Embracing oligodendrocyte diversity in the context of perinatal injury
2017-11-08JessieNewvilleLaurenJantzieLeeAnnaCunningham
Jessie Newville, Lauren L. Jantzie,, Lee Anna Cunningham,
1 Department of Neurosciences, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
2 Department of Pediatrics, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
How to cite this article: Newville J, Jantzie LL, Cunningham LA (2017) Embracing oligodendrocyte diversity in the context of perinatal injury.Neural Regen Res 12(10):1575-1585.
Embracing oligodendrocyte diversity in the context of perinatal injury
Jessie Newville1, Lauren L. Jantzie1,2, Lee Anna Cunningham1,*
1 Department of Neurosciences, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
2 Department of Pediatrics, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
How to cite this article: Newville J, Jantzie LL, Cunningham LA (2017) Embracing oligodendrocyte diversity in the context of perinatal injury.Neural Regen Res 12(10):1575-1585.
Emerging evidence is fueling a new appreciation of oligodendrocyte diversity that is overturning the traditional view that oligodendrocytes are a homogenous cell population. Oligodendrocytes of distinct origins,maturational stages, and regional locations may differ in their functional capacity or susceptibility to injury.One of the most unique qualities of the oligodendrocyte is its ability to produce myelin. Myelin abnormalities have been ascribed to a remarkable array of perinatal brain injuries, with concomitant oligodendrocyte dysregulation. Within this review, we discuss new insights into the diversity of the oligodendrocyte lineage and highlight their relevance in paradigms of perinatal brain injury. Future therapeutic development will be informed by comprehensive knowledge of oligodendrocyte pathophysiology that considers the particular facets of heterogeneity that this lineage exhibits.
oligodendrogenesis; oligodendrocyte progenitor cell; myelination; central nervous system development;ontogenetic origin; white matter; white matter injury; preterm birth; glia; macroglia
Introduction
Cellular diversity within the central nervous system is the evolutionary trend that underlies the brain’s ability to orchestrate complex behaviors and cognitive tasks. To this end, the oligodendrocyte lineage contributes greatly to the evolutionary success of vertebrates through its unique production of myelin sheaths, lipid rich multilamellar membranes that envelop axons in the central nervous system(Zalc and Colman, 2000; Schweigreiter et al., 2006; Zalc et al., 2008). Myelination is largely a developmental process that is preceded by waves of oligodendrocyte generation,proliferation, migration, and differentiation (Mitew et al.,2014; Bercury and Macklin, 2015). During embryonic development, oligodendrocytes originate from various pools of progenitor populations derived from separate germinal zones (Kessaris et al., 2006).ree waves of ontogenetically distinct populations of oligodendrocyte progenitor cells disseminate throughout the grey and white matter regions of the central nervous system (Tsai et al., 2009; Armati and Mathey, 2010). Ultimately, migratory oligodendrocyte progenitors settle and differentiate into mature oligodendrocyte cells that provide local myelination and enable rapid impulse propagation through saltatory conduction (Susuki et al., 2013). Additionally, through their myelin sheaths,oligodendrocytes deliver trophic support for maintenance of axonal integrity (Funfschilling et al., 2012; Simons and Nave, 2015). Recent transcriptome data demonstrating new subtypes of oligodendrocytes in the central nervous system has considerably expanded our appreciation of oligodendrocyte diversity (Marques et al., 2016).ese new insights into basic oligodendrocyte biology highlight the importance of adding to the paucity of research that truly addresses the heterogeneous nature of the oligodendrocyte lineage.
Oligodendrocytes of distinct origins, maturational stages,or regional locations may differ in their functional capacity and susceptibility to any type of neural injury. However,oligodendrocyte diversity is particularly relevant to perinatal brain injury, as this stage in neurodevelopment exhibits heightened vulnerability to white matter injury.roughout this review, perinatal brain injury is defined as damage to the brain acquired before or immediately following birth.Oligodendrocyte dysregulation leading to white matter injury is a predominant pattern observed in survivors of perinatal brain injury (Iida et al., 1995; Back et al., 2001; Robinson et al., 2006; Billiards et al., 2008; Buser et al., 2012; Jantzie et al., 2015). Myelination in the human central nervous system begins during the second half of gestation in an inferior to superior, posterior to anterior pattern, whereby myelination begins in the occipital lobe and continues through the temporal and frontal lobes (Jakovcevski and Zecevic, 2005;Tasker, 2006). The extraordinary metabolic demands required during myelination and the complexity of oligodendrogenesis render this neurodevelopmental stage vulnerable to insult (Nave, 2010a). Perinatal brain injury sustained from preterm birth is one specific example that classically involves white matter injury and subsequent myelin deficits.Preterm birth is the leading cause of infant morbidity and mortality in the United States (Wilson-Costello et al., 2005;Shapiro-Mendoza, 2016; Liu et al., 2017). The major form of brain injury in contemporary cohorts of preterm infants is diffuse white matter injury characterized by selective oligodendrocyte dysregulation that precipitates abnormal my-elination and cognitive impairment (Anderson and Doyle,2008; Aarnoudse-Moens et al., 2009; Buser et al., 2012).Oligodendrocytes are implicated in many preclinical models of perinatal brain injury including: in utero and postnatal hypoxic-ischemia (Robinson et al., 2005; Segovia et al.,2008; Riddle et al., 2011; Jantzie et al., 2013; Davidson et al.,2014), hyperoxia or hypoxia exposure (Gerstner et al., 2008;Schmitz et al., 2011; Brehmer et al., 2012; Jablonska et al.,2012; Ritter et al., 2013; Deng et al., 2014; Pham et al., 2014;Scafidi et al., 2014; Yuen et al., 2014), ischemia (Falahati et al., 2013; Ahrendsen et al., 2016), fetal growth restriction(Tolcos et al., 2011; Reid et al., 2012; Rideau Batista Novais et al., 2016), hyperbilirubinemia (Barateiro et al., 2012, 2013,2014), exposure to myelin debris (Robinson and Miller,1999; Baer et al., 2009), leukodystrophy (Baracskay et al.,2002), in addition to infection and inflammation (Valerio et al., 2002; Vela et al., 2002; Pang et al., 2003; Taylor et al.,2010; Favrais et al., 2011; Nobuta et al., 2012). Preclinical models of toxic exposure during developmental myelination, such as gestational ethanol or isoflurane exposure,also involve white matter injury sustained from oligodendrocyte dysregulation (Brambrink et al., 2012; Creeley et al.,2013, 2014; Newville et al., 2017). Interestingly, some models suggest that early insult to the oligodendrocyte lineage may permanently alter oligodendrocyte and immune function, ultimately contributing to adult pathogenesis (Jalabi et al., 2005;Benardais et al., 2014; Graf et al., 2014; Traka et al., 2016; Patra et al., 2017). Additional evidence that suggests early injury to oligodendrocyte lineage impacts cognition later in life is demonstrated by clinical studies of preterm cohorts, wherein persisting white matter structural abnormalities, as assessed by diffusion tensor imaging, correlate to an increased incidence of neuropsychiatric disorders (Hagberg et al., 2012;Pyhala et al., 2014; Guy et al., 2015).
Within this review, we lay out evidence that elucidates a deeper understanding of oligodendrocyte function and susceptibility to injury that is dependent on origin, maturational status, and location within the central nervous system. It is clear that the oligodendrocytes play a major role in perinatal brain injury and that the path toward developing appropriate therapeutics will target oligodendrocyte cells (Olivier et al., 2009; Jantzie et al., 2013). Furthermore, future therapeutic development will be informed by comprehensive knowledge of oligodendrocyte pathophysiology that considers the particular facets of heterogeneity that this lineage exhibits.
Ontogenetic Origin
Oligodendrocyte cells that populate the central nervous system are derived from spatially and temporally distinct waves of oligodendrogenesis (Figure 1). Within the developing forebrain, gradients of organizing signals emanate from specific centers to pattern the tissue, creating specialized regions that produce different neuronal and glial cell types(Rowitch and Kriegstein, 2010). Sonic hedgehog (SHH) secreted from the ventral center along with bone morphogenic proteins (BMPs) from the dorsal cortical hem signaling center, regulate the specification of oligodendrocyte progenitor cells (OPCs) within the ventricular zone (Orentas et al., 1999; Samanta and Kessler, 2004; Feigenson et al., 2011).The three distinct waves of OPCs that arise from the developing forebrain ventricular zone follow a ventral-dorsal temporal progression (Ivanova et al., 2003; Chojnacki and Weiss, 2004; Kessaris et al., 2006). The first wave of OPC production is dependent on SHH signaling, whereas the later waves of OPC production occur in an SHH independent manner (Pringle and Richardson, 1993; Pringle et al., 1996;Nery et al., 2001; Tekki-Kessaris et al., 2001). Cre-LoxP fate mapping studies in transgenic mice have shown that the first wave of OPCs starting at embryonic day 12.5 is generated by Nkx2.1 expressing progenitors from the medial ganglionic eminence and entopeduncular area (Kessaris et al., 2006).As these ventrally derived progenitors migrate tangentially and dorsally to populate the entire developing telencephalon, the second wave of Gsh2 (also referred to as Gsx2)progenitors from the lateral and medial ganglionic eminences begins at embryonic day 15.5 (Kessaris et al., 2006;Chapman et al., 2013). Finally, at birth, a third wave occurs from Emx1 expressing progenitors arising from the dorsal ventricular zone underlying the developing cortex (Kessaris et al., 2006). By postnatal day 10, the Nkx2.1 expressing OPCs derived from the earliest wave have disappeared.e mechanisms behind the elimination and replacement of these early Nkx2.1 oligodendrocytes are unclear. One likely possibility is that subsequent populations of oligodendrocytes outcompete these early oligodendrocytes for survival factors such as platelet-derived growth factor (PDGF).is process would reflect how the overabundance of OPCs is balanced during myelination as these cells compete for the limited survival factors produced by axons and astrocytes(Barres et al., 1992; Trapp et al., 1997; Barres and Raff,1999).e oligodendrocytes that remain in the forebrain are the Gsh2 ventrally derived and Emx1 dorsally derived oligodendrocytes at an approximate ratio of 1 to 4, respectively(Tripathi et al., 2011). These oligodendrocyte progenitors that arise during development have greater motility, more rapid cell cycle and better survival relative to oligodendrocytes generated later in life (Tang et al., 2000; Ruffini et al.,2004).roughout postnatal life, new oligodendrocytes are generated from a reservoir of nestin-expressing neural stem cells that occupy the subventricular zone (SVZ) of the lateral ventricle (Levison et al., 1993, 1999; Marshall et al., 2003,2005; Quinones-Hinojosa et al., 2006; Jablonska et al., 2010;Fiorelli et al., 2015).ese neural stem cells exist in spatially segregated microdomains that produce different ratios of oligodendrocytes depending on their rostrocaudal coordinates along the ventricular zone, with more caudal domains having a greater proclivity towards generating oligodendrocytes (Azim et al., 2016). Under normal conditions, rostral SVZ domains produce approximately one oligodendrocyte per thirty cells, whereas caudal domains produce one oligodendrocyte per three cells (Menn et al., 2006). Once generated, SVZ derived OPCs migrate dorsally or laterally into the corpus callosum, fornix, or striatum, usually remaining at the same rostrocaudal level of their original precursor (Menn et al., 2006). Neural stem cells in the SVZ increase their production of oligodendrocytes in pathological circumstances such as stroke (Li et al., 2010), or demyelinating lesion (Menn et al., 2006; Aguirre et al., 2007; Mecha et al., 2013; Xing et al., 2014; Brousse et al., 2015). Following a demyelinating injury in the central nervous system, oligodendrocytes are also generated from adult OPCs that are distributed uniformly throughout the postnatal parenchyma (Tripathi et al., 2010;Richardson et al., 2011). Considering that the populations of oligodendrocytes arise from diverse origins under differing transcriptional control (Bergles and Richardson, 2015), it is important to investigate possible functional heterogeneity within these described subpopulations.
Functional heterogeneity between ontogenetically distinct populations of oligodendrocytes is paramount to the context of neural injury and repair. Identification of potential differences may guide therapeutic targeting of certain populations that demonstrate greater propensity toward remyelination or survival during perinatal brain injury paradigms. Diphtheria toxin mediated ablation of any one of these developmental oligodendrocyte waves showed that the other unaffected waves compensated for the loss numerically without any significant neurological consequences (Kessaris et al.,2006). This finding contributed to the understanding that despite being derived from spatially and temporally distinct origins, all oligodendrocytes in the forebrain are seemingly functionally analogous. However, recent research has suggested ontogenetic-dependent heterogeneity regarding developmental myelination, remyelination capacity, and susceptibility to developmental insult. Indeed, researchers have found functional differences between dorsally and ventrally derived oligodendrocytes in this respect. Dorsal OPCs,despite having indistinguishable electrophysiological properties from their ventrally derived counterparts, as assessed by whole-cell patch clamping, differ in their migration and settling patterns (Tripathi et al., 2011; Clarke et al., 2012).is was demonstrated in the spinal cord, whereby dorsally derived OPCs were less migratory and were able to outcompete ventrally derived OPCs in dorsal territory (Tripathi et al., 2011). In response to demyelination in the mature central nervous system, dorsally derived OPCs outperformed ventrally derived OPCs in measures of proliferation, migration, and differentiation (Zhu et al., 2011; Crawford et al.,2016).is important finding, that dorsal OPCs contributed more to remyelination, underscores the need for future studies to determine if a similar dynamic occurs in perinatal myelin deficits. Interestingly, investigators revealed that these dorsal oligodendrocytes were more susceptible to age-associated differentiation impairment (Crawford et al.,2016). Another study found that developmental injury in the form of ethanol exposure during the brain growth spurt in mice elicited acute oligodendrocyte cell loss dependent on ontogenetic origin, where embryonically derived (ventral)oligodendrocytes were depleted whereas the pool of postnatally derived (dorsal) oligodendrocytes were numerically unaffected (Newville et al., 2017).
Emerging evidence that demonstrates functional differences between oligodendrocytes of distinct origins has important implications in the setting of developmental central nervous system injury. Particularly, the understanding of certain developmental pathologies that include oligodendrocyte dysregulation, such as preclinical models of neonatal encephalopathy, would be greatly improved if researchers considered ontogenetic origin as a factor of oligodendrocyte performance. The manner in which these sub populations of oligodendrocytes respond to therapeutic interventions that target oligodendrocytes, such as erythropoietin (EPO),Darbepoetin (a hyperglycosylated analogue of recombinant EPO), or melatonin, should also be investigated (Olivier et al.,2009; Jantzie et al., 2013).ese are treatments that are being used in clinical trials to improve outcomes in infants born preterm (Wu et al., 2012; Juul and Pet, 2015; McAdams and Juul, 2016; An et al., 2017). If in fact, oligodendrocytes are distinctly susceptible to developmental white matter injury associated with preterm birth, perhaps one subpopulation is more responsive to therapeutic manipulation than the other.
Maturational Stage
Oligodendrocytes are comprised of a continuous lineage of progressive maturational cell stages (Marques et al., 2016).ese stages can be defined according to proliferative capacity, temporal expression of cell surface markers, and morphological complexity.ese distinctions yield four separate maturational stages within the human and rodent forebrain:oligodendrocyte progenitor cells, pre-oligodendrocytes,immature pre-myelinating oligodendrocytes, and mature myelinating oligodendrocytes (Figure 2) (Kinney and Back,1998; Back et al., 2001; Baumann and Pham-Dinh, 2001;Butts et al., 2008). Specification, proliferation, differentiation, and maturation of oligodendrocyte lineage cells are regulated through expression of various transcription factors that act on oligodendrocyte lineage genes (Emery and Lu, 2015). Once specified from multipotent stem cells, OPCs migrate radially and tangentially away from their respective germinal zone along the vascular network to populate the developing CNS (Tsai et al., 2016).is first committed oligodendrocyte lineage stage has simple bipolar morphology and is identified by the specific expression of platelet-derived growth factor receptor alpha (PDGFRα) (Pringle et al., 1992). PDGFRα expression is regulated by Olig1, Olig2,and Mash transcription factors, which are influenced by the gradient expression of SHH morphogen (Butts et al., 2008).Other markers such as nuclear Olig1, Sox10, or sulfated proteoglycan NG2 are used to identify OPCs. However,these are also expressed in the subsequent pre-oligodendrocyte stage (Tolcos et al., 2016). Nuclear expression of the transcription factor Olig2, important for oligodendrocyte specification, is expressed throughout the oligodendrocyte lineage stages (Lu et al., 2002; Emery and Lu, 2015).
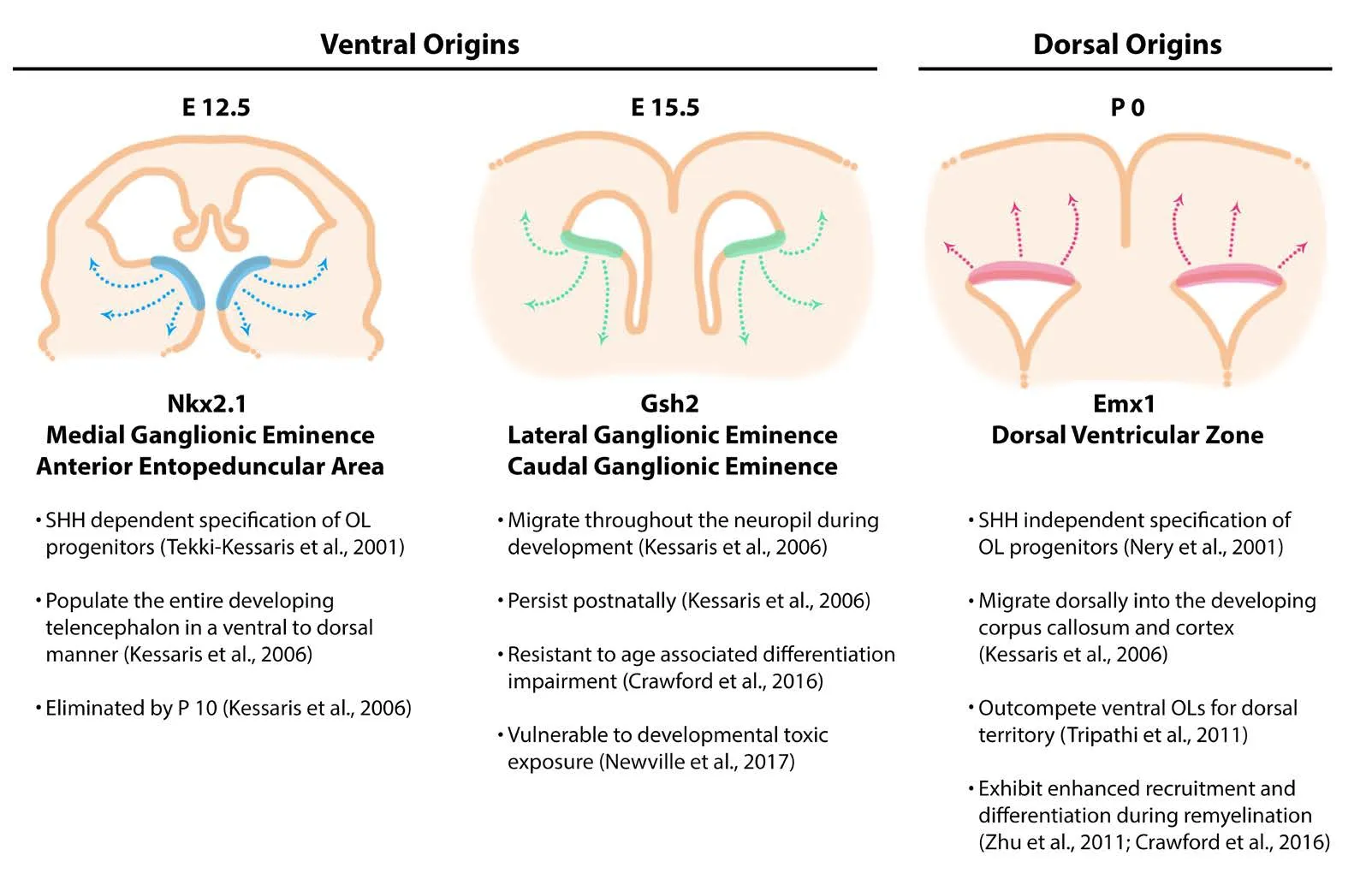
Figure 1 Oligodendrcytes(OLs) arise from three regionally and temporally distinct waves during neurodevelopment.
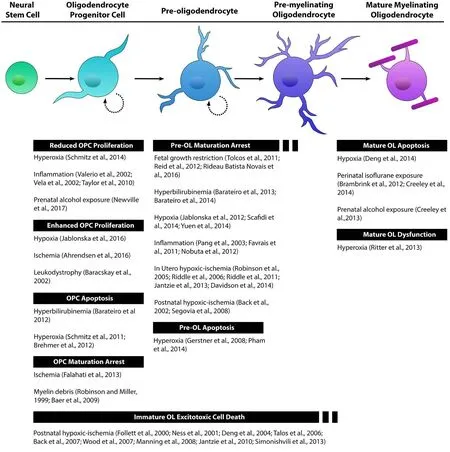
Figure 2 e lineage of oligodendrocytes (OLs) is a continuous series of maturational stages.
Proliferation of the progenitor pool is stimulated by locally expressed mitogens, in addition to environmental cues such as chemokines (Robinson et al., 1998; Armati and Mathey,2010). Once an OPC has arrived at its final destination within the white or grey matter, the OPC can differentiate into a mitotically active, multipolar pre-oligodendrocyte, whereby it loses its migratory ability. Identification of this oligodendrocyte stage is defined as expression of O4 in the absence of O1 (Warrington and Pfeiffer, 1992; Reynolds and Hardy,1997; Back et al., 2001).ese pre-oligodendrocyte cells differentiate into immature pre-myelinating oligodendrocytes of increased multipolar morphological complexity. This third cell stage in the oligodendrocyte lineage is post-mitotic and expresses O4 and O1 in the absence of myelin proteins.Additionally, this stage expresses MAG and CNP, which are also expressed in later myelinating oligodendrocytes.Ultimately, immature pre-myelinating oligodendrocytes produce mature myelinating oligodendrocyte cells. A small fraction of pre-myelinating oligodendrocytes undergo programmed cell death under conditions of normal development (Barres et al., 1992; Trapp et al., 1997).e expression of major myelin proteins, such as myelin basic protein (MBP)and proteolipid protein (PLP), denotes the final stage of the oligodendrocyte lineage. Mature oligodendrocytes are also commonly identified by their expression of Olig1, and the adenomatous polyposis coli antigen (APC, often referred to as CC1) (Kitada and Rowitch, 2006). The identification of stage specific markers was an important milestone in oligodendrocyte lineage research. Additional maturational stratification of oligodendrocytes has been suggested by recent transcriptome data from forebrain oligodendrocytes indicating a narrow path of differentiation from oligodendrocyte progenitor cell to myelin forming oligodendrocyte that then diversifies into six separate mature states (Marques et al., 2016). This new evidence suggests that limiting the maturational stages to four subtypes may depreciate the true diversity of the lineage. Categorizing oligodendrocytes into stages has been conducive for investigations into maturation dependent vulnerability and function. Describing how different paradigms of neuronal injury effect these newly identified oligodendrocyte populations will be important in future research efforts.
Functional capacity changes as an oligodendrocyte matures. Famously, the most mature oligodendrocyte stage generates myelin that enables rapid impulse propagation along axons, via sodium ion fluxes at the nodes of Ranvier.Myelin wrapping by mature oligodendrocytes dictates the spatial organization of these nodes and the sequestration of ion channels within them (Kaplan et al., 1997; Susuki et al., 2013). Oligodendrocytes provide trophic support to neurons, especially to long axons that are isolated from their respective neuronal soma (Nave, 2010a, b; Simons and Nave, 2015). The function of OPCs, other than generating additional myelinating oligodendrocytes, is less obvious.OPCs receive synaptic inputs from neighboring axons and express numerous voltage-gated ion channels (Bergles et al., 2000; Chittajallu et al., 2004; Jabs et al., 2005; Ge et al.,2006; De Biase et al., 2010; Clarke et al., 2012). Synaptic input onto OPCs is lost as these oligodendrocyte cells progress into more mature stages (Kukley et al., 2010).e functional significance of electrical input from neurons has remained obscure. However, it has been demonstrated that neuronal spiking influences oligodendrogenesis and myelination, suggesting that myelin biogenesis could be linked to the unique properties of OPC physiology (Wake et al., 2011; Gibson et al., 2014). In the specific case of glutamate, the manner in which this neurotransmitter effects OPC function is dependent on pathological circumstance. Glutamate mediates a host of oligodendrocyte functions related to brain development, such as migration, differentiation and myelination(Gallo et al., 1996; Gudz et al., 2006; Dimou and Gallo, 2015;Gautier et al., 2015). In brain injury associated with hypoxic ischemia, extracellular glutamate negatively impacts OPC survival by means of excitotoxicity (Johnston, 2005).
Oligodendrocyte maturational state is another layer of ever-growing oligodendrocyte heterogeneity that is important to consider in creating a more informed story of oligodendrocyte-associated pathogenesis in the context of developmental brain injury. Solely focusing on the mature myelinating endpoint, or choosing not to distinguish between the multiple stages of cellular maturation that exist, risks overlooking important information regarding the susceptibility of these distinct populations. Experimental paradigms of perinatal injury that examine the entire oligodendrocyte lineage, taking into consideration the selective vulnerabilities of cells at various stages of oligodendrocyte maturation, are able to parse out individual susceptibility profiles. Models of in utero and postnatal hypoxia ischemia aimed at determining the cellular underpinnings and molecular mechanisms behind diffuse white matter injury in preterm infants have shown that white matter injury is largely due to selective maturational arrest of pre-oligodendrocytes (Back et al.,2002; Robinson et al., 2005; Riddle et al., 2006, 2011; Segovia et al., 2008; Jantzie et al., 2013; Davidson et al., 2014).Interference in oligodendrocyte lineage progression at the pre-oligodendrocyte stage has also been demonstrated in models of hypoxia (Jablonska et al., 2012; Scafidi et al., 2014;Yuen et al., 2014), postnatal inflammation (Pang et al., 2003;Favrais et al., 2011; Nobuta et al., 2012), hyperbilirubinemia(Barateiro et al., 2013, 2014), and fetal growth restriction(Tolcos et al., 2011; Reid et al., 2012; Rideau Batista Novais et al., 2016). Under the pathological conditions of preterm birth pre-oligodendrocyte cells fail to mature into myelinating oligodendrocytes resulting in white matter deficits and poor clinical outcomes (van Tilborg et al., 2016). This is a cellular maturational stage-specific property that is independent of the developmental age of the animal or location of these cells within the cerebral white matter (Back, 2017).Further analysis has revealed that pre-oligodendrocytes are particularly vulnerable to apoptotic cell death under conditions of hyperoxia (Gerstner et al., 2008; Pham et al., 2014).Another mechanism of perinatal injury that specifically targets immature oligodendrocytes is excitotoxicity. OPCs and pre-oligodendrocytes are vulnerable to excitotoxic cell death in models of postnatal hypoxic-ischemia (Follett et al., 2000;Ness et al., 2001; Deng et al., 2004; Talos et al., 2006; Back et al., 2007; Wood et al., 2007; Manning et al., 2008; Jantzie et al., 2010; Simonishvili et al., 2013). Developmentally regulated expression patterns of glutamate receptors on OPCs render these cells in their early stage of oligodendrocyte maturation more susceptible to glutamate toxicity compared to their more mature, myelinating, counterparts (Rosenberg et al., 2003; Matute et al., 2006; Marinelli et al., 2016). In other experimental conditions of injury, OPCs show stage specific vulnerability to apoptosis in hyperbilirubinemia (Barateiro et al., 2012), and hyperoxia (Schmitz et al., 2011; Brehmer et al., 2012). Pathogenesis can also effect OPC proliferative capacity. OPCs show reduced proliferation in models of hyperoxia (Schmitz et al., 2014), inflammation (Valerio et al., 2002;Vela et al., 2002; Taylor et al., 2010), and prenatal alcohol exposure (Newville et al., 2017). Conversely, models of devel-opmental hypoxia, leukodystrophy, and ischemia augment the proliferation of OPCs (Baracskay et al., 2002; Ahrendsen et al., 2016; Jablonska et al., 2016). OPC maturational arrest has also been demonstrated in models of ischemia and exposure to myelin debris (Robinson and Miller, 1999; Baer et al.,2009; Falahati et al., 2013). Lastly, mature oligodendrocytes show stage specific susceptibility to apoptosis in models of hypoxia (Deng et al., 2014), and perinatal exposure to isoflurane or alcohol (Brambrink et al., 2012; Creeley et al., 2013,2014). Hyperoxia induced myelination deficits demonstrate functional impairment in mature oligodendrocytes (Ritter et al., 2013). Disruptions to early oligodendrogenesis could negatively affect downstream progeny and mature oligodendrocyte function.e evidence from studies outlined inFigure 2strongly demonstrates that the oligodendrocyte lineage cells are vulnerable to perinatal brain injury in a maturation-dependent manner.ese findings underscore the importance of considering this aspect of heterogeneity in future investigations (Butts et al., 2008; Back, 2017).
Regional Specificity
Migration of OPCs into distinct neuronal environments dictates their functional properties and vulnerability to injury. Once specified, OPCs assume a bipolar morphology and depart their germinal zones to populate the grey and white matter regions of the central nervous system. OPCs associate with the abluminal surface of the vasculature mediated by Wnt-chemokine receptor 4 interaction and use the vessels as scaffolding to reach their destinations (Tsai et al.,2016). Other factors that regulate migration are contact-mediated molecules that repel and attract OPCs, in combination with growth factor availability (de Castro and Bribián,2005; Bergles and Richardson, 2015). OPCs express a host of receptors for contact-mediated molecules also involved in neuronal migration such as Netrin-1 receptors (Jarjour et al., 2003; Tsai et al., 2003), Neuropilin-1 and -2 receptors(Sugimoto et al., 2001; Spassky et al., 2002; Syed et al., 2011),and Eph receptors (Prestoz et al., 2004). Furthermore, extracellular glutamate has been shown to induce OPC migration (Gudz et al., 2006; Harlow et al., 2015). OPCs exhibit self-avoidance, whereby they dictate their own tiling by constantly surveying their local territory and retracting when their processes contact an adjacent OPCs (Zhang and Miller, 1996; Hughes et al., 2013). Ultimately, oligodendrocyte progenitors evenly distribute themselves across grey matter regions and are only slightly outnumbered by progenitors within the white matter, 1 to 1.5 respectively (Chang et al.,2000; Dawson et al., 2003).e OPC that persist into adulthood steadily proliferate and differentiate to generate new myelinating oligodendrocytes (Rivers et al., 2008; Psachoulia et al., 2009; Young et al., 2013).e degree to which these cells proliferate and differentiate, in addition to other functional properties, is dependent on their regional location within the central nervous system.
Illuminating the differences between white matter and grey matter OPCs has demonstrated functional heterogeneity dependent on location. For instance, despite equivalent levels of PDGFRα expression, white matter OPCs demonstrate a greater proliferative response to PDGF than grey matter OPCs (Hill et al., 2013). OPCs within the developing grey and white matter have distinct electrophysiological properties and express different profiles of membrane K+and Na+channels (Chittajallu et al., 2004). During development and throughout postnatal life, OPCs generate mature oligodendrocyte cells. It is not definitively known if these diverse populations of OPCs give rise to functionally distinct mature oligodendrocytes, however, one study suggests that oligodendrogenesis is regulated differently in grey and white matter regions (Baracskay et al., 2002). In adulthood, OPCs located in white matter differentiate into mature myelin producing oligodendrocyte with greater proficiency then those in the grey matter (Dimou et al., 2008). Furthermore,when white matter or grey matter derived OPCs are transplanted into the cerebral cortex, white matter derived OPCs differentiated into mature oligodendrocytes with greater proficiency (Vigano et al., 2013). Although evidence is mounting that demonstrates region-specific properties, the extent to which these functional differences are dependent on cell intrinsic factors or extrinsic control remains speculative (Mayoral and Chan, 2016). Morphological differences have been observed between grey matter and white matter pre-myelinating oligodendrocytes. The cellular processes of pre-myelinating oligodendrocytes in the callosal white matter were more numerous and shorter in length than their cortical counterparts (Trapp et al., 1997). Additionally, white matter oligodendrocytes myelinate more axons than grey matter oligodendrocyte (Trapp et al., 1997). Within the cortical layers,researchers showed that the distinct myelin profiles produced within certain levels was influenced by the neuronal subtype in the immediate proximity suggesting extrinsic control (Tomassy et al., 2014). As reviewed previously, extrinsic factors mediated by neuron and astrocyte heterogeneity also contribute to myelin diversity (Ornelas et al., 2016; Tomassy et al.,2016). On the other hand, new evidence has emerged showing that myelin sheath length was determined by intrinsic oligodendrocyte control (Bechler et al., 2015). Regardless of whether intrinsic or extrinsic control prevails in determining oligodendrocyte regional specific functioning, it will be important for future researchers to consider this aspect of oligodendrocyte diversity in their analysis.
Regional heterogeneity is an important aspect of oligodendrocyte biology and is relevant in oligodendrocyte susceptibility to perinatal injury.e patterning of pre-oligodendrocyte lineage cells across grey and white matter regions of the developing brain dictates regional vulnerability to perinatal white matter injury associated with hypoxic-ischemia and inflammation, both common occurrences in preterm infants(Hagberg et al., 2002; Khwaja and Volpe, 2008; Ferriero and Miller, 2010; Anblagan et al., 2016). For example, during development the increased distribution of pre-oligodendrocytes in the germinal matrix, a region that includes the ventricular and subventricular zones, makes this area more susceptible to hypoxic-ischemic injury. Although intrinsically regulated mechanisms underlie the vulnerability ofpre-oligodendrocyte cells in these conditions of injury, other factors can influence oligodendrocyte vulnerability to injury that are imposed by local niches. One study that used tissue explants from early postnatal pups demonstrated that white matter OPCs were more responsive to PDGF than grey matter OPCs (Hill et al., 2013), supporting the hypothesis that regional location may be important for oligodendrocyte function in the perinatal brain. Overall, information regarding extrinsic control of oligodendrocytes by regional niche factors within normal and injured perinatal brain is remarkably absent. Recent studies of multiple sclerosis lesions reveal differences in the pathology and the extent of remyelination by oligodendrocytes within grey matter or white matter (Albert et al., 2007; Stadelmann et al., 2008; Gudi et al., 2009). Although these observations pertain to an adult pathology, they further the idea that regional specificities exist within the oligodendrocyte population.
Conclusion
The diversity of neuronal subtypes throughout the many brain regions is well defined regarding function, morphology, and susceptibility to injury. Only recently, has there been significant investigation into different aspects of glial diversity despite their critical role in brain physiology and pathology. Diversity within the three classes of glia is only just coming into perspective (Tomassy and Fossati, 2014;Grabert et al., 2016; Ben Haim and Rowitch, 2017). New research supports that oligodendrocytes are far more diverse than previously held.e importance of dissecting the oligodendrocyte lineage into subtypes will help our understanding of oligodendrocyte biology and will inform our clinical approach to neuropathologies. Perinatal brain injury oen involves oligodendrocyte dysregulation. As described in this review, emerging evidence indicates that ontogenetic origin, maturational stage, and regional location determine functional differences between populations of oligodendrocytes. These three factors also influence oligodendrocyte susceptibility to perinatal injury.us, oligodendrocyte heterogeneity must be considered in future research aimed at developing appropriate therapeutic options.
Author contributions:JN, LLJ and LAC prepared, edited, and approved the final version of the manuscript.
Conflicts of interest: None declared.
Plagiarism check:Checked twice by ienticate.
Peer review: Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review report:
Reviewer:Joanna Czarnecka, Nicholaus Copernicus University, Poland.
Comments to authors:e paper is clear and accessible systematization of information about the oligodendrocytes diversity. The oligodendrocytes, cells able to myelin produce, are not a homogenous population.e paper is clear and accessible systematization of information about the oligodendrocytes diversity. Knowledge of oligodendrocytes pathophysiology, particular facets of their heterogeneity are important for therapeutic development.
Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J (2009) Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124:717-728.
Aguirre A, Dupree JL, Mangin JM, Gallo V (2007) A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci 10:990-1002.
Ahrendsen JT, Grewal HS, Hickey SP, Culp CM, Gould EA, Shimizu T,Strnad FA, Traystman RJ, Herson PS, Macklin WB (2016) Juvenile striatal white matter is resistant to ischemia-induced damage. Glia 64:1972-1986.
Albert M, Antel J, Bruck W, Stadelmann C (2007) Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol 17:129-138.
An G, Ohls RK, Christensen RD, Widness JA, Mock DM, Veng-Pedersen P (2017) Population pharmacokinetics of darbepoetin in infants following single intravenous and subcutaneous dosing. J Pharm Sci 106:1644-1649.
Anblagan D, Pataky R, Evans MJ, Telford EJ, Serag A, Sparrow S, Piyasena C, Semple SI, Wilkinson AG, Bastin ME, Boardman JP (2016)Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci Rep 6:37932.
Anderson PJ, Doyle LW (2008) Cognitive and educational deficits in children born extremely preterm. Semin Perinatol 32:51-58.
Armati P, Mathey E (2010) The Biology of Oligodendrocytes. Cambridge University Press: Cambridge University Press.
Azim K, Berninger B, Raineteau O (2016) Mosaic subventricular origins of forebrain oligodendrogenesis. Front Neurosci 10:107.
Back SA (2017) White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol doi: 10.1007/s00401-017-1718-6.
Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC(2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21:1302-1312.
Back SA, Craig A, Kayton RJ, Luo NL, Meshul CK, Allcock N, Fern R(2007) Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab 27:334-347.
Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J,Arvin KL, Holtzman DM (2002) Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci 22:455-463.
Baer AS, Syed YA, Kang SU, Mitteregger D, Vig R, Ffrench-Constant C, Franklin RJ, Altmann F, Lubec G, Kotter MR (2009) Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 132:465-481.
Baracskay KL, Duchala CS, Miller RH, Macklin WB, Trapp BD (2002)Oligodendrogenesis is differentially regulated in gray and white matter of jimpy mice. J Neurosci Res 70:645-654.
Barateiro A, Vaz AR, Silva SL, Fernandes A, Brites D (2012) ER stress,mitochondrial dysfunction and calpain/JNK activation are involved in oligodendrocyte precursor cell death by unconjugated bilirubin.Neuromolecular Med 14:285-302.
Barateiro A, Domingues HS, Fernandes A, Relvas JB, Brites D (2014)Rat cerebellar slice cultures exposed to bilirubin evidence reactive gliosis, excitotoxicity and impaired myelinogenesis that is prevented by AMPA and TNF-α inhibitors. Mol Neurobiol 49:424-439.
Barateiro A, Miron VE, Santos SD, Relvas JB, Fernandes A,Ffrench-Constant C, Brites D (2013) Unconjugated bilirubin restricts oligodendrocyte differentiation and axonal myelination. Mol Neurobiol 47:632-644.
Barres BA, Raff MC (1999) Axonal control of oligodendrocyte development. J Cell Biol 147:1123-1128.
Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD,Raff MC (1992) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70:31-46.
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871.
Bechler Marie E, Byrne L, ffrench-Constant C (2015) CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr Biol 25:2411-2416.
Ben Haim L, Rowitch DH (2017) Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18:31-41.
Benardais K, Gudi V, Gai L, Nessler J, Singh V, Prajeeth CK, Skripuletz T, Stangel M (2014) Long-term impact of neonatal inflammation on demyelination and remyelination in the central nervous system. Glia 62:1659-1670.
Bercury Kathryn K, Macklin Wendy B (2015) Dynamics and mechanisms of CNS myelination. Dev Cell 32:447-458.
Bergles DE, Richardson WD (2015) Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol 8:a020453.
Bergles DE, Roberts JDB, Somogyi P, Jahr CE (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405:187-191.
Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL,Rowitch DH, Ligon KL, Volpe JJ, Kinney HC (2008) Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia.Brain Pathol 18:153-163.
Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, Dissen GA,Creeley CE, Dikranian KT, Olney JW (2012) Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol 72:525-535.
Brehmer F, Bendix I, Prager S, van de Looij Y, Reinboth BS, Zimmermanns J, Schlager GW, Brait D, Sifringer M, Endesfelder S, Sizonenko S, Mallard C, Buhrer C, Felderhoff-Mueser U, Gerstner B (2012)Interaction of inflammation and hyperoxia in a rat model of neonatal white matter damage. PLoS One 7:e49023.
Brousse B, Magalon K, Durbec P, Cayre M (2015) Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol Open 4:980-992.
Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P,Grafe MR, Back SA (2012) Arrested pre-oligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 71:93-109.
Butts BD, Houde C, Mehmet H (2008) Maturation-dependent sensitivity of oligodendrocyte lineage cells to apoptosis: implications for normal development and disease. Cell Death Differ 15:1178-1186.
Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD (2000)NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 20:6404-6412.
Chapman H, Waclaw RR, Pei Z, Nakafuku M, Campbell K (2013)e homeobox gene Gsx2 controls the timing of oligodendroglial fate specification in mouse lateral ganglionic eminence progenitors. Development 140:2289-2298.
Chittajallu R, Aguirre A, Gallo V (2004) NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol 561:109-122.
Chojnacki A, Weiss S (2004) Isolation of a novel platelet-derived growth factor-responsive precursor from the embryonic ventral forebrain. J Neurosci 24:10888-10899.
Clarke LE, Young KM, Hamilton NB, Li H, Richardson WD, Attwell D(2012) Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J Neurosci 32:8173-8185.
Crawford Abbe H, Tripathi Richa B, Richardson William D, Franklin Robin JM (2016) Developmental origin of oligodendrocyte lineage cells determines response to demyelination and susceptibility to age-associated functional decline. Cell Rep 15:761-773.
Creeley CE, Dikranian KT, Johnson SA, Farber NB, Olney JW (2013)Alcohol-induced apoptosis of oligodendrocytes in the fetal macaque brain. Acta Neuropathol Commun 1:23.
Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM (2014) Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology 120:626-638.
Davidson JO, Drury PP, Green CR, Nicholson LF, Bennet L, Gunn AJ(2014) Connexin hemichannel blockade is neuroprotective aer asphyxia in preterm fetal sheep. PLoS One 9:e96558.
Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24:476-488.
De Biase LM, Nishiyama A, Bergles DE (2010) Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci 30:3600-3611.
de Castro F, Bribián A (2005)e molecular orchestra of the migration of oligodendrocyte precursors during development. Brain Res Brain Res Rev 49:227-241.
Deng W, Wang H, Rosenberg PA, Volpe JJ, Jensen FE (2004) Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci U S A 101:7751-7756.
Deng Y, Xie D, Fang M, Zhu G, Chen C, Zeng H, Lu J, Charanjit K(2014) Astrocyte-derived proinflammatory cytokines induce hypomyelination in the periventricular white matter in the hypoxic neonatal brain. PloS One 9:e87420.
Dimou L, Gallo V (2015) NG2-glia and their functions in the central nervous system. Glia 63:1429-1451.
Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M (2008) Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci 28:10434-10442.
Emery B, Lu QR (2015) Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb Perspect Biol 7:a020461.
Falahati S, Breu M, Waickman AT, Phillips AW, Arauz EJ, Snyder S,Porambo M, Goeral K, Comi A, Wilson MA, Johnston MV, Fatemi A (2013) Ischemia induced neuroinflammation is associated with disrupted development of oligodendrocyte progenitors in a model of periventricular leukomalacia. Dev Neurosci 35:182-196.
Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, Lacaud A, Saliba E, Dammann O, Gallego J,Sizonenko S, Hagberg H, Lelievre V, Gressens P (2011) Systemic inflammation disrupts the developmental program of white matter.Ann Neurol 70:550-565.
Feigenson K, Reid M, See J, Crenshaw IE, Grinspan JB (2011) Canonical Wnt signalling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN Neuro 3:e00061.
Ferriero DM, Miller SP (2010) Imaging selective vulnerability in the developing nervous system. J Anat 217:429-435.
Fiorelli R, Azim K, Fischer B, Raineteau O (2015) Adding a spatial dimension to postnatal ventricular-subventricular zone neurogenesis.Development 142:2109-2120.
Follett PL, Rosenberg PA, Volpe JJ, Jensen FE (2000) NBQX attenuates excitotoxic injury in developing white matter. J Neurosci 20:9235-9241.
Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J,Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F,Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J,Goebbels S, Nave KA (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517-521.
Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC(1996) Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+channel block. J Neurosci 16:2659-2670.
Gautier HO, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I, James F, Lao-Peregrin C, Reynolds R, Franklin RJ, Káradóttir RT(2015) Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat Commun 6:8518-8518.
Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S(2006) Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science 312:1533-1537.
Gerstner B, DeSilva TM, Genz K, Armstrong A, Brehmer F, Neve RL,Felderhoff-Mueser U, Volpe JJ, Rosenberg PA (2008) Hyperoxia causes maturation-dependent cell death in the developing white matter. J Neurosci 28:1236-1245.
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS,Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H,Monje M (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304.
Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW (2016) Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci 19:504-516.
Graf AE, Haines KM, Pierson CR, Bolon BN, Houston RH, Velten M,Heyob KM, Rogers LK (2014) Perinatal inflammation results in decreased oligodendrocyte numbers in adulthood. Life Sci 94:164-171.
Gudi V, Moharregh-Khiabani D, Skripuletz T, Koutsoudaki PN, Kotsiari A, Skuljec J, Trebst C, Stangel M (2009) Regional differences between grey and white matter in cuprizone induced demyelination.Brain Res 1283:127-138.
Gudz TI, Komuro H, Macklin WB (2006) Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci 26:2458-2466.
Guy A, Seaton SE, Boyle EM, Draper ES, Field DJ, Manktelow BN,Marlow N, Smith LK, Johnson S (2015) Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J Pediatr 166:269-275.e3.
Hagberg H, Peebles D, Mallard C (2002) Models of white matter injury:comparison of infectious, hypoxic-ischemic, and excitotoxic insults.Ment Retard Dev Disabil Res Rev 8:30-38.
Hagberg H, Gressens P, Mallard C (2012) Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 71:444-457.
Harlow DE, Saul KE, Komuro H, Macklin WB (2015) Myelin proteolipid protein complexes with alphav integrin and AMPA receptors in vivo and regulates AMPA-dependent oligodendrocyte progenitor cell migration through the modulation of cell-surface GluR2 expression. J Neurosci 35:12018-12032.
Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A (2013) NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci 33:14558-14566.
Hughes EG, Kang SH, Fukaya M, Bergles DE (2013) Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci 16:668-676.
Iida K, Takashima S, Ueda K (1995) Immunohistochemical study of myelination and oligodendrocyte in infants with periventricular leukomalacia. Pediatr Neurol 13:296-304.
Ivanova A, Nakahira E, Kagawa T, Oba A, Wada T, Takebayashi H,Spassky N, Levine J, Zalc B, Ikenaka K (2003) Evidence for a second wave of oligodendrogenesis in the postnatal cerebral cortex of the mouse. J Neurosci Res 73:581-592.
Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA,Ming GL, Song H, Gallo V (2010) Chordin-induced lineage plasticity of adult SVZ neuroblasts aer demyelination. Nat Neurosci 13:541-550.
Jablonska B, Scafidi J, Aguirre A, Vaccarino F, Nguyen V, Borok E, Horvath TL, Rowitch DH, Gallo V (2012) Oligodendrocyte regeneration aer neonatal hypoxia requires FoxO1-mediated p27Kip1 expression.J Neurosci 32:14775-14793.
Jablonska B, Gierdalski M, Chew LJ, Hawley T, Catron M, Lichauco A,Cabrera-Luque J, Yuen T, Rowitch D, Gallo V (2016) Sirt1 regulates glial progenitor proliferation and regeneration in white matter aer neonatal brain injury. Nature Commun 7:13866.
Jabs R, Pivneva T, Huttmann K, Wyczynski A, Nolte C, Kettenmann H,Steinhauser C (2005) Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J Cell Sci 118:3791-3803.
Jakovcevski I, Zecevic N (2005) Sequence of oligodendrocyte development in the human fetal telencephalon. Glia 49:480-491.
Jalabi W, Boehm N, Grucker D, Ghandour MS (2005) Recovery of myelin aer induction of oligodendrocyte cell death in postnatal brain. J Neurosci 25:2885-2894.
Jantzie LL, Miller RH, Robinson S (2013) Erythropoietin signaling promotes oligodendrocyte development following prenatal systemic hypoxic-ischemic brain injury. Pediatr Res 74:658-667.
Jantzie LL, Talos DM, Selip DB, An L, Jackson MC, Folkerth RD, Deng W, Jensen FE (2010) Developmental regulation of group I metabotropic glutamate receptors in the premature brain and their protective role in a rodent model of periventricular leukomalacia. Neuron Glia Biol 6:277-288.
Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE (2015) Developmental expression of N-methyl-D-aspartate (NMDA) receptor subunits in human white and gray matter: potential mechanism of increased vulnerability in the immature brain. Cereb Cortex 25:482-495.
Jarjour AA, Manitt C, Moore SW,ompson KM, Yuh SJ, Kennedy TE(2003) Netrin-1 is a chemorepellent for oligodendrocyte precursor cells in the embryonic spinal cord. J Neurosci 23:3735-3744.
Johnston MV (2005) Excitotoxicity in perinatal brain injury. Brain Pathol 15:234-240.
Juul SE, Pet GC (2015) Erythropoietin and Neonatal Neuroprotection.Clinics in perinatology 42:469-481.
Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA (1997) Induction of sodium channel clustering by oligodendrocytes. Nature 386:724-728.
Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD (2006) Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 9:173-179.
Khwaja O, Volpe JJ (2008) Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed 93:F153-F161.
Kinney HC, Back SA (1998) Human oligodendroglial development: relationship to periventricular leukomalacia. Semin Pediatr Neurol 5:180-189.
Kitada M, Rowitch DH (2006) Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia 54:35-46.
Kukley M, Nishiyama A, Dietrich D (2010)e fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci 30:8320-8331.
Levison SW, Young GM, Goldman JE (1999) Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res 57:435-446.
Levison SW, Chuang C, Abramson BJ, Goldman JE (1993)e migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development 119:611-622.
Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA(2010) Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 58:1610-1619.
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S,Mathers C, Black RE (2017) Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388:3027-3035.
Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH (2002)Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109:75-86.
Manning SM, Talos DM, Zhou C, Selip DB, Park HK, Park CJ, Volpe JJ,Jensen FE (2008) NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci 28:6670-6678.
Marinelli C, Bertalot T, Zusso M, Skaper SD, Giusti P (2016) Systematic review of pharmacological properties of the oligodendrocyte lineage.Front Cell Neurosci 10:27.
Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A,Xiao L, Li H, Haring M, Hochgerner H, Romanov RA, Gyllborg D,Munoz-Manchado AB, La Manno G, Lonnerberg P, Floriddia EM,Rezayee F, Ernfors P, Arenas E, Hjerling-Leffler J, Harkany T, et al.(2016) Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352:1326-1329.
Marshall CA, Suzuki SO, Goldman JE (2003) Gliogenic and neurogenic progenitors of the subventricular zone: who are they, where did they come from, and where are they going? Glia 43:52-61.
Marshall CA, Novitch BG, Goldman JE (2005) Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells.J Neurosci 25:7289-7298.
Matute C, Domercq M, Sanchez-Gomez MV (2006) Glutamate-mediated glial injury: mechanisms and clinical importance. Glia 53:212-224.
Mayoral SR, Chan JR (2016) The environment rules: spatiotemporal regulation of oligodendrocyte differentiation. Curr Opin Neurobiol 39:47-52.
McAdams RM, Juul SE (2016) Neonatal encephalopathy: update on therapeutic hypothermia and other novel therapeutics. Clin Perinatol 43:485-500.
Mecha M, Feliu A, Carrillo-Salinas FJ, Mestre L, Guaza C (2013) Mobilization of progenitors in the subventricular zone to undergo oligodendrogenesis in theeiler’s virus model of multiple sclerosis: implications for remyelination at lesions sites. Exp Neurol 250:348-352.
Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D,Alvarez-Buylla A (2006) Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 26:7907-7918.
Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B (2014)Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276:29-47.
Nave KA (2010a) Myelination and support of axonal integrity by glia.Nature 468:244-252.
Nave KA (2010b) Myelination and the trophic support of long axons.Nat Rev Neurosci 11:275-283.
Nery S, Wichterle H, Fishell G (2001) Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 128:527-540.
Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW (2001)Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev Neurosci 23:203-208.
Newville J, Valenzuela CF, Li L, Jantzie LL, Cunningham LA (2017)Acute oligodendrocyte loss with persistent white matter injury in a third trimester equivalent mouse model of fetal alcohol spectrum disorder. Glia 65:1317-1332.
Nobuta H, Ghiani CA, Paez PM, Spreuer V, Dong H, Korsak RA, Manukyan A, Li J, Vinters HV, Huang EJ, Rowitch DH, Sofroniew MV,Campagnoni AT, de Vellis J, Waschek JA (2012) STAT3-mediated astrogliosis protects myelin development in neonatal brain injury. Ann Neurol 72:750-765.
Olivier P, Fontaine RH, Loron G, Van Steenwinckel J, Biran V, Massonneau V, Kaindl A, Dalous J, Charriaut-Marlangue C, Aigrot MS,Pansiot J, Verney C, Gressens P, Baud O (2009) Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats.PLoS One 4:e7128.
Orentas DM, Hayes JE, Dyer KL, Miller RH (1999) Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development 126:2419-2429.
Ornelas IM, McLane LE, Saliu A, Evangelou AV, Khandker L, Wood TL(2016) Heterogeneity in oligodendroglia: Is it relevant to mouse models and human disease? J Neurosci Res 94:1421-1433.
Pang Y, Cai Z, Rhodes PG (2003) Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain aer intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res 140:205-214.
Patra A, Huang H, Bauer JA, Giannone PJ (2017) Neurological consequences of systemic inflammation in the premature neonate. Neural Regen Res 12:890-896.
Pham H, Vottier G, Pansiot J, Duong-Quy S, Bollen B, Dalous J, Gallego J,Mercier JC, Dinh-Xuan AT, Bonnin P, Charriaut-Marlangue C, Baud O (2014) Inhaled NO prevents hyperoxia-induced white matter damage in neonatal rats. Exp Neurol 252:114-123.
Prestoz L, Chatzopoulou E, Lemkine G, Spassky N, Lebras B, Kagawa T, Ikenaka K, Zalc B,omas JL (2004) Control of axonophilic migration of oligodendrocyte precursor cells by Eph-ephrin interaction.Neuron Glia Biol 1:73-83.
Pringle NP, Richardson WD (1993) A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development 117:525-533.
Pringle NP, Mudhar HS, Collarini EJ, Richardson WD (1992) PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115:535-551.
Pringle NP, Yu WP, Guthrie S, Roelink H, Lumsden A, Peterson AC,Richardson WD (1996) Determination of neuroepithelial cell fate:induction of the oligodendrocyte lineage by ventral midline cells and sonic hedgehog. Dev Biol 177:30-42.
Psachoulia K, Jamen F, Young KM, Richardson WD (2009) Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol 5:57-67.
Pyhala R, Hovi P, Lahti M, Sammallahti S, Lahti J, Heinonen K, Pesonen AK, Strang-Karlsson S, Eriksson JG, Andersson S, Jarvenpaa AL, Kajantie E, Raikkonen K (2014) Very low birth weight, infant growth,and autism-spectrum traits in adulthood. Pediatrics 134:1075-1083.
Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A (2006) Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol 494:415-434.
Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA, Grinspan JB (2012) Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol 71:640-653.
Reynolds R, Hardy R (1997) Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res 47:455-470.
Richardson WD, Young KM, Tripathi RB, McKenzie I (2011) NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 70:661-673.
Riddle A, Luo NL, Manese M, Beardsley DJ, Green L, Rorvik DA, Kelly KA, Barlow CH, Kelly JJ, Hohimer AR, Back SA (2006) Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J Neurosci 26:3045-3055.
Riddle A, Dean J, Buser JR, Gong X, Maire J, Chen K, Ahmad T, Cai V,Nguyen T, Kroenke CD, Hohimer AR, Back SA (2011) Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol 70:493-507.
Rideau Batista Novais A, Pham H, Van de Looij Y, Bernal M, Mairesse J,Zana-Taieb E, Colella M, Jarreau PH, Pansiot J, Dumont F, Sizonenko S, Gressens P, Charriaut-Marlangue C, Tanter M, Demene C, Vaiman D, Baud O (2016) Transcriptomic regulations in oligodendroglial and microglial cells related to brain damage following fetal growth restriction. Glia 64:2306-2320.
Ritter J, Schmitz T, Chew LJ, Buhrer C, Mobius W, Zonouzi M, Gallo V(2013) Neonatal hyperoxia exposure disrupts axon-oligodendrocyte integrity in the subcortical white matter. J Neurosci 33:8990-9002.
Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD (2008) PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice.Nat Neurosci 11:1392-1401.
Robinson S, Miller RH (1999) Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Dev Biol 216:359-368.
Robinson S, Li Q, Dechant A, Cohen ML (2006) Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg 104:396-408.
Robinson S, Tani M, Strieter RM, Ransohoff RM, Miller RH (1998)e chemokine growth-regulated oncogene-alpha promotes spinal cord oligodendrocyte precursor proliferation. J Neurosci 18:10457-10463.
Robinson S, Petelenz K, Li Q, Cohen ML, Dechant A, Tabrizi N, Bucek M,Lust D, Miller RH (2005) Developmental changes induced by graded prenatal systemic hypoxic-ischemic insults in rats. Neurobiol Dis 18:568-581.
Rosenberg PA, Dai W, Gan XD, Ali S, Fu J, Back SA, Sanchez RM, Segal MM, Follett PL, Jensen FE, Volpe JJ (2003) Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res 71:237-245.
Rowitch DH, Kriegstein AR (2010) Developmental genetics of vertebrate glial-cell specification. Nature 468:214-222.
Ruffini F, Arbour N, Blain M, Olivier A, Antel JP (2004) Distinctive properties of human adult brain-derived myelin progenitor cells. Am J Pathol 165:2167-2175.
Samanta J, Kessler JA (2004) Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development 131:4131-4142.
Scafidi J, Hammond TR, Scafidi S, Ritter J, Jablonska B, Roncal M, Szigeti-Buck K, Coman D, Huang Y, McCarter RJ Jr, Hyder F, Horvath TL,Gallo V (2014) Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature 506:230-234.
Schmitz T, Ritter J, Mueller S, Felderhoff-Mueser U, Chew LJ, Gallo V(2011) Cellular changes underlying hyperoxia-induced delay of white matter development. J Neurosci 31:4327-4344.
Schmitz T, Krabbe G, Weikert G, Scheuer T, Matheus F, Wang Y, Mueller S, Kettenmann H, Matyash V, Buhrer C, Endesfelder S (2014) Minocycline protects the immature white matter against hyperoxia. Exp Neurol 254:153-165.
Schweigreiter R, Roots BI, Bandtlow CE, Gould RM (2006) Understanding myelination through studying its evolution. Int Rev Neurobiol 73:219-273.
Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A,Craig A, Struve J, Sherman LS, Back SA (2008) Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol 63:520-530.
Shapiro-Mendoza CK (2016) CDC grand rounds: public health strategies to prevent preterm birth. MMWR Morb Mortal Wkly Rep 65:826-830.
Simonishvili S, Jain Mohit R, Li H, Levison Steven W, Wood Teresa L(2013) Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia-schemia. ASN Neuro 5:e00131.
Simons M, Nave KA (2015) Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect Biol 8:a020479.
Spassky N, de Castro F, Le Bras B, Heydon K, Queraud-LeSaux F,Bloch-Gallego E, Chedotal A, Zalc B,omas JL (2002) Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci 22:5992-6004.
Stadelmann C, Albert M, Wegner C, Bruck W (2008) Cortical pathology in multiple sclerosis. Curr Opin Neurol 21:229-234.
Sugimoto Y, Taniguchi M, Yagi T, Akagi Y, Nojyo Y, Tamamaki N(2001) Guidance of glial precursor cell migration by secreted cues in the developing optic nerve. Development 128:3321-3330.
Susuki K, Chang K-J, Zollinger Daniel R, Liu Y, Ogawa Y, Eshed-Eisenbach Y, Dours-Zimmermann María T, Oses-Prieto Juan A, Burlingame Alma L, Seidenbecher Constanze I, Zimmermann Dieter R,Oohashi T, Peles E, Rasband Matthew N (2013)ree mechanisms assemble ventral nervous system nodes of ranvier. Neuron 78:469-482.
Syed YA, Hand E, Mobius W, Zhao C, Hofer M, Nave KA, Kotter MR(2011) Inhibition of CNS remyelination by the presence of semaphorin 3A. J Neurosci 31:3719-3728.
Talos DM, Fishman RE, Park H, Folkerth RD, Follett PL, Volpe JJ,Jensen FE (2006) Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J Comp Neurol 497:42-60.
Tang DG, Tokumoto YM, Raff MC (2000) Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol 148:971-984.
Tasker RC (2006) Changes in white matter late after severe traumatic brain injury in childhood. Dev Neurosci 28:302-308.
Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C, Diemel LT, Gveric D, Yeung D, Mehmet H (2010) Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. J Neurosci Res 88:1632-1644.
Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD (2001) Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development 128:2545-2554.
Tolcos M, Rowitch DH, Dean J (2016) Oligodendrocytes: cells of origin for white matter injury in the developing brain. In: Prenatal and Postnatal Determinants of Development (Walker DW, ed), pp 281-301.New York, NY: Springer New York.
Tolcos M, Bateman E, O’Dowd R, Markwick R, Vrijsen K, Rehn A, Rees S (2011) Intrauterine growth restriction affects the maturation of myelin. Exp Neurol 232:53-65.
Tomassy GS, Fossati V (2014) How big is the myelinating orchestra?Cellular diversity within the oligodendrocyte lineage: facts and hypotheses. Front Cell Neurosci 8:201.
Tomassy GS, Dershowitz LB, Arlotta P (2016) Diversity matters: A revised guide to myelination. Trends Cell Biol 26:135-147.
Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P (2014) Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344:319-324.
Traka M, Podojil JR, McCarthy DP, Miller SD, Popko B (2016) Oligodendrocyte death results in immune-mediated CNS demyelination.Nat Neurosci 19:65-74.
Trapp BD, Nishiyama A, Cheng D, Macklin W (1997) Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol 137:459-468.
Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD (2010)NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci 30:16383-16390.
Tripathi RB, Clarke LE, Burzomato V, Kessaris N, Anderson PN,Attwell D, Richardson WD (2011) Dorsally and ventrally derived oligodendrocytes have similar electrical properties but myelinate preferred tracts. J Neurosci 31:6809-6819.
Tsai HH, Tessier-Lavigne M, Miller RH (2003) Netrin 1 mediates spinal cord oligodendrocyte precursor dispersal. Development 130:2095-2105.
Tsai HH, Macklin WB, Miller RH (2009) Distinct modes of migration position oligodendrocyte precursors for localized cell division in the developing spinal cord. J Neurosci Res 87:3320-3330.
Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, Fancy SP (2016) Oligodendrocyte precursors migrate along vasculature in the developing nervous system.Science 351:379-384.
Valerio A, Ferrario M, Dreano M, Garotta G, Spano P, Pizzi M (2002)Soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro differentiation of purified rat oligodendroglial lineage cells.Mol Cell Neurosci 21:602-615.
van Tilborg E, Heijnen CJ, Benders MJ, van Bel F, Fleiss B, Gressens P,Nijboer CH (2016) Impaired oligodendrocyte maturation in preterm infants: Potential therapeutic targets. Prog Neurobiol 136:28-49.
Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C(2002) Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci 20:489-502.
Vigano F, Mobius W, Gotz M, Dimou L (2013) Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci 16:1370-1372.
Wake H, Lee PR, Fields RD (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science 333:1647-1651.
Warrington AE, Pfeiffer SE (1992) Proliferation and differentiation of O4+ oligodendrocytes in postnatal rat cerebellum: analysis in unfixed tissue slices using anti-glycolipid antibodies. J Neurosci Res 33:338-353.
Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M(2005) Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 115:997-1003.
Wood TL, Loladze V, Altieri S, Gangoli N, Levison SW, Brywe KG,Mallard C, Hagberg H (2007) Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Dev Neurosci 29:302-310.
Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, Chang T, Durand DJ, Song D, Bonifacio SL (2012) Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics 130:683-691.
Xing YL, Roth PT, Stratton JA, Chuang BH, Danne J, Ellis SL, Ng SW,Kilpatrick TJ, Merson TD (2014) Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci 34:14128-14146.
Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD (2013) Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77:873-885.
Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP,Zahed H, Maltepe E, Rowitch DH (2014) Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158:383-396.
Zalc B, Colman DR (2000) Origins of vertebrate success. Science 288:271-272.
Zalc B, Goujet D, Colman D (2008)e origin of the myelination program in vertebrates. Curr Biol 18:R511-512.
Zhang H, Miller RH (1996) Density-dependent feedback inhibition of oligodendrocyte precursor expansion. J Neurosci 16:6886-6895.
Zhu Q, Whittemore SR, Devries WH, Zhao X, Kuypers NJ, Qiu M(2011) Dorsally-derived oligodendrocytes in the spinal cord contribute to axonal myelination during development and remyelination following focal demyelination. Glia 59:1612-1621.
*Correspondence to:
Lee Anna Cunningham, Ph.D.,leeanna@salud.unm.edu.
orcid:
0000-0003-0294-5262
(Lee Anna Cunningham)
10.4103/1673-5374.217320
Accepted: 2017-09-12
杂志排行
中国神经再生研究(英文版)的其它文章
- Can we treat neurodegenerative diseases by preventing an age-related decline in microRNA expression?
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
- Beta secretase activity in peripheral nerve regeneration
- On the road towards the global analysis of human synapses
- Non-invasive electrical brain stimulation: from acute to late-stage treatment of central nervous system damage