Baicalin protects neonatal rat brains against hypoxicischemic injury by upregulating glutamate transporter 1 via the phosphoinositide 3-kinase/protein kinase B signaling pathway
2017-11-08ZhiqingZhouYongliangLiZhenboAoZhiliwenQiwenChenZhenggangJuangBingXiaoXiaohuaYan
Zhi-qing Zhou, Yong-liang Li, Zhen-bo Ao, Zhi-li wen, Qi-wen Chen, Zheng-gang Juang, Bing Xiao, Xiao-hua Yan,
1 Department of Pediatrics, the Second People’s Hospital of Huaihua City, Huaihua, Hunan Province, China
2 Department of Oncology, the Second People’s Hospital of Huaihua City, Huaihua, Hunan Province, China
3 Department of Gastroenterology, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, China
4 Department of Pediatrics, the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, China
5 Department of Neurosurgery, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, China
How to cite this article: Zhou ZQ, Li YL, Ao ZB, Wen ZL, Chen QW, Huang ZG, Xiao B, Yan XH (2017) Baicalin protects neonatal rat brains against hypoxic-ischemic injury by upregulating glutamate transporter 1 via the phosphoinositide 3-kinase/protein kinase B signaling pathway.Neural Regen Res 12(10):1625-1631.
Funding: is study was supported by the Chinese Medicine Research Foundation of Jiangxi Provincial Health Department of China, No.2013A040; the Science and Technology Program of Jiangxi Provincial Health Department of China, No. 20123023; and the Science and Technology Support Program of Jiangxi Province of China, No. 2009BSB11209.
Baicalin protects neonatal rat brains against hypoxicischemic injury by upregulating glutamate transporter 1 via the phosphoinositide 3-kinase/protein kinase B signaling pathway
Zhi-qing Zhou1, Yong-liang Li2, Zhen-bo Ao1, Zhi-li wen3, Qi-wen Chen4, Zheng-gang Juang4, Bing Xiao5, Xiao-hua Yan4,*
1 Department of Pediatrics, the Second People’s Hospital of Huaihua City, Huaihua, Hunan Province, China
2 Department of Oncology, the Second People’s Hospital of Huaihua City, Huaihua, Hunan Province, China
3 Department of Gastroenterology, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, China
4 Department of Pediatrics, the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, China
5 Department of Neurosurgery, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, China
How to cite this article: Zhou ZQ, Li YL, Ao ZB, Wen ZL, Chen QW, Huang ZG, Xiao B, Yan XH (2017) Baicalin protects neonatal rat brains against hypoxic-ischemic injury by upregulating glutamate transporter 1 via the phosphoinositide 3-kinase/protein kinase B signaling pathway.Neural Regen Res 12(10):1625-1631.
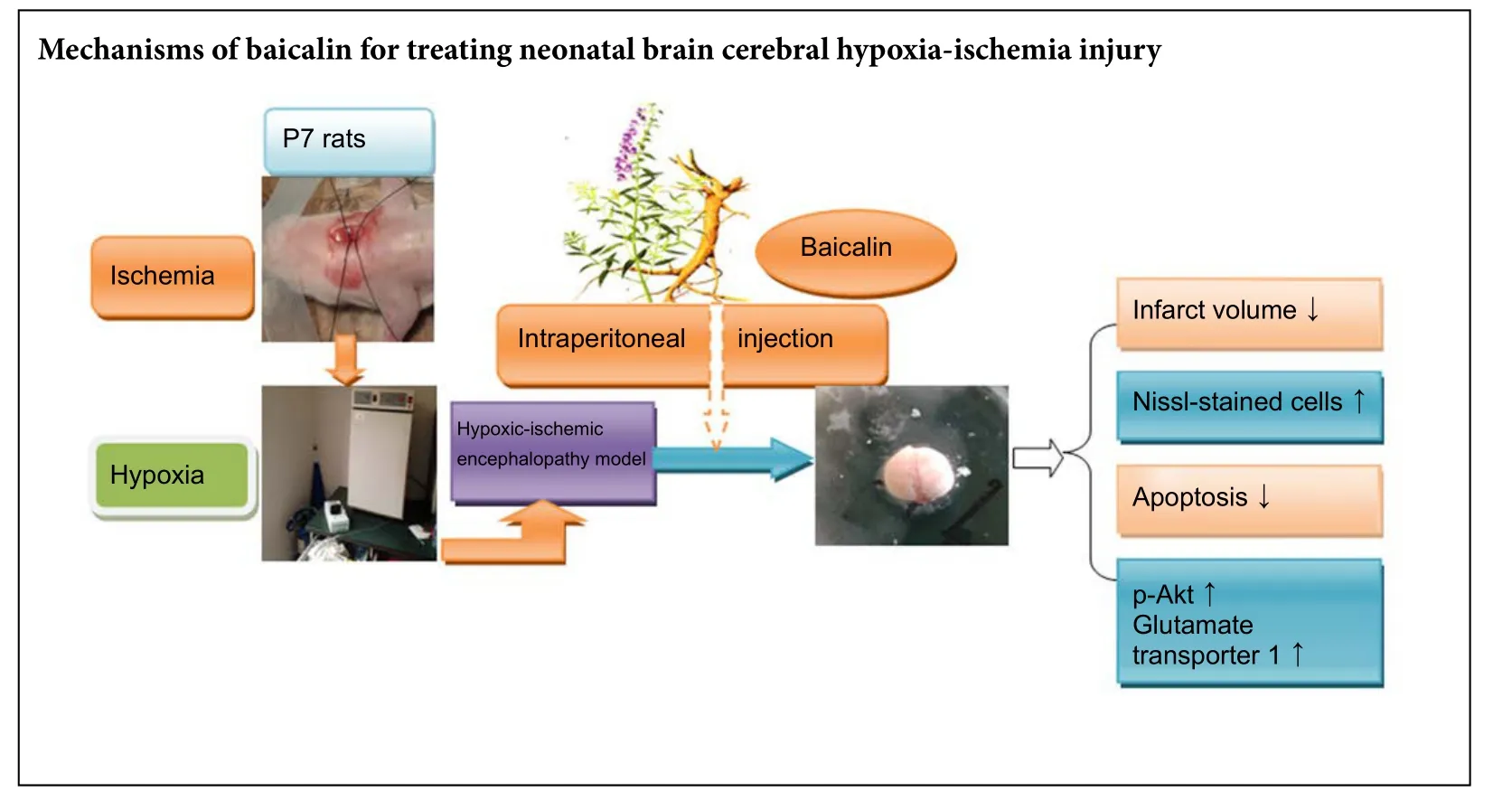
Baicalin is a flavonoid compound extracted from Scutellaria baicalensis root. Recent evidence indicates that baicalin is neuroprotective in models of ischemic stroke. Here, we investigate the neuroprotective effect of baicalin in a neonatal rat model of hypoxic-ischemic encephalopathy. Seven-day-old pups underwent lecommon carotid artery ligation followed by hypoxia (8% oxygen at 37°C) for 2 hours, before being injected with baicalin (120 mg/kg intraperitoneally) and examined 24 hours later. Baicalin effectively reduced cerebral infarct volume and neuronal loss, inhibited apoptosis, and upregulated the expression of p-Akt and glutamate transporter 1. Intracerebroventricular injection of the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) inhibitor LY294002 30 minutes before injury blocked the effect of baicalin on p-Akt and glutamate transporter 1, and weakened the associated neuroprotective effect. Our findings provide the first evidence,to our knowledge that baicalin can protect neonatal rat brains against hypoxic-ischemic injury by upregulating glutamate transporter 1 via the PI3K/Akt signaling pathway.
nerve regeneration; baicalin; hypoxia ischemia; PI3K/Akt signaling pathway; glutamate transporter 1; excitotoxicity; neonatal rats;apoptosis; neural regeneration
Introduction
Perinatal hypoxia-ischemia causes permanent damage to the immature brain, defined as hypoxic-ischemic encephalopathy (HIE). Neonatal HIE affects one in every 500 term births (Kurinczuk et al., 2010), causing a series of irreversible brain injuries and neurological disorders including epilepsy,mental retardation and cerebral palsy (Johnston et al., 1995;Vannucci and Hagberg, 2004). However, the pathophysiological mechanisms of HIE are not fully understood. Energy failure, intracellular calcium overload, glutamate-mediated excitotoxicity, oxidative stress, and inflammation have all been suggested to contribute to HIE (Vannucci et al., 1999;Perlman, 2006; Li et al., 2015; Lin, 2015); of these, glutamate-mediated excitotoxicity is considered the trigger for ischemic neuronal injury (Kostandy, 2012).
Excessive extracellular glutamate is mainly removed by glutamate transporters, because there are no extracellular enzymes to degrade glutamate. Five subtypes of glutamate transporters have been identified in the rat brain (EAAT1–5). EAAT2, also called glial glutamate transporter-1 (GLT-1), is expressed mainly in glial cells and is responsible for most glutamate transport, maintaining extracellular glutamate concentrations in the brain below neurotoxic levels(Rao et al., 2001). More than 90% of glutamate reuptake is mediated by GLT-1 in the forebrain (Danbolt, 2001).Knockout of the GLT-1 gene increases concentrations of extracellular glutamate and causes enlarged infarct volumes(Rao et al., 2001). Some pathological conditions have been associated with GLT-1 expression, including stroke (Yeh et al., 2005) and Alzheimer’s disease (Lauderback et al., 2001).Therefore, increasing the clearance of glutamate via GLT-1 is a promising therapeutic target for such conditions.Indeed, some drugs and pretreatments, such as ceriaxone and hypoxia preconditioning, have already shown neuroprotective effects via GLT-1 upregulation (Cimarosti et al.,2005; Lai et al., 2011).
Baicalin is an isolated flavonoid compound extracted from the dry root of Scutellaria baicalensis Georgi, a member of the Labiatae family. Baicalin possesses anti-inflammatory (Li et al., 2000), anti-oxidant and anti-apoptotic properties (Cao et al., 2011) and is widely used in traditional herbal medicine for the treatment of various diseases.Interestingly, baicalin ameliorates the damage caused by global cerebral ischemia/reperfusion in gerbils (Cao et al.,2011), as well as attenuating cerebral ischemia-induced glutamate increases and exerting neuroprotection (Li et al.,2003). In our previous study, we found that baicalin was able to reduce brain edema and decrease glutamate levels in neonatal rat brain aer intracerebral hemorrhage (Yan et al., 2004).erefore, baicalin shows promise as a neuroprotective agent against cerebral injury. However, the mechanisms underlying this neuroprotective action are incompletely understood. Baicalin activates the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling pathway in vivo and in vitro (Liu et al., 2010; Sun et al., 2013), and several recent studies have suggested that the PI3K/Akt pathway is involved in the regulation of GLT-1 expression in astrocytes (Zelenaia et al., 2000; Lee et al., 2012). However, the relationship between baicalin and GLT-1 expression remains unclear.erefore, in the present study, we investigated the effects of baicalin on GLT-1 expression and the PI3K/Akt pathway in a neonatal rat model of HIE.
Materials and Methods
Animals
All animal research was approved by the Animal Ethics Committee of the First Affiliated Hospital of Nanchang University, China (approval No. 2015-086).irty pregnant specific-pathogen-free Sprague-Dawley rats, aged 2 months and weighing 250–350 g, were provided by the Center for Animal Testing of Nanchang University, China (license No. SYXK (Gan) 2010-0002). All rats were housed in individual cages under controlled illumination (12-hour light/dark cycle) and with free access to food and water.e day each litter was born was considered postnatal day 0 (P0).We chose P7 pups because the brain maturation of pups at this age is equivalent to that of 32 to 34-week-old human infants (Vannucci et al., 1999, 2005). Seventy-two P7 pups were randomly allocated to four groups (n = 18 per group):sham-operated, HIE, HIE + baicalin + dimethyl sulfoxide(DMSO) group, and HIE + baicalin + LY294002 (a PI3K/Akt pathway inhibitor).
HIE models
HIE was produced as described previously (Rice et al.,1981). Briefly, P7 rat pups were anesthetized with 3% halothane and the lecommon carotid artery was ligated.e pups were returned to the dam for 2 hours to recover from anesthesia, then placed into a glass jar, which was partially submerged in a 37°C water bath and contained 8% O2and 92 % N2for 2 hours of hypoxia. The pups were then returned to their dams.e sham group underwent the same procedures, except the left carotid artery was not ligated and the jar contained air. Criteria for HIE were as follows:behavioral disability (decreased righting reflex and balance,spontaneous rotation to the leor holding the tail curled,and convulsions); mostly liquefied and necrotic lesions of the lateral brain; loss and apoptosis of neurons under the microscope.
Drug administration
In the HIE + baicalin + LY294002 group, rats received an intracerebroventricular (i.c.v.) injection of LY294002 (0.2 M in DMSO; 5 μL; Sigma-Aldrich, St. Louis, MO, USA) 30 minutes before HIE. In the HIE + baicalin + DMSO group, 2%DMSO (5 μL i.c.v.) was injected 30 minutes before HIE.e dose and site (2 mm rostral, 1.5 mm lateral to bregma, and 2.5 mm deep to the skull surface) of the LY294002 injection were guided by previous studies in a neonatal rat model of HIE (Xiong et al., 2012). Rats in the HIE + baicalin + DMSO and HIE + baicalin + LY294002 groups received baicalin(120 mg/kg intraperitoneally; Food and Drug Inspection Institute, Beijing, China; lot number 110715-201318; purity, 98.5%) immediately after HIE. Baicalin was dissolved in saline and diluted to 3.6 mg/mL and the dose was based on our previous study (Yan et al., 2004).e sham and HIE groups received 0.5 mL physiological saline. Twenty-four hours aer HIE, all rats were sacrificed and the brain tissue was obtained as described below.
Measurement of infarct volume
Six pups were anesthetized with 3% halothane and decapitated 24 hours aer HIE.e brains were quickly removed and placed at −20°C for 8 minutes. Five 2-mm-thick coronal slices were cut from the frontal pole, incubated in 2%2,3,5-triphenyltetrazolium chloride (TTC) for 10 minutes at 37°C in the dark, and fixed in 4% formalin overnight.The stained slices were digitally photographed and the volume of each section was determined using ImageJ so-ware (National Institutes of Health, Bethesda, MD, USA).The percentage infarction was calculated by dividing the infarct volume by the total volume of all the slices (Liu et al., 2010).
Nissl staining
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining
TUNEL staining was performed on 4 μm paraffin-embedded coronal sections in the same way as Nissl staining but using the In Situ Cell Death Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany), according to the manufacturer’s instructions. Nuclei were stained with DAPI. Fluorescein (green) was visualized using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany) at excitation and emission wavelengths of 485 nm and 535 nm, respectively. DAPI fluorescence (blue) was visualized at excitation and emission wavelengths of 365 nm and 450 nm, respectively.
Counting of Nissl- and TUNEL-stained cells
Six visual fields in the cerebral cortex (400× magnification)were photographed per section and the stained cells were counted. Data are presented as the number of cells per visual field. Images of the cortex from each rat were captured using a Leica DMLB microscope (Wetzlar, Germany), and Image-Pro Plus (Media Cybernetics, Inc. Silver Spring, MD,USA) was used to perform quantitative analysis of Nissl-positive cells. TUNEL-stained cells were visualized using a fluorescence microscope.
western blot assay
The brains (n = 6) were removed and the left peri-infarct cortex was rapidly isolated. Total proteins were extracted and protein concentration was determined using the bicinchoninic acid assay (Solarbio, Beijing, China). Total proteins(30 μg) were loaded and separated in a 10% sodium dodecyl sulfate-polyacrylamide gel and then electrotransferred to a polyvinylidene difluoride membrane. The membrane was blocked in 5 % fat-free milk for 1 hour at room temperature and incubated for 24 hours at 4°C with the following primary antibodies: rabbit anti-rat Akt monoclonal antibody(1:1,000; Cell Signaling Technology, Danvers, MA, USA),rabbit anti-rat phospho-Ser473Akt monoclonal antibody(1:1,000; Cell Signaling Technology), rabbit anti-rat GLT-1 monoclonal antibody (1:1,000; Proteintech Group, Rosemont, IL, USA) and rabbit anti-β-actin polyclonal antibody(1:1,000; Zhongshan Golden Bridge Biotechnology, Beijing,China). Subsequently, blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000; Zhongshan Golden Bridge Biotechnology) for 1 hour at room temperature. All blots were developed using an enhanced chemiluminescence kit (ermo Scientific, Waltham, MA, USA).The integrated optical density of each band was measured using a Bio-Image Analysis System (Bio-Rad, Hercules, CA,USA).e relative integrated optical density ratio of p-Akt/Akt and GLT-1/β-actin in each group was normalized to the value from the sham group.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analysis was performed using SPSS 13.0 soware (SPSS, Chicago, IL,USA). The differences among groups were analyzed using one-way analysis of variance followed by the Student-Newman-Keuls tests if there was a significant difference between groups. P < 0.05 was considered statistically significant.
Results
Effect of baicalin on morbidity and mortality rates of rat models of HIE
Twenty-four hours after injury, the morbidity rate of the animals was 0.00% (n = 0) in the sham group, 75.00% (n =12) in the HIE group, 80.00% (n = 12) in the HIE + baicalin+ DMSO group and 80.00% (n = 12) in the HIE + baicalin +LY294002 group. Mortality was 11.76% (n = 2) in the sham group, 25.00% (n = 4) in the HIE group, 20.00% (n = 3) in the HIE + baicalin + DMSO group and 20.00% (n = 3) in the HIE + baicalin + LY294002 group. Mortality rate in the sham group was significantly lower than in the other three groups(P < 0.01).ere were no significant differences in mortality rate among the other three groups (P > 0.05).
Baicalin decreased infarct volume in rat models of HIE
TTC staining revealed no infarction in the sham group 24 hours aer injury, whereas there was high infarct percentage in brains in the HIE group (Figure 1A).e infarct percentage in the HIE + baicalin + DMSO group was significantly smaller than that in the HIE group (P < 0.01). In the HIE +baicalin + LY294002 group, the infarct percentage was significantly higher than that in the HIE + baicalin + DMSO group (P < 0.01), but lower than in the HIE group (P < 0.01;Figure 1B).
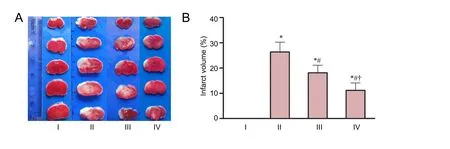
Figure 1 Effect of baicalin on infarct volume in a rat model of HIE 24 hours aer injury.
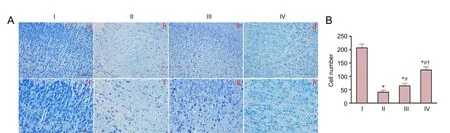
Figure 2 Effect of baicalin on pathological damage in the cerebral cortex of a model of HIE in rat pups 24 hours aer injury.
Baicalin improved pathological damage in the cerebral cortex of rat models of HIE
Figure 2Ashows representative Nissl-stained cerebral cortex in rats 24 hours after hypoxic-ischemic injury. In the HIE group, extensive morphological changes were observed in the neurons, including features of pyknosis, disappearance of Nissl bodies, and nuclear condensation, fragmentation and dissolution (Figure 2A). Significantly more Nissl-positive cells were observed in the baicalin-treated group than in the HIE group (P < 0.01;Figure 2B). However, there were fewer Nissl-positive cells in the HIE + baicalin + LY294002 group than in the HIE + baicalin + DMSO group (P < 0.01;Figure 2B).
Baicalin reduced the number of TUNEL-positive cells in the cerebral cortex of rat models of HIE 24 hours aer injury
Almost no TUNEL-positive cells were observed in brains from sham-operated rats. Rats in the HIE + baicalin +DMSO group had significantly fewer TUNEL-positive cells in the cerebral cortex than those in the HIE and HIE +baicalin + LY294002 groups (P < 0.01). The ameliorating effect of baicalin on HIE-induced neuronal apoptosis was weakened by pretreatment with LY294002. The number of TUNEL-positive cells was markedly greater 24 hours after injury in the HIE + baicalin + LY294002 group than in the HIE + baicalin + DMSO group (P < 0.01;Figure 3).
Baicalin activated phosphorylation of Akt and upregulated GLT-1 expression in peri-infarct cortex in models of HIE 24 hours aer injury
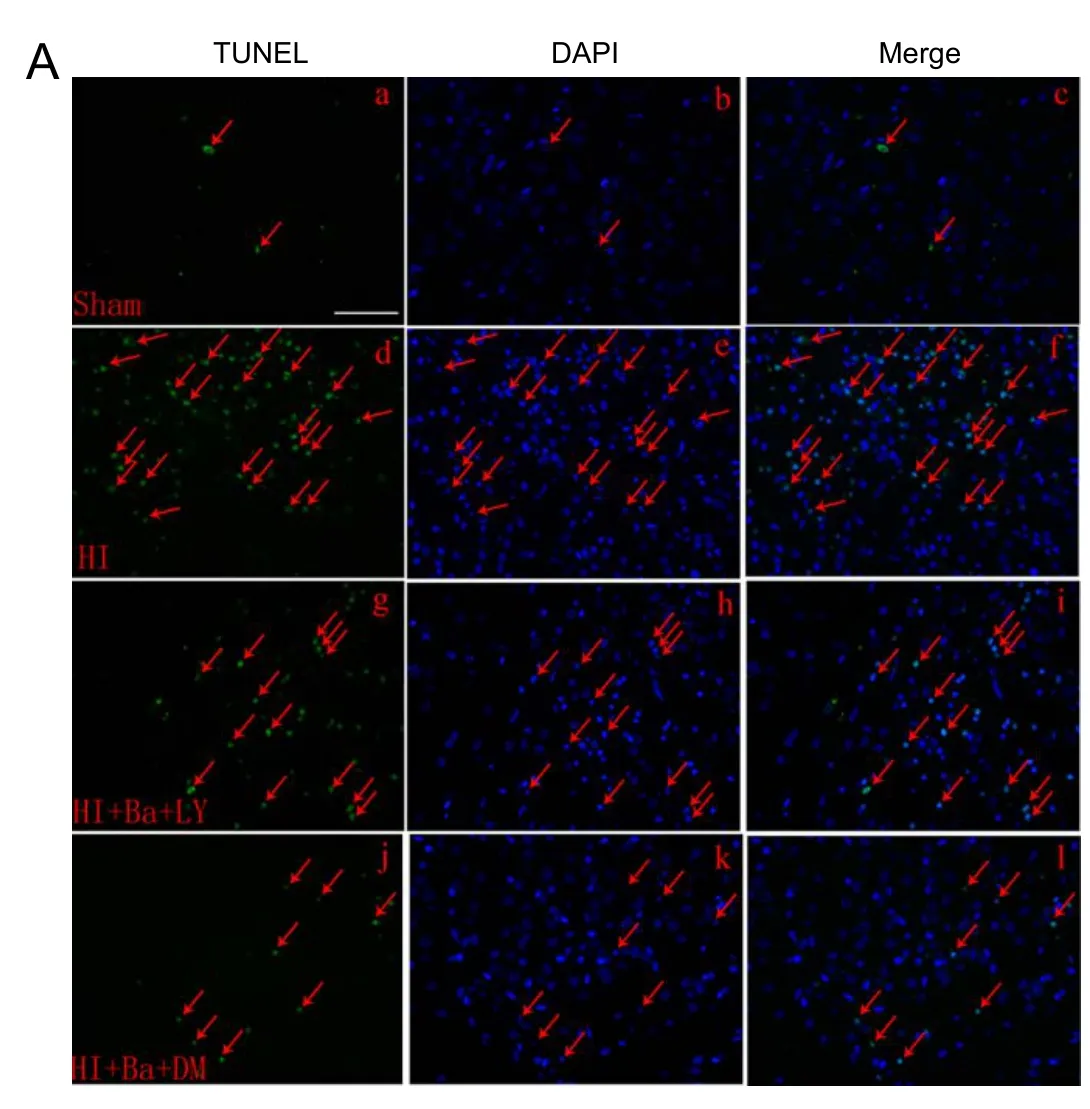
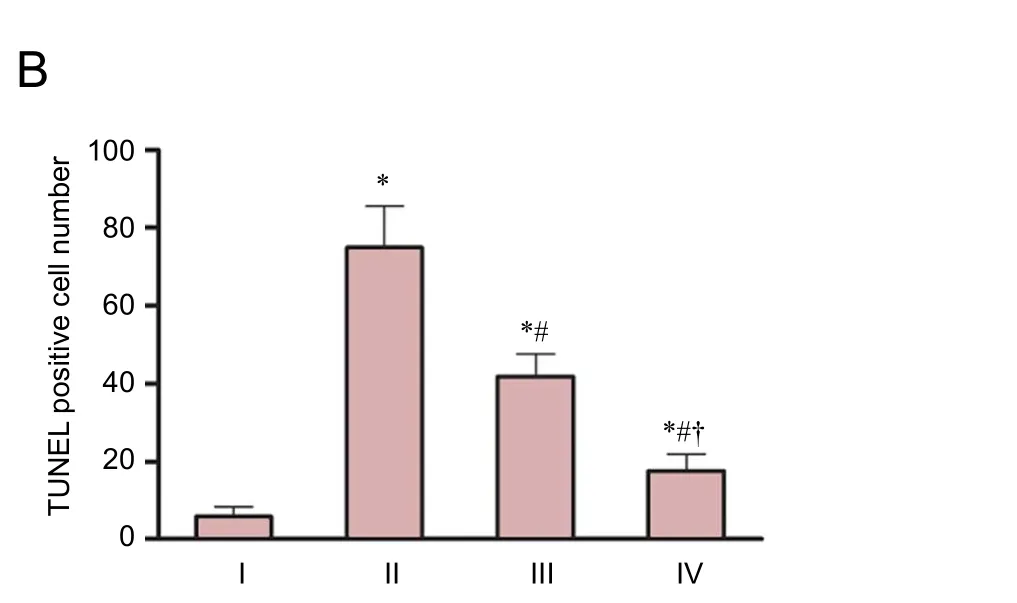
Figure 3 Effect of baicalin on the number of TUNEL-positive cells in the cerebral cortex of a rat model of HIE 24 hours aer injury.
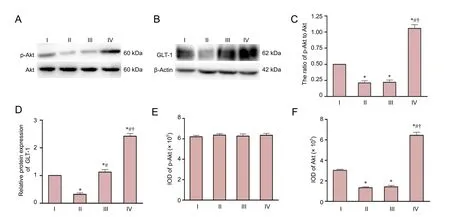
Figure 4 Effect of baicalin on expression of p-Akt and GLT-1 in the ischemic hemisphere in a rat model of HIE 24 hours aer injury.
Western blot analysis of total Akt, p-Akt and GLT-1 is shown inFigure 4A,B. p-Akt and GLT-1 in the HIE group were significantly lower than in the sham group 24 hours aer HIE (P < 0.01;Figure 4C,D,E). Protein levels of total Akt were not significantly different between the four groups (P > 0.05;Figure 4A,F). However, baicalin upregulated the expression of p-Akt and GLT-1 in the HIE+ baicalin + DMSO group compared with the sham and HIE groups (P < 0.01).e PI3K/Akt inhibitor LY294002 completely abolished the baicalin-induced elevation in Akt phosphorylation (P < 0.01;Figure 4C,E), with no statistical difference in p-Akt between the HIE and HIE + baicalin + LY294002 groups (P > 0.05). GLT-1 expression was higher in the HIE + baicalin + LY294002 group than in the HIE group (P < 0.01;Figure 4D), but LY294002 only partially blocked the baicalin-induced elevation in GLT-1 expression (P < 0.01).
Discussion
We have investigated the potential neuroprotective effect of baicalin in a neonatal rat model of HIE.ere were no significant differences in mortality rate among the HIE groups, but the injury from the intracerebroventricular injection must be taken into account in the two baicalin groups, which might mask any baicalin-induced improvement in survival rate. Morphological evidence obtained from the present study also shows the beneficial effect of baicalin therapy on the reduction of ischemic brain damage. Our findings indicate that post-treatment with baicalin markedly decreased cerebral infarct volume,neuronal loss, and the number of apoptotic cells, but also increased GLT-1 expression and PI3K/Akt pathway activation in the ischemic perifocal region.is suggests that the PI3K/Akt pathway and GLT-1 are involved in the neuroprotective role of baicalin in neonatal rats subjected to hypoxic-ischemic injury.
The PI3K/Akt signaling pathway plays a critical role in modulating the activity of many transcriptional factors and regulating neuronal survival (Dudek et al., 1997). Several studies reported that this pathway can regulate GLT-1 expression in astrocytes in vitro (Zelenaia et al., 2000; Lee et al., 2012). In addition, baicalin has been reported to activate the PI3K/Akt signaling pathway (Liu et al., 2010; Sun et al.,2013). To further investigate the mechanism of baicalin in the developing brain subjected to hypoxic-ischemic injury,we explored the effects of baicalin on the expression of GLT-1 and the PI3K/Akt signaling pathway in a neonatal rat model of HIE. p-Akt expression was significantly lower in rats subjected to HIE than in the sham group, and administration of baicalin markedly upregulated GLT-1 and p-Akt levels.
Limitations of this study were that we did not measure GLT-1 activity, explore signal transduction downstream of GTL-1, or conduct behavioral tests. Future studies should examine these factors and evaluate the long-term effects of baicalin in the neonatal rat model of HIE. The precise mechanisms of the increase in GLT-1 expression by baicalin remain unclear. Several studies have shown that phosphorylation of Akt activates the transcription factor NF-κB.Activation of NF-κB signaling contributes to the induction of GLT-1 through an interaction of NF-κB subunits with specific sites in the GLT-1 promoter (Li et al., 2006; Lee et al., 2012). We suspect that baicalin may activate the PI3K/Akt signaling pathway, then activate NF-κB signaling, and subsequently affect GLT-1 expression.
In conclusion, baicalin protects neonatal rat brain against hypoxic-ischemic injury by upregulating GLT-1 via the PI3K/Akt pathway. This suggests that baicalin may be a promising drug for the amelioration of hypoxic-ischemic injury in neonates during the perinatal stage, and provides new insight into the mechanisms of baicalin in treating neonatal brain injury in vivo.
Acknowledgments:We thank Yan-chang Lei, Ph.D. and Zhen-zhen Hu,Ph.D. for their skillful technical assistance.
Author contributions:XHY was responsible for the design of the experiments. QWC was responsible for establishing the animal model.ZQZ and YLL were responsible for molecular biology experiment. BX was responsible for experimental operation in 2,3,5-triphenyltetrazolium chloride, Nissl staining and terminal deoxynucleotidyl transferase dUTP nick end labeling staining. ZGH was responsible for data collection. ZLW was responsible for statistical analysis. ZQZ wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:e study protocol was approved by the Animal Ethics Committee of First Affiliated Hospital of Nanchang University of China(approval number: 2015-086).e experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, Min D, Sun H, Xie N,Cai J (2011) Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways.Brain Res Bull 85:396-402.
Cimarosti H, Jones NM, O’Shea RD, Pow DV, Salbego C, Beart PM(2005) Hypoxic preconditioning in neonatal rat brain involves regulation of excitatory amino acid transporter 2 and estrogen receptor alpha. Neurosci Lett 385:52-57.
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1-105.
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM,Segal RA, Kaplan DR, Greenberg ME (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661-665.
Fukamachi S, Furuta A, Ikeda T, Ikenoue T, Kaneoka T, Rothstein JD,Iwaki T (2001) Altered expressions of glutamate transporter subtypes in rat model of neonatal cerebral hypoxia-ischemia. Brain Res Dev Brain Res 132:131-139.
Gong SJ, Chen LY, Zhang M, Gong JX, Ma YX, Zhang JM, Wang YJ, Hu YY, Sun XC, Li WB, Zhang Y (2012) Intermittent hypobaric hypoxia preconditioning induced brain ischemic tolerance by up-regulating glial glutamate transporter-1 in rats. Neurochem Res 37:527-537.
Johnston MV, Trescher WH, Taylor GA (1995) Hypoxic and ischemic central nervous system disorders in infants and children. Adv Pediatr 42:1-45.
Kurinczuk JJ, White-Koning M, Badawi N (2010) Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum Dev 86:329-338.
Lai PC, Huang YT, Wu CC, Lai CJ, Wang PJ, Chiu TH (2011) Ceriaxone attenuates hypoxic-ischemic brain injury in neonatal rats. J Biomed Sci 18:69.
Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA (2001) The glial glutamate transporter,GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1-42. J Neurochem 78:413-416.
Lee E, Sidoryk-Wêgrzynowicz M, Wang N, Webb A, Son DS, Lee K,Aschner M (2012) GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem 287:26817-26828.
Li BQ, Fu T, Gong WH, Dunlop N, Kung H, Yan Y, Kang J, Wang JM(2000)e flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology 49:295-306.
Li H, Wang H, Chen JH, Wang LH, Zhang HS, Fan Y (2003) Determination of amino acid neurotransmitters in cerebral cortex of rats administered with baicalin prior to cerebral ischemia by capillary electrophoresis-laser-induced fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 788:93-101.
Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB (2006) Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem 97:759-771.
Li YB, Wang Y, Tang JP, Chen D, Wang SL (2015) Neuroprotective effects of ginsenoside Rg1-induced neural stem cell transplantation on hypoxic-ischemic encephalopathy. Neural Regen Res 10:753-759.
Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, Chen J (2010) Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem 112:1500-1512.
Perlman JM (2006) Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Cliner 28:1353-1365.
Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD,Dempsey RJ (2001) Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci 21:1876-1883.
Rice JE, 3rd, Vannucci RC, Brierley JB (1981)e influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9:131-141.
Silverstein FS, Buchanan K, Johnston MV (1986) Perinatal hypoxia-ischemia disrupts striatal high-affinity [3H]glutamate uptake into synaptosomes. J Neurochem 47:1614-1619.
Sun YY, Lin SH, Lin HC, Hung CC, Wang CY, Lin YC, Hung KS, Lien CC, Kuan CY, Lee YH (2013) Cell type-specific dependency on the PI3K/Akt signaling pathway for the endogenous Epo and VEGF induction by baicalein in neurons versus astrocytes. PLoS One 8:e69019.
Vannucci RC, Vannucci SJ (2005) Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci 27:81-86.
Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ (1999) Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res 55:158-163.
Vannucci SJ, Hagberg H (2004) Hypoxia-ischemia in the immature brain. J Exp Biol 207:3149-3154.
Verma R, Mishra V, Sasmal D, Raghubir R (2010) Pharmacological evaluation of glutamate transporter 1 (GLT-1) mediated neuroprotection following cerebral ischemia/reperfusion injury. Eur J Pharmacol 638:65-71.
Xiong T, Tang J, Zhao J, Chen H, Zhao F, Li J, Qu Y, Ferriero D, Mu D (2012) Involvement of the Akt/GSK-3beta/CRMP-2 pathway in axonal injury aer hypoxic-ischemic brain damage in neonatal rat.Neuroscience 216:123-132.
Yan XH, Yang YJ, Liu Y (2004)erapeutic effects of baicalin on encephaledema followed by the laboratory brain blood swelling in neonatal rats. Xiaoer Jijiu Yixue 11:221-223.
Yeh TH, Hwang HM, Chen JJ, Wu T, Li AH, Wang HL (2005) Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol Dis 18:476-483.
Yin Y, Sun W, Li Z, Zhang B, Cui H, Deng L, Xie P, Xiang J, Zou J (2013)Effects of combining methylprednisolone with rolipram on functional recovery in adult rats following spinal cord injury. Neurochem Int 62:903-912.
Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS,Grinspan JB, Rothstein JD, Robinson MB (2000) Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol 57:667-678.
Graphical Abstract
*Correspondence to:
Xiao-hua Yan, M.D.,yanxiaohua456@aliyun.com.
orcid:
0000-0002-0972-8431
(Xiao-hua Yan)
10.4103/1673-5374.217335
Accepted: 2017-09-04
considerable attention for its crucial role in removing excess glutamate from the extracellular space (Rao et al., 2001). It is responsible for more than 90% of glutamate reuptake in the forebrain (Danbolt, 2001). Therefore, enhancing GLT-1 expression may be a pertinent strategy for treating cerebral ischemic disease accompanying excitotoxic insults. Several studies showed that ceftriaxone, a third generation cephalosporin, exerted neuroprotection by increasing GLT-1 expression in a neonatal rat model of HIE (Lai et al., 2011)and in a focal cerebral ischemia model in adult rats (Verma et al., 2010). Additionally, Gong et al. (2012) reported that GLT-1 plays a significant role in the acquisition of ischemic tolerance in the brain aer preconditioning with intermittent hypobaric hypoxia.e expression of GLT-1 in the rat brain is downregulated aer ischemic insult (Fukamachi et al., 2001). Together, this suggests GLT-1 plays a neuroprotective role during ischemic stroke. In the present study,our results revealed significant decreases in GLT-1 protein levels in neonatal rats 24 hours after hypoxic-ischemic brain injury, consistent with a previous study (Fukamachi et al., 2001). Administration of baicalin markedly upregulated protein levels of GLT-1, increased the number of surviving neurons and reduced the number of apoptotic cells.Baicalin also significantly reduced the infarct volume in HIE rats compared with rats that did not receive baicalin.Excitotoxicity related to extracellular accumulation of glutamate plays a vital role in neonatal hypoxic-ischemic brain injury (Silverstein et al., 1986; Kostandy, 2012) and GLT-1 is responsible for more than 90% of glutamate reuptake(Danbolt, 2001). This indicates that baicalin alleviates excitotoxicity and reduces apoptosis at least in part via the upregulation of GLT-1 expression.
Copyedited by Slone-Murphy J, Haase R, Yu J, Li CH, Qiu Y, Song LP, Zhao M
杂志排行
中国神经再生研究(英文版)的其它文章
- Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Using 3D bioprinting to produce mini-brain
- Beta secretase activity in peripheral nerve regeneration
- Embracing oligodendrocyte diversity in the context of perinatal injury
- On the road towards the global analysis of human synapses