昆虫水通道蛋白的研究进展
2017-11-07陆明星潘丹丹王桂荣杜予州
陆明星,潘丹丹,徐 静,刘 杨,王桂荣,杜予州*
(1. 扬州大学园艺与植物保护学院&扬州大学应用昆虫研究所,江苏扬州 225009;2. 中国农业科学院植物病虫害生物学国家重点实验室,北京 100193)
昆虫水通道蛋白的研究进展
陆明星1,潘丹丹1,徐 静1,刘 杨2,王桂荣2,杜予州1*
(1. 扬州大学园艺与植物保护学院&扬州大学应用昆虫研究所,江苏扬州 225009;2. 中国农业科学院植物病虫害生物学国家重点实验室,北京 100193)
昆虫水通道蛋白(Aquaporins, AQPs)是一种膜蛋白,它们是昆虫维持体内水分平衡的必要蛋白,有关它们的研究不断深入。因此,本文对昆虫水通道蛋白的最新研究成果进行了概述,旨在引起人们对该类蛋白的兴趣,以便系统了解和研究该类蛋白。目前研究表明:昆虫典型的AQPs是由250-300个氨基酸残基组成,其分子量在23-35 kDa,包含6个疏水性横跨膜区域、两个NPA结构单元(asparagine-proline-alanine)等。系统发育分析发现:已知昆虫AQPs可分为5大类,分别为DRIP、BIB、PRIP、RPIPs和LHIPs。昆虫AQPs除了运送水分子外,还可以运输其他的一些小分子溶质,如尿素、甘油、海藻糖等。它们还具有组织特异性表达特性,可能在昆虫的多个生理活动中起到重要的作用,因此它们的功能仍需进一步研究证实。此外,昆虫AQPs的深入研究还将会给害虫综合治理提供新的思路。
昆虫;水通道蛋白;功能;水分;分类
水是所有生物体中最重要的组成成分,活细胞含有80%以上的水分,其维持着细胞内外一切生化反应,保护细胞内大分子以及膜的结构,在生物体体液平衡方面起着重要的作用。从细胞和组织中吸收或释放水是一个最基本的生理过程(Klowden, 2002; O'Donnell, 2008)。干燥对于昆虫以及地球上其它生物体来说是一个生理学难题。昆虫为了维持其体内的水分平衡,常常会形成一系列结构和功能上的适应,例如大多数昆虫通过形成坚硬的外骨骼来保护体表防止水分的散失就是这些结构中的一种。在昆虫维持体内水分平衡的各种结构中,水通道蛋白就是其中的一种重要结构。它的存在使得细胞内水的运输相对水分子通过简单扩散的方式缓慢穿过细胞膜的磷脂双分子层而言变得更加容易,因此它在昆虫体内水分平衡中起着不可替代的作用(徐文彦等, 2015)。
早在1987年,Agre就偶然发现了一种水通道蛋白,其分子量为28 kDa,因此将其命名为“形成通道的28 kDa膜整合蛋白”,简称CHIP28,并且发现它大量存在于红细胞和肾脏细胞的膜上(Agreetal., 1987; Denkeretal., 1988)。Preston 等1992年利用非洲爪蟾(Xenopuslaevis)卵母细胞证实了这种膜蛋白确实具有转运水分的功能,随后将这种膜蛋白命名为水通道蛋白(aquaporins, AQPs)(Prestonetal., 1992; Agreetal., 1993)。Agre 等人也因发现了水通道蛋白及其功能,获得了2003年诺贝尔化学奖(Agreetal., 2004)。水通道蛋白与离子通道、甘油通道、多功能通道蛋白同属于重要的内在蛋白(major intrinsic protein,简称MIP)家族。目前,已经发现13种哺乳动物的水通道蛋白(AQP0-AQP12),统称“aquaporins”,AQPs(Fushimietal., 1993; Morishitaetal., 2005; Itohetal., 2005)。水通道蛋白除了能高效转运水分子外,还能转运其他的一些小分子物质,其中包括甘油和尿素等具有重要生理学功能的中性溶质。它们可能还参与某些激素的调节过程,如参与动物体中抗利尿激素、糖皮质激素、胰岛素等激素调节(Kingetal., 1996; Christensenetal., 2000; Kishidaetal., 2000; Benoitetal., 2014);一些研究表明:在植物上发现各种胁迫(低温、干旱、盐等)都会对植物水通道蛋白的mRNA丰度产生影响(Gasparetal., 2003; Liuetal., 2003; Jangetal., 2004)。
1 昆虫水通道蛋白的发现
LeCaherec等人在大青叶蝉(Cicadellaviridis)体内发现了昆虫的第一个水通道蛋白基因(LeCaherecetal., 1996a, b)。但是,随后的十几年来,在昆虫体内仅记录有42个水通道蛋白基因全长,而鳞翅目仅在家蚕Bombyxmori体内发现3个、梨小心食虫Grapholitamolesta中发现2个、斜纹夜蛾Spodopteralitura体内发现 1个、茶尺蠖Ectropisobliqua体内发现1个(表1)(LeCaherecetal., 1996a, b; Echevarriaetal., 2001; Yanochko & Yool, 2002; Duchesneetal., 2003; Kaufmannetal., 2005; Kikawadaetal., 2008; Shakesbyetal., 2009; Balletal., 2009; Kataokaetal., 2009a, b; Drakeetal., 2010; Gotoetal., 2011; Mathewetal., 2011; Philipetal., 2011; Herraizetal., 2011; Azumaetal., 2012; Ishidaetal., 2012; Wallaceetal., 2012; Nagaeetal., 2013; Fabricketal., 2014; Drakeetal., 2015; Liuetal., 2016;刘海远等, 2013; 钟鸣, 2014; 李良德等, 2016)。目前我们已在水稻二化螟Chilosuppressalis体内克隆并发现3种水通道蛋白基因全长(未发表资料)。总之,昆虫的AQPs是由250-300个氨基酸残基组成,其分子量在23-35 kDa(Campbelletal., 2008)。典型的昆虫水通道蛋白都拥有6个疏水性横跨膜区域,大部分昆虫水通道蛋白都包含两个NPA结构单元(Asn-Pro-Ala)(图1)。对于数量庞大的昆虫来说,有关它们的水通道蛋白研究还远远不够。
2 昆虫水通道蛋白的分类
Campbell和Goto等人通过比较12种昆虫的基因组和已得到的昆虫AQPs基因全长序列将昆虫体内的AQP大体分为3类,分别是DRIP,BIB和PRIP(Campbelletal., 2008; Gotoetal., 2011)。昆虫AQPs的第一大类包括从黑腹果蝇Drosophilamelanogaster体内获得的DRIP基因(Dowetal., 1995)。因此将该类AQP基因称为DRIPs家族。该类家族是水渗透型的AQP,拥有六个跨膜区域,氨基和羧基末端都在细胞内以及和其他类AQP一样的两个NPA基序;和哺乳动物的AQP4最为相似。第二大类因为在黑腹果蝇大脑内发现的BIB基因,而称为BIB家族。除了具有两个NPA基序外,BIB家族还具有延伸的羧基段(约350-450个氨基酸),该类不运输水,与哺乳动物晶状体中发现的AQP0最为接近,而AQP0已经被证实具有离子通道的功能(Drakeetal., 2002)。昆虫PRIPs家族与DRIPs的关系要比BIBs更近。PRIPs家族是以窗萤Pyrocoeliarufa内源性蛋白命名(Leeetal., 2001)。有研究表明:该类家族成员既能运输水也能运输甘油(Kikawadaetal., 2008; Herraizetal., 2011)。目前,有关PRIPs家族的AQPs的结构和功能还有待进一步研究。这三大类昆虫水通道蛋白在结构和功能上有一定的相似性,但都具有其独特的结构和功能。最近,Fabrick等人利用Genbank中西部牧草盲蝽Lygushesperus的水通道蛋白和57种其他无脊椎动物的水通道蛋白序列进行系统发育分析表明,昆虫至少包括 5种不同类型的水通道蛋白,除上面提到的3大类外,还有以长红锥蝽Rhodniusprolixus的水通道蛋白为代表的RPIPs和以西部牧草盲蝽的水通道蛋白为代表的LHIPs(Fabricketal., 2014)。Fabric等人(2014)的这种划分仅仅是通过现有种类的水通道蛋白所构建的系统发育树划分的,因此这种分类还有待商榷。随着研究的不断深入,将会鉴定出更多的昆虫水通道蛋白,新鉴定出来的昆虫水通道蛋白有可能不属于上面的任何一类,因此应该考虑多方面的因素,比如进化关系以及结构功能等,不断更新和完善它们的分类系统。

图1 金针瘿蚊Eurosta solidaginis水通道蛋白拓扑预测图(引自Philip et al., 2011)Fig.1 Topology prediction of aquaporin of Eurosta solidaginis(Philip et al., 2011)I-VI 表示6个跨膜区域;A-E表示连接6个跨膜区域的5个颈环结构;红色方框表示2个保守的NPA。The six transmembrane regions (I-VI)were connected by five loops (A-E). Red squares stood for conserved NPA motif.
3 昆虫水通道蛋白的功能
自Preston等人利用非洲爪蟾卵母细胞表达系统验证红细胞中的AQPs功能以来,后续各个生物体包括昆虫的AQPs都利用这个表达系统探究其功能(Prestonetal., 1992)。目前,已经通过非洲爪蟾卵母细胞表达系统验证功能的昆虫AQPs有28种,其中26种有运送水分子的功能。可见,AQPs的主要功能之一是维持昆虫体内的水分平衡。所有的AQPs结构都是由6个跨膜的α螺旋组成的四聚体构成。每一个单聚体都可形成一个水通道,包含2个呈倒置的重复结构,在每一个重复有2个保守的天冬氨酸—脯氨酸—丙氨酸(NPA)基序(图2)。这二个NPA基序是整个水通道的核心部分,它们形成了指引水分子转运的狭窄的空间,从而排除其他物质和离子的通过(Abramson and Vartanian, 2013)。在NPA附近还有一个非常保守的芳香族氨基酸/精氨酸限制位点(ar/R),它是主要限制和选择水分子进入细胞的结构(Chenetal., 2006)。专一性转运水的昆虫AQPs主要就是利用上面的2个保守结构负责细胞水分的转运。同时,许多AQPs都存在对汞化物敏感的位点。

图2 水通道蛋白(AQP)的结构模型(引自Abramson and Vartanian, 2013)Fig.2 Structural model of aquaporin (AQP)(Abramson and Vartanian, 2013 )AQP包含两个保守的NPA标志特征,红色的环表示水分子。Aquaporins contain two conserved NPA signature motif, red circles represent water molecules.
除了运送水分子外,昆虫AQPs还可以运输其他的一些小分子溶质,但是不同的AQPs其转运类型也不一致。例如,在黑腹果蝇体内有一种AQP可以运送单价阳离子;而在家蚕和梨小心食虫体内的一种AQP不但可以转运水还可以转运甘油和尿素;在埃及伊蚊Aedesaegypti体内有一种AQP甚至还可以转运甘油、赤藓糖醇、海藻糖等物质。与专一转水型AQPs相比,这些昆虫AQPs在氨基酸序列和蛋白结构上发生了一定程度的变化,例如,家蚕转甘油性AQP的一个保守的“NPA”变成了“NPS”;有些AQPs还可以增大2个“NPA”结构之间的直径,从而使其能够转运甘油、尿素等(Jensenetal., 2001;Kingetal., 2004;Kataokaetal., 2009a)。
现有的研究表明,每种昆虫都拥有多条AQPs,并且不同种类的昆虫其体内存在的AQPs数目也不一样,例如冈比亚按蚊Anopholesgambiae体内至少有7种AQPs(Liuetal., 2011),果蝇属Drosophila体内可能包含有7种AQPs;从意大利蜜蜂Apismellifera、丽蝇蛹金小蜂Nasovitripennis和赤拟谷盗Triboleumcastaneum基因组推测有6种AQPs,埃及伊蚊和致倦库蚊Culexpipiens有5种,豌豆蚜Aphispisum有4种,家蚕有3种(Campbelletal., 2008)。目前,已经通过试验克隆出家蚕体内的3种AQPs基因(Kataokaetal., 2009a; Azumaetal., 2012),而在烟粉虱Bemisiatabaci中已经克隆了8种AQPs基因(Ekertetal., 2016)。此外,同一种昆虫的不同AQPs在功能上也有差别,如在埃及伊蚊中有一种AQP(基因登录号:XP_001650168)不可以转运水分子,而其他3种AQPs均可以转运水分子(表一)。昆虫水通道蛋白在数目和功能上的差异,可能是其所处环境长期进化的结果。因此,昆虫体内的每一种AQP的功能都与其生态适应有着密切的联系。Kikawada等人发现嗜睡摇蚊Polypedilumvanderplanki之所以能够在缺水的环境中生存,其体内的两种水通道蛋白发挥着重要的作用(Kikawadaetal., 2008)。金针瘿蚊在菊科植物中越冬,其越冬幼虫可以忍受-50℃的低温是因为其体内AQPs在起作用(Philipetal., 2008)。最近发现生活在南极地区的南极摇蚊Belgicaantarctica体内也有多种AQPs的存在,这些AQPs在南极摇蚊适应南极低温干燥环境中起到重要的作用(Gotoetal., 2011; Gotoetal., 2015)。昆虫的AQPs还具有组织表达特异性,不同的昆虫有着不同的组织分布这与其体内的AQPs参与相应的生理功能密切相关。昆虫的马氏管是昆虫排泄和吸收水分的重要器官,因此在许多昆虫的马氏管中AQPs都有很高的表达量,例如长红锥蝽、埃及伊蚊、黑腹果蝇等。对于一些吸血性和取食植物汁液的昆虫来说,它们由于取食大量的液体食物,维持体内的水分和渗透压对于它们正常的生长发育有着重要的意义。因此,这类昆虫在其滤室和后肠中都有很高的AQPs表达量,如豌豆蚜、烟粉虱等(Shakesbyetal., 2009; Ekertetal., 2016)。而德国小蠊的AQP在卵巢中表达量高,金针瘿蚊的AQP在大脑中表达量最高(表1)(Philipetal., 2011; Herraizetal., 2011)。在埃及伊蚊和长红猎蝽体内还发现,水通道蛋白受到血清素的调节(Leeetal., 2003; Martinietal., 2004)。在蚜虫体内发现AQP还可以调节水分和多元醇来控制其体内共生菌生长环境的渗透压(Wallaceetal., 2012)。同时,有些研究还表明:昆虫的AQPs还与生殖、滞育等有着密切的联系(Benoitetal., 2014; Liuetal., 2017)。但是,有关昆虫的AQPs研究起步较晚,研究也不够深入,因此需要加强对昆虫AQPs的研究,特别是其未知功能的研究。
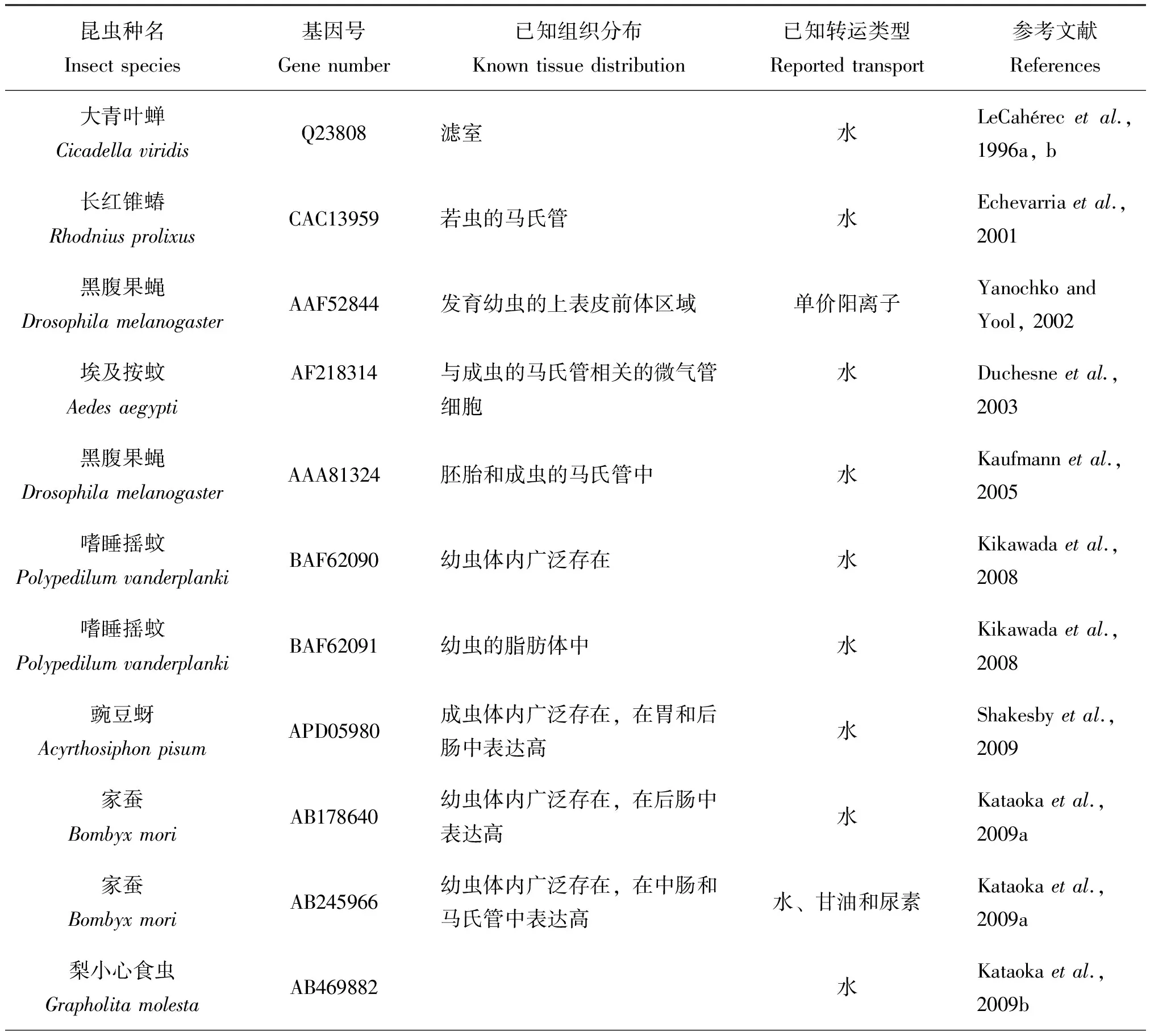
表1 目前已知昆虫的水通道蛋白(AQPs)
续上表

昆虫种名Insectspecies基因号Genenumber已知组织分布Knowntissuedistribution已知转运类型Reportedtransport参考文献References梨小心食虫GrapholitamolestaAB469883水、甘油和尿素Kataokaetal.,2009b冈比亚按蚊AnophelesgambiaeAB523397成虫体内广泛存在,在肠道、马氏管和卵巢中表达高水Liuetal.,2010南极摇蚊BelgicaantarcticaAB602340,AB602341幼虫体内广泛存在,在唾液腺和马氏管中表达高水Gotoetal.,2011金针瘿蚊EurostasolidaginisFJ489680幼虫体内广泛存在,大脑中表达最高水Philipetal.,2011德国小蠊BlattellagermanicaFR744897成虫体内广泛存在,卵巢中表达高水和尿素Herraizetal.,2011烟粉虱BemisiatabaciEU127479 1成虫体内广泛存在,在成虫的滤室和后肠中表达高水Mathewetal.,2011家蚕BombyxmoriAB458833主要在幼虫后肠表达水Azumaetal.,2012伏蝇PhormiareginaAB713909在成虫触角中表达水Ishidaetal.,2012豌豆蚜AcyrthosiphonpisumACYPI009194若虫体内广泛存在,在脂肪体和含菌细胞中表达高水和直链醇Wallaceetal.,2012斜纹夜蛾SpodopteralituraKC999953,KC999954幼虫体内广泛存在,血淋巴中表达高未验证功能刘海远等,2013丽金龟AnomalacupreaAB741517水Nagaeetal.,2013西部牧草盲蝽LygushesperusKF048092KF048101成虫体内广泛存在水Fabricketal.,2014家蝇MuscaDomesticKJ599672幼虫体内广泛存在,在涎腺中表达高未验证功能钟鸣,2014埃及按蚊AedesaegyptiAAF64037成虫体内广泛存在,在马氏管中表达高水Drakeetal.,2010,2015埃及按蚊AedesaegyptiXP_001656932成虫体内广泛存在,在马氏管中表达高水Drakeetal.,2010,2015埃及按蚊AedesaegyptiXP_001650168成虫体内广泛存在,在马氏管中表达高甘油、尿素、赤藓糖醇、核糖醇、甘露醇、海藻糖Drakeetal.,2010,2015埃及按蚊AedesaegyptiXP_001650169成虫体内广泛存在,在马氏管中表达高水Drakeetal.,2010,2015
续上表
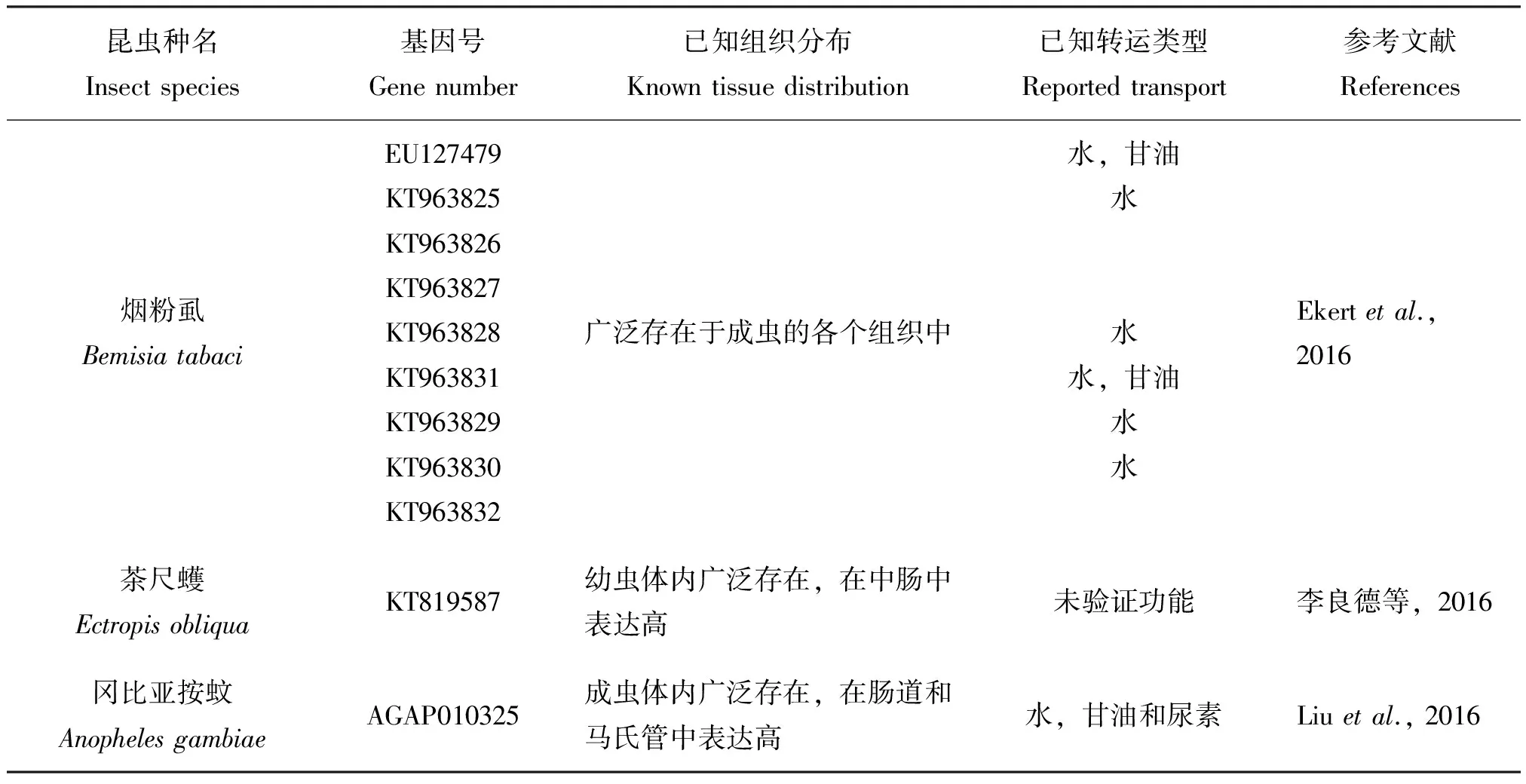
昆虫种名Insectspecies基因号Genenumber已知组织分布Knowntissuedistribution已知转运类型Reportedtransport参考文献References烟粉虱BemisiatabaciEU127479KT963825KT963826KT963827KT963828KT963831KT963829KT963830KT963832广泛存在于成虫的各个组织中水,甘油水水水,甘油水水Ekertetal.,2016茶尺蠖EctropisobliquaKT819587幼虫体内广泛存在,在中肠中表达高未验证功能李良德等,2016冈比亚按蚊AnophelesgambiaeAGAP010325成虫体内广泛存在,在肠道和马氏管中表达高水,甘油和尿素Liuetal.,2016
4 结语
水是生命之源,而所有活细胞维持胞内水分平衡,必须要依赖水通道蛋白所形成的通道。因此,在所有的生命体中至少拥有一种水通道蛋白(AQP),并且许多生物体拥有多种AQPs。其中,以哺乳动物的13种AQPs研究最为深入,目前已经发现这13种AQPs功能各异,在哺乳动物的许多生理活动中起到重要的作用。作为全球繁衍最为成功的昆虫,遍布在地球的各个角落,它们无疑会遇到各种各样的不利环境。其中,水和温度是昆虫得以生存的最重要的因素。昆虫的AQPs可以帮助其进行水分调节和适应不良环境。研究表明每种昆虫体内拥有多种AQPs,并且有的AQP还存在多种转录型。昆虫是如何合理地协调这些AQPs呢?是否昆虫的AQPs还参与了其他的生理活动?在长期进化过程中,不同类型的昆虫AQPs功能上有何差异?目前,虽然已经报道了几种哺乳动物AQPs结构,但是有关昆虫的AQPs蛋白结构未见报道。那么,昆虫的AQPs的蛋白结构和哺乳动物AQPs的蛋白结构有哪些差别呢?因此昆虫的AQPs还有很多问题亟待解决。目前有关昆虫的AQPs研究还很少,尤其我国仅见数篇报道,这可能是由于AQPs属于膜蛋白,研究难度大有关。但是,昆虫AQPs的系统研究无疑将会给整个AQPs的研究带来全新的视角,同时,也给害虫治理带来崭新的契机。
References)
Abramson J, Vartanian AS. Watch water flow[J].Science, 2013, 340(6138): 1294-1295.
Agre P, Preston GM, Smith BL,etal. Aquaporin CHIP—the archetypal molecular water channel[J].AmericanJournalofPhysiology-HeartandCirculatoryPhysiology, 1993, 265(4Pt2): F463-F476.
Agre P, Saboori AM, Asimos A,etal. Purification and partial characterization of the Mr 30,000 integral membrane-protein associated with the erythrocyte Rh(d)antigen[J].TheJournalofBiologicalChemistry, 1987, 262(36): 17497-17503.
Agre P. Aquaporin water channels[J].BioscienceReports, 2004, 24(3): 127-163.
Azuma M, Nagae T, Maruyama M,etal. Two water-specific aquaporins at the apical and basal plasma membranes of insect epithelia: Molecular basis for water recycling through the cryptonephric rectal complex of lepidopteran larvae[J].JournalofInsectPhysiology, 2012, 58(4): 523-533.
Benga G. Water channel proteins (later called aquaporins)and relatives: Past, present, and future[J].IubmbLife, 2009, 61(2):112-133.
Benoit JB, Hansen IA, Attardo GM,etal. Aquaporins are critical for provision of water during lactation and intrauterine progeny hydration to maintain tsetse fly reproductive success[J].PLoSNeglectedTropicalDiseases, 2014, 8(4): e2517.
Campbell EM, Ball A, Hoppler S,etal. Invertebrate aquaporins: A review[J].JournalofComparativePhysiologyB-BiochemicalSystemicandEnvironmentalPhysiology, 2008, 178(8): 935-955.
Chen H, Wu Y, Voth GA. Origins of proton transport behavior from selectivity domain mutations of the Aquaporin-1 channel[J].BiophysicalJournal, 2006, 90(10): L73-L75.
Christensen BM, Zelenina M, Aperia A,etal. Localization and regulation of PKA-phosphorylated AQP2 in response to V(2)-receptor agonist/antagonist treatment[J].AmericanJournalofPhysiology-renalPhysiology, 2000, 278(1): F29-42.
Denker BM, Smith BL, Kuhajda FP,etal. Identification, purification, and partial characterization of a novel Mr 28, 000 integral membrane protein from erythrocytes and renal tubules[J].JournalofBiologicalChemistry, 1988, 263(30):15634-15642.
Dow JAT, Kelly DC, Davies SA,etal. A novel member of the major intrinsic protein family inDrosophila―are aquaporins involved in insect Malpighian (renal)tubule fluid secretion[J].TheJournalofPhysiology, 1995, 489: 110-111.
Drake KD, Schuette D, Chepelinsky AB,etal. pH-Dependent channel activity of heterologously-expressed main intrinsic protein (MIP)from rat lens[J].FEBSLetters, 2002, 512(1-3): 199-204.
Drake LL, Rodriguez SD, Hansen IA. Functional characterization of aquaporins and aquaglyceroporins of the yellow fever mosquito,Aedesaegypti[J].ScientificReports, 2015, 5: 7795.
Drake LL, Boudko DY, Marinotti O,etal. The aquaporin gene family of the yellow fever mosquito,Aedesaegypti[J].PLoSONE, 2010, 5(12): e15578.
Duchesne L, Hubert JF, Verbavatz JM,etal. Mosquito (Aedesaegypti)aquaporin, present in tracheolar cells, transports water, not glycerol, and forms orthogonal arrays inXenopusoocytemembranes[J].EuropeanJournalofBiochemistry, 2003, 270(3): 422-429.
Echevarria M, Ramirez-Lorca R, Hernandez CS,etal. Identification of a new water channel (Rp-MIP)in the Malpighian tubules of the insectRhodniusprolixus[J].EuropeanJournalofBiochemistry, 2001, 442(1): 27-34.
Ekert EV, François Chauvigné F, Finn RN,etal. Molecular and functional characterization ofBemisiatabaciaquaporins reveals the water channel diversity of hemipteran insects[J].InsectBiochemistryandMolecularBiology, 2016, 77: 39-51.
Fabrick JA, Pei JX, Hull JJ,etal. Molecular and functional characterization of multiple aquaporin water channel proteins from the western tarnished plant bug,Lygushesperus[J].InsectBiochemistryandMolecularBiology, 2014, 45: 125-140.
Fushimi K, Uchida S, Hara Y,etal. Cloning and expression of apical membrane water channel of rat kidney collecting tubule[J].Nature, 1993, 361(6412): 549-552.
Gaspar M, Bousser A, Sissoff I,etal. Cloning and characterization of ZmPIP1-5b, an aquaporin transporting water and urea[J].PlantScience, 2003, 165(1):21-31.
Goto SG, Philip BN, Teets NM,etal. Functional characterization of an aquaporin in the Antarctic midgeBelgicaAntarctica[J].JournalofInsectPhysiology, 2011, 57(8): 1106-1114.
Goto SG, Lee, JR. RE,etal. Aquaporins in the antarctic midge, an extremophile that relies on dehydration for cold survival[J].TheBiologicalBulletin, 2015, 229(1): 47-57.
Herraiz A, Chauvigné, CerdJ,etal. Identification and functional characterization of an ovarian aquaporin from the cockroachBlattellagermanicaL. (Dictyoptera, Blattellidae)[J].TheJournalofExperimentalBiology,2011, 214(Pt21): 3895-3903.
Ishida Y, Nagae T, Azuma MA. Water-specific aquaporin is expressed in the olfactory organs of the Blowfly,Phormiaregina[J].JournalofChemicalEcology, 2012, 38(7): 1057-1061.
Ishiguro S, Li YP, Nakano K,etal. Seasonal changes in glycerol content and cold hardiness in two ecotypes of the rice stem borer,Chilosuppressalis, exposed to the environment in the Shonai district, Japan[J].JournalofInsectPhysiology, 2007, 53(4): 392-397.
Itoh T, Rai T, Kuwahara M, Ko SB,etal. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells[J].BiochemicalandBiophysicalResearchCommunications, 2005, 330(3):832-838.
Jang JY, Kim DG, Kim YO,etal. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses inArabidopsisthaliana[J].PlantMolecularBiologyReporter, 2004, 54(5): 713-725.
Jensen MO, Tajkhorshid E, Schulten K. The mechanism of glycerol conduction in aquaglyceroporins[J].Structure, 2001, 9(11): 1083-1093.
Kataoka N, Miyake S, Azuma M. Aquaporin and aquaglyceroporin in silkworms, differently expressed in the hindgut and midgut ofBombyxmori[J].InsectMolecularBiology, 2009, 18(3): 303-314.
Kataoka N, Miyake S, Azuma M. Molecular characterization of aquaporin and aquaglyceroporin in the alimentary canal ofGrapholitamolesta(the oriental fruit moth)—comparison withBombyxmoriaquaporins[J].JournalofInsectBiotechnologyandSericology, 2009, 78(2): 81-90.
Kaufmann N, Mathai JC, Hill WG,etal. Developmental expression and biophysical characterization of aDrosophilamelanogasteraquaporin[J].AmericanJournalofPhysiology-CellPhysiology, 2005, 289(2): C397-C407.
Kikawada T, Saito A, Kanamori Y,etal. Dehydration-inducible changes in expression of two aquaporins in the sleeping Chironomid,Polypedilumvanderplanki[J].BiochimicaetBiophysicaActa, 2008, 1778(2): 514-520.
King LS, Nielsen S, Agre P. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat[J].JournalofClinicalInvestigation, 1996, 97(10): 2183-2191.
King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology[J].NatureReviewsMolecularCellBiology, 2004, 5(9): 687-698.
Kishida K, Kuyiyama H, Funahashi T,etal. Aquaporin adipose, a putative glycerol channel in adipocytes[J].JournalofBiologicalChemistry, 2000, 275(27): 20896-20902.
Klowden MJ. Physiological systems in insects[M]. San Diego: San Diego Academic Press. 2002,231-251.
LeCahérec F, Bron P, Verbavatz JM,etal. Incorporation of proteins into (Xenopus)oocytes by proteoliposome microinjection: functional characterization of a novel aquaporin[J].JournalofCellScience, 1996, 109(Pt6): 1285-1295.
LeCahérec F, Deschamps S, Delamarche C,etal. Molecular cloning and characterization of an insect aquaporin—functional comparison with aquaporin 1[J].EuropeanJournalofBiochemistry, 1996, 241(3): 707-715.
Lee DW, Pietrantonio PV. In vitro expression and pharmacology of the 5-HT7-like receptor present in the mosquitoAedesaegyptitracheolar cells and hindgut-associated nerves[J].InsectMolecularBiology, 2003, 12(6): 561-569.
Lee KS, Kim SR, Lee SM,etal. Molecular cloning and expression of a cDNA encoding the aquaporin homologue from the firefly,Pyrocoeliarufa[J].KoreanJournalofEntomology, 2001, 31(4): 269-279.
Li LD, Wang DF, Liu FJ,etal. cDNA cloning, preparation of polyclonal antibody and subcellular localization of aquaporin 1 (AQP1)inEctropisobliqua(Lepidoptera: Geoqmetridae)[J].ActaEntomologySinica, 2016, 59(4): 382-391. [李良德, 王定锋, 刘丰静, 等. 茶尺蠖水通道蛋白EoAQP1的cDNA克隆、多克隆抗体制备及亚细胞定位[J]. 昆虫学报, 2016, 59(4): 382-391]
Liu HY, Shu BH, Jiang CL,etal. Molecular cloning, characterization and expression analysis of aquaporin 1 (AQP1)gene inSpodopteralitura(Lepidoptera: Noctuidae)[J].ActaEntomologySinica, 2013, 56(4): 339-349. [刘海远, 舒本水, 姜春来, 等. 斜纹夜蛾水通道蛋白1(AQP1)基因的克隆、分子特性和表达分析[J]. 昆虫学报, 2013, 56(4): 339-349]
Liu K, Tsujimoto H, Cha S,etal. Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation[J].ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 2011, 108(15): 6062-6066.
Liu K, Tsujimoto H, Huang YZ,etal. Aquaglyceroporin function in the malaria mosquitoAnophelesgambiae[J].BiologyoftheCell, 2016, 108: 294-305.
Liu LH, Ludewig U, Gassert B,etal. Urea transport by nitrogen-regulated tonoplast intrinsic proteins inArabidopsis[J].PlantPhysiology, 2003, 133(3): 1220-1228.
Liu Y, Denlinger DL, Piermarini PM. The diapause program impacts renal excretion and molecular expression of aquaporins in the northern house mosquito,Culexpipiens[J].JournalofInsectPhysiology, 2017, 98: 141-148.
Martini SV, Goldenberg RC, Fortes FSA,etal.RhodniusprolixusMalpighian tubule’s aquaporin expression is modulated by 5-hydroxytryptamine[J].ArchiveInsectBiochemistryPhysiology, 2004, 57(3): 133-141.
Mathew LG, Campbell EM, Yool AJB,etal. Identification and characterization of functional aquaporin water channel protein from alimentary tract of whitefly,Bemisiatabaci[J].InsectBiochemistryandMolecularBiology, 2011, 41(3): 178-190.
Morishita Y, Matsuzaki T, Hara-chikuma M,etal. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule[J].MolecularandCellularBiology, 2005, 25(17): 7770-7779.
Nagae T, Miyake S, Kosaki S,etal. Identification and characterisation of a functional aquaporin water channel (AnomalacupreaDRIP)in a coleopteran insect[J].TheJournalofExperimentalBiology, 2013, 216: 2564-2572.
O’Donnell M. Insect excretory mechanisms[J].AdvancesinInsectPhysiology, 2008, 35: 1-122.
Philip BN, Kiss AJ, Lee RE. The protective role of aquaporins in the freeze-tolerant insectEurostasolidaginis: functional characterization and tissue abundance of EsAQP1[J].TheJournalofExperimentalBiology, 2011, 214(Pt5): 848-857.
Philip BN, Yi SX, Elnitsky MA,etal. Aquaporins play a role in desiccation and freeze tolerance in larvae of the goldenrod gall fly,Eurostasolidaginis[J].TheJournalofExperimentalBiology, 2008, 211(Pt7): 1114-1119.
Preston GM, Carroll TP, Guggino WB,etal. Appearance of water channels inXenopusoocytes expressing red-cell CHIP28 protein[J].Science, 1992, 256(5055): 385-387.
Tang JX, Zhang C, Bai L,etal. Cloning and sequence analysis of cDNA encoding aquaporin (AQP)gene fromAnophelessinensis[J].ChineseJournalofSchistosomiasisControl, 2012, 24(6): 663-667. [唐建霞, 张超, 白亮, 等. 中华按蚊水通道蛋白(AsAQP)cDNA克隆与序列分析[J]. 中国血吸虫病防治杂志, 2012, 24(6): 663-667]
Shakesby AJ, Wallace IS, Isaacs HV,etal. Water-specific aquaporin involved in aphid osmoregulation[J].InsectBiochemistryandMolecularBiology, 2009, 39(1): 1-10.
Xu WY, Tan Y, Shang HW,etal. Advances in understanding of the mechanisms of water regulation of insects[J].BulletinofScienceandTechnology, 2015, 31(11): 89-96. [徐文彦,谭椰, 商晗武, 等. 昆虫体水分调控机制的研究进展[J]. 科技通报, 2015, 31(11): 89-96]
Yanochko GM, Yool AJ. Regulated cationic channel function inXenopusoocytes expressingDrosophilabig brain[J].InternationalJournalofNeuroscience, 2002, 22(7): 2530-2540.
Yi SX, Joshua BB, Michael AE,etal. Function and immunolocalization of aquaporins in the Antarctic midgeBelgicaAntarctica[J].JournalofInsectPhysiology, 2011, 57(8): 1106-1114.
Zhong M. Identification and functional analysis of the aquaporin inMuscaDomestic[D]. Changshai: Doctoral dissertation of Central South University. 2014. [钟鸣. 家蝇水通道蛋白克隆、鉴定及其功能的研究[D]. 长沙: 中南大学博士论文. 2014]
Researchprogressininsectaquaporins
LU Ming-Xing1, PAN Dan-Dan1,XU Jing1, LIU Yang2, WANG Gui-Rong2, DU Yu-Zhou1*
(1. College of Horticulture and Plant Protection & Institute of Applied Entomology, Yangzhou University, Yangzhou 225009, China; 2. State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, 100193)
Insect aquaporins are integral membrane proteins. It’s necessary to maintain the balance of water by aquaporins in insect, and more and more studies on the insect aquaporins were found, Therefore, in this article, the newest research progresses were summarized systematically, in order to draw scientist’s interesting. This review will be also helpful to researchers, who intend to know and study the aquaporins. Current researches demonstrated insect typical aquaporins consisted of 250-300 amino acids, with a molecular weight of 23-35 kDa, and they included six hydrophobic membrane-spanning helices, two conserved asparagine-proline-alanine (NPA)amino acid triplet motif etc. Phylogenetic analyses exhibited all known insect aquaporins were segregated into five subfamilies, including DRIP, BIB, PRIP, RPIPs and LHIPs. In addition to water, insect aquaporins could transport low-molecular weight solutes, such as urea, glycerol, trehalose and so on. Insect aquaporins also possessed tissue-specific characteristics, and they played very important roles in many physiological functions of insect. Therefore, the further functions should be demonstrated. Moreover, the deep study of insect aquaporins will also give new sight of the integrated pest management.
Insect; aquaporins; function; water; classification
陆明星,潘丹丹,徐静,等.昆虫水通道蛋白的研究进展[J].环境昆虫学报,2017,39(5):983-991.
Q968
A
1674-0858(2017)05-0983-09
国家自然科学基金项目(31371937,31401733);植物病虫害生物学国家重点实验室开放基金(SKLOF201406); 国家重大基础研究规划(“973”计划)项目(2012CB114100)
陆明星, 男, 1984年生, 江苏盐城人, 副教授,博士, 研究方向为昆虫分子生态及系统进化, E-mail: lumx@yzu.edu.cn
*通讯作者Corresponding author, E-mail: yzdu@yzu.edu.cn
Received: 2016-12-16;接受日期Accepted: 2017-07-02
