques for dissecting plastic and structural properties of human synapses. However, these approaches cannot be used for early diagnosis or therapeutic monitoring, as these methods have only been validated in brain tissue obtained from autopsy. Recently,a first-in-human study showed that synaptic density can be monitored noninvasively by PET (Finnema et al., 2016), a well-established technique for detecting a wide range of brain molecules including receptors, transporters and enzymes.Quantification of human synaptic density by PET is based on the detection of the novel synaptic-specific radio-ligand[11C]UCB-J, (chemical name: [11C]UCB-J[(R)-1-((3-(11C-methyl-11C)pyridin-4-yl)methyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one]), which labels the synaptic vesicle glycoprotein 2A (SV2A) (Finnema et al., 2016) (Figure 1C). The synaptic marker SV2A, ubiquitously present in synapses across the brain, is an integral membrane protein located in presynaptic vesicles membranes. PET studies on synaptic density were first validated in baboons. Validation of [11C]UCB-J as a synaptic radio-ligand included a PET analysis of putative SV2A regional densities relative to signal obtained with synaptophysin, a bona fide synaptic marker. Importantly,the authors confirmed that [11C]UCB-J binds specifically to SV2A by pharmacological and biochemical assays (Finnema et al., 2016). Using [11C]UCB-J, SV2A-PET imaging allowed the quantification of synaptic density in over 10 healthy humans and, remarkably, this method provided in vivo evidence of synaptic loss in patients with temporal lobe epilepsy (Finnema et al., 2016).us, SV2A-PET analysis may be used to monitor changes in synaptic density non-invasively in a living brain.
Striving towards the Big Picture by Combining Functional and Anatomical Analysis of Human Synapses
Based on classic methods, preparations and molecular tools in neuroscience (e.g., EM, synaptosomes, and radio-ligands),FASS-LTP, AT, and SV2A-PET offer a new scientific avenue for studying the human brain, in particular, for the detailed characterization of the synaptic component of brain circuitries. FASS-LTP, AT and SV2A-PET have been validated in animal models, and subsequently tested in the human brain.Also, all three novel approaches have proven useful for detecting synaptic changes in brains from patients, affected either by neurodegenerative (FASS-LTP and AT in AD cases)or neurological disorders (SV2A-PET in epilepsy).Table 1compares the advantages and capabilities of FASS-LTP, AT,and SV2A-PET. It is evident that SV2A-PET has the unique advantage of being an in vivo and minimally invasive method,but this approach is not suited for single-synapse or multi-parameter analysis. In contrast, via in vitro procedures in postmortem tissue, FASS-LTP and AT allow detailed multi-parametric analysis of subpopulation of synapses (e.g., excitatory vs. inhibitory), at the single-event level. It is noteworthy that dye development is undergoing exponential technological expansion (e.g., novel fluorescent dyes, quantum dots and metal tags) which in the future could increase the analytical power of both FASS-LTP and AT. In addition, FASS-LTP and AT can be scaled for high throughput analysis, and some steps on these methods could be automatized (e.g., automated flow cytometry). FASS-LTP is an unique approach as it tests a major functional response of the synapse: its plasticity. In contrast to the analysis of physically isolated synapses by FASS-LTP,AT analyzes molecular signatures of synapses in their native circuitry, thus preserving valuable anatomical information, at the 3D level.us, FASS-LTP and AT data from same tissue sample may provide an unprecedented set of functional and anatomical information at both cellular and molecular levels.Whether combining the information provided by FASS-LTP,AT and SV2A-PET will facilitate the global analysis of human synapses deserves further research.
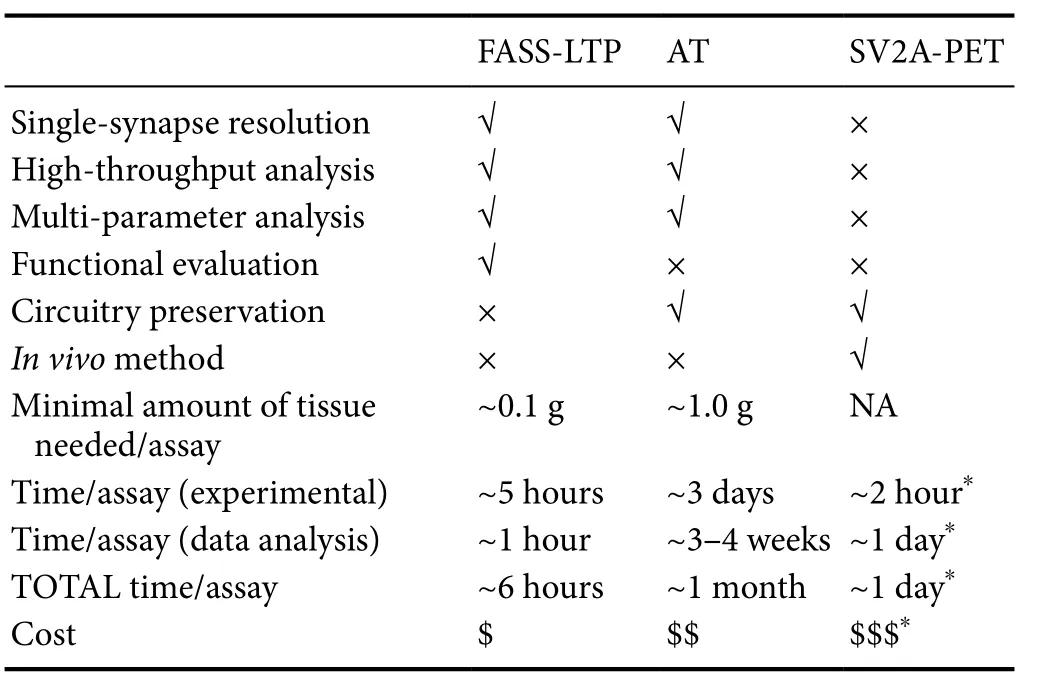
Table 1 Comparison of current approaches for the analysis of human synapses.
We envision that new functional and anatomical data on human synapses, the essential building blocks of the brain,may significantly contribute to several ongoing projects for brain mapping (Glasser et al., 2016). Also, this new information of human synapses may set the basis to the human synaptome, and lead to detailed studies on the role of major age-related factors that compromise cognition such as amyloid-β, oxidative damage, and inflammation.e information provided by these studies may offer a global perspective of synaptic function in diseased human brains, thus directly impacting translational research.
Acknowledgments:We appreciate N. Berchtold’s comments on our manuscript.
Author contributions:GAP and CWC both contributed to manuscript preparation, editing and review.
Conflicts of interest:None declared.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewers:Kentaro Hatano, University of Tsukuba, Japan; Noela Rodriguez-Losada, University of Malaga, Spain.
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331-356.
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31-39.
de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A (2009) LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron 64:799-806.
Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, Lynch G (2007)Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J Neurosci 27:8031-8039.
Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, Dhaher R, Matuskey D, Baum E, Holden D, Spencer DD, Mercier J, Hannestad J, Huang Y, Carson RE (2016) Imaging synaptic density in the living human brain. Sci Transl Med 8:348ra396.
Fu Y, Huang ZJ (2010) Differential dynamics and activity-dependent regulation of alpha- and beta-neurexins at developing GABAergic synapses.Proc Natl Acad Sci U S A 107:22699-22704.
Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, Coalson TS, Harms MP, Jenkinson M, Moeller S, Robinson EC,Sotiropoulos SN, Xu J, Yacoub E, Ugurbil K, Van Essen DC (2016)e Human Connectome Project’s neuroimaging approach. Nat Neurosci 19:1175-1187.
Grant SG (2012) Synaptopathies: diseases of the synaptome. Curr Opin Neurobiol 22:522-529.
Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K,Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE (2006)Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 12:824-828.
Hahn CG, Banerjee A, Macdonald ML, Cho DS, Kamins J, Nie Z, Borgmann-Winter KE, Grosser T, Pizarro A, Ciccimaro E, Arnold SE, Wang HY, Blair IA (2009)e post-synaptic density of human postmortem brain tissues: an experimental study paradigm for neuropsychiatric illnesses. PLoS One 4:e5251.
Huganir RL, Nicoll RA (2013) AMPARs and synaptic plasticity: the last 25 years. Neuron 80:704-717.
Kay KR, Smith C, Wright AK, Serrano-Pozo A, Pooler AM, Koffie R, Bastin ME, Bak TH, Abrahams S, Kopeikina KJ, McGuone D, Frosch MP,Gillingwater TH, Hyman BT, Spires-Jones TL (2013) Studying synapses in human brain with array tomography and electron microscopy. Nat Protoc 8:1366-1380.
Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML,Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL (2009) Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A 106:4012-4017.
Lynch G, Rex CS, Gall CM (2007) LTP consolidation: substrates, explanatory power, and functional significance. Neuropharmacology 52:12-23.
Micheva KD, Smith SJ (2007) Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron 55:25-36.
Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ (2010) Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron 68:639-653.
Mondin M, Labrousse V, Hosy E, Heine M, Tessier B, Levet F, Poujol C, Blanchet C, Choquet D, Thoumine O (2011) Neurexin-neuroligin adhesions capture surface-diffusing AMPA receptors through PSD-95 scaffolds. J Neurosci 31:13500-13515.
Morris RG, Anderson E, Lynch GS, Baudry M (1986) Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319:774-776.
Morrison JH, Baxter MG (2012)e ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13:240-250.
Muller D, Joly M, Lynch G (1988) Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science 242:1694-1697.
Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R (2014) Engineering a memory with LTD and LTP. Nature 511:348-352.
Prieto GA, Trieu BH, Dang CT, Bilousova T, Gylys KH, Berchtold NC,Lynch G, Cotman CW (2017) Pharmacological rescue of long-term potentiation in Alzheimer diseased synapses. J Neurosci 37:1197-1212.
Prieto GA, Snigdha S, Baglietto-Vargas D, Smith ED, Berchtold NC,Tong L, Ajami D, LaFerla FM, Rebek J, Jr., Cotman CW (2015) Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1beta in the aged hippocampus. Proc Natl Acad Sci U S A 112:E5078-5087.
Roman F, Staubli U, Lynch G (1987) Evidence for synaptic potentiation in a cortical network during learning. Brain Res 418:221-226.
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789-791.
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL,Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122:1316-1338.
Whitlock JR, Heynen AJ, Shuler MG, Bear MF (2006) Learning induces long-term potentiation in the hippocampus. Science 313:1093-1097.
*Correspondence to:
G. Aleph Prieto, Ph.D.,aleph.prieto@uci.edu.
orcid:
0000-0001-9517-6989
(G. Aleph Prieto)
10.4103/1673-5374.217321
Accepted: 2017-09-19
杂志排行
中国神经再生研究(英文版)的其它文章
- Can we treat neurodegenerative diseases by preventing an age-related decline in microRNA expression?
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
- Beta secretase activity in peripheral nerve regeneration
- Embracing oligodendrocyte diversity in the context of perinatal injury
- Non-invasive electrical brain stimulation: from acute to late-stage treatment of central nervous system damage