miRNA-519d对卵巢癌的生长抑制作用及其对XIAP表达的影响
2017-11-02张卫霞
杨 婷 张卫霞
miRNA-519d对卵巢癌的生长抑制作用及其对XIAP表达的影响
杨 婷 张卫霞
目的探讨MicroRNA-519d(miRNA-519d)对卵巢癌细胞生长抑制的作用及其对XIAP表达的影响。方法
对人卵巢癌细胞系OVCAR3瞬时转染miR-519d mimic(实验组)和NC(阴性对照组),不进行任何转染为空白对照组,转染48 h后进行MTT实验、流式细胞实验、Transwell侵袭实验,观察卵巢癌细胞生长抑制状况,WB实验测定XIAP的表达变化。结果定量Real-time PCR检测显示:miR-519d的表达水平均较对阴性对照组与空白对照组有明显的增高(P<0.05)。MTT检测显示:上调OVCAR3细胞内miR-519d水平后,细胞增殖抑制率为(29.81±8.24)%,而阴性照组与空白对照组分别为(2.49±0.28)%和(1.98±0.34)%,对比差异有统计学意义(P<0.05)。流式细胞术检测显示实验组OVCAR3细胞凋亡率为(8.33±1.39)%,而阴性对照组与空白对照组分别为(2.13±2.11)%和(1.89±1.08)%,对比差异有统计学意义(P<0.05)。Transwell侵袭实验显示:转染miR-519dmimics后实验组、阴性对照组、空白对照组的三组的细胞转移能力对比无明显差异(P>0.05)。Western实验结果显示:处理后实验组的XIAP蛋白相对表达量为(0.98±0.34),阴性对照组为(7.54±1.22),空白对照组为(7.67±1.32),对比差异有统计学意义(P<0.05)。结论增强miR-519d的表达能对卵巢癌细胞的增殖起抑制作用,并促进其凋亡,而对卵巢癌细胞的转移没有影响,能抑制XIAP的表达,表明miR-519d在卵巢癌的生长中起到一定的抑制作用。
MicroRNA-519d;卵巢癌;细胞增殖;细胞侵袭;XIAP
(ThePracticalJournalofCancer,2017,32:1596~1599)
上皮性卵巢癌(epithelialovariancancer,EOC)是卵巢癌中最常见的类型,浸润、转移、扩散是死亡的主要原因[1-2]。且卵巢癌发病隐匿,大于2/3的患者出现症状时已经属于晚期,为此对于治疗的要求比较高[3]。在传统治疗中,肿瘤细胞减灭术和以铂类化疗药物为主的联合化疗应用比较多,但易复发,使得患者5年生存率大大下降[4]。miRNA通过碱基配对与靶mRNA序列的3’非翻译区或编码区结合,促进目标mRNA降解或抑制蛋白翻译,调控靶基因的表达[5]。miRNA在肿瘤中的表达与其他肿瘤相似,卵巢癌也有其特异的miRNA表达谱,可调节卵巢癌的生长、侵袭和转移[6-7]。MicroRNA-519d(miR-519d)是在人类组织或细胞中发现较早、广泛存在的miRNAs之一[8-9],但是在卵巢癌的应用报道还比较少见。XIAP连锁凋亡抑制蛋白作为凋亡抑制蛋白(IAPs)家族的一个重要成员,在多数肿瘤中呈现高表达状况[10]。本文具体探讨了miRNA-519d对卵巢癌的生长抑制作用及其对XIAP的表达影响,现报告如下。
1 材料与方法
1.1 实验材料
人卵巢癌细胞OVCAR3(上皮性卵巢癌)购于中国科学院典型培养物保藏委员会上海细胞库,培养体系为RPMI-1640培养基+10%胎牛血清培养条件为37 ℃、5%CO2、饱和湿度培养。
1.2 细胞培养
待OVCAR3细胞密度至80%~90%时,用0.25%的胰酶与0.02%EDTA进行消化传代,继续培养,取对数生长期的肿瘤细胞进行实验。
1.3 瞬时转染miR-519d
根据lipofectamine2000的转染试剂盒的说明书,对人卵巢癌细胞系OVCAR3瞬时转染miR-519d mimic(实验组)和NC(阴性对照组),不进行任何转染为空白对照组,具体步骤如下:将5 μl的miR-519d mimic及阴性对照分别加入250 μl的无血清培养基液中。将5 μl的lipofectamine2000加入到250 μl的无血清培养基液中;将上述液体混匀,室温放置20 min。各孔加入无血清培养基1 ml,再加入混悬液500 μl进行细胞培养。在转染细胞的鉴定中,分别从实验组、阴性对照组、空白对照组细胞中提取RNA,采用RT-PCR进行miR-519d的表达量的测定。
1.4 MTT实验
取转染后48 h的细胞,调整实验组、阴性对照组、空白对照组细胞密度为2×104个/ml,接种于96孔板,接种后48 h进行MTT检测。每孔加入20 μl的MTT,使其终浓度为0.5 mg/ml。37 ℃孵育4~6 h,570 nm处测定吸光度,计算细胞存活率与细胞增殖抑制率。
1.5 流式细胞实验
将细胞胰酶消化后接种于6孔板中,待细胞密度至30%时,转染miR-519d mimics作用48 h。采用100 μl FITC-Annexin与binding buffer悬浮细胞,每组加Annexin V-FITC 5 μl和PI 5 μl,混匀避光。流式细胞仪上机检测,检测细胞凋亡情况。
1.6 Transwell侵袭实验
取转染后48 h的细胞,调整实验组、阴性对照组、空白对照组细胞密度为3×105个/ml,取细胞悬液200 μl加入Transwell小室上室。24孔板下室加入550 μl含10%FBS的培养基进行培养,37 ℃孵育36 h,PBS洗细胞3次,3.7%的甲醛固定10 min,结晶紫染色5 min,显微镜下计算每个视野的平均细胞数量。
1.7 XIAP的基因及蛋白表达
采用RIPA裂解液提取细胞总蛋白,BCA法定量。取100 μg总蛋白行10%SDS-PAGE电泳,将蛋白转移到PVDF膜上,用5%脱脂奶封闭1h后,与XIAP一抗(1∶500,Abcam)4 ℃孵育过夜;常规进行二抗杂交、洗膜、ECL显影,实验以β-actin为内参,计算XIAP蛋白的相对表达量。所有实验重复3次。
1.8 统计学方法
2 结果
2.1 转染miR-519d mimics后细胞的miR-152水平过表达
对人卵巢癌细胞系OVCAR3转染miR-519d mimics 48 h后,定量Real-time PCR检测,结果发现miR-519d的表达水平均较阴性对照组与空白对照组有明显的增高(P<0.05),见表1。
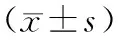
表1 转染miR-519d mimics的人卵巢癌细胞系中miR-519d的相对表达水平
注:与实验组对比,△为P<0.05。
2.2 转染miR-519d mimics对卵巢癌细胞增殖的影响
在OVCAR3细胞中转染miR-519d mimics 48 h后,应用MTT检测显示上调OVCAR3细胞内miR-519d水平后,细胞增殖抑制率为(29.81±0.24)%,而阴性对照组与空白对照组分别为(2.49±0.28)%和(1.98±0.34)%,对比差异有统计学意义(P<0.05)。
2.3 转染miR-519d mimics对卵巢癌细胞凋亡作用的影响
在卵巢癌细胞中转染miR-519d mimics 48 h后,流式细胞术检测显示实验组OVCAR3细胞凋亡率为(8.33±1.39)%,而阴性对照组与空白对照组分别为(1.13±2.11)%和(1.89±1.08)%,对比差异有统计学意义(P<0.05)。
2.4 转染miR-519d mimic对卵巢癌细胞侵袭的影响
转染miR-519d mimics后,观察实验组、阴性对照组、空白对照组在接种后第48 h细胞的转移能力,发现细胞转移能力没有明显下降(P>0.05)。
2.5 XIAP表达分析
Western实验结果显示:处理后实验组的XIAP蛋白相对表达量为(0.98±0.34),阴性对照组为(7.54±1.22),空白对照组为(7.67±1.32),对比差异有统计学意义(P<0.05)。
3 讨论
现代研究表明肿瘤的发生是1个涉及多种肿瘤基因功能改变的多步骤过程,肿瘤抑制基因的功能失活为细胞的恶性转化所必须[11-12]。miRNA是1类内源性的非编码的小分子RNA,miRNA在细胞增殖、免疫反应、凋亡、肿瘤发生及侵袭、转移、血管生成等各个过程中均发挥了重要的调控作用[13-14]。研究证实,microRNA在细胞增殖、分化、凋亡、基因调控及疾病的发生中扮演重要的角色,iR-222能抑制血管内皮细胞的增殖和迁移,miR-23、miR-27b和miR-130a能促血管生成,miR-155、miR-21和miR-126参与血管炎症的调控[15-16]。
研究显示卵巢癌组织中有35个miRNA的表达与正常对照标本相比有统计学差异,其中31个miRNA呈现低表达[17]。有研究发现了miR-10b参与乳腺癌的侵袭和转移过程,其表达量的增加可以特异性的增强肿瘤细胞的侵袭转移能力[18]。miR-519d具有调控细胞增殖的功能,敲除小鼠miR-519d基因簇会引起心脏、肺的发育缺陷,从而导致新生小鼠致死率,但是miR-519d在肿瘤的浸润和转移过程中的具体作用机制尚不清楚[19]。因此我们选取卵巢瘤细胞系OVCAR3,通过过表达miR-519d的功能研究其对卵巢癌细胞的增殖及转移能力的影响。实时定量RT-PCR检显示过表达miR-519d后,miR-519d的表达水平随之升高;MTT实验结果证明,过表达miR-519d 48 h能影响细胞的活性,对细胞长期的增殖能力有抑制作用,且能促进细胞凋亡。但是miR-519d促进OVCAR3增殖及转移的靶基因以及调控机制仍需进一步研究。
肿瘤的转移过程是原发性肿瘤细胞浸润邻近组织、进入体循环,然后最终由微小转移灶增殖成为继发性肿瘤的过程。尽管已经证明一些miRNA具有癌基因或者抑癌基因的功能,但是miRNA在介导肿瘤转移的研究还有待研究[20]。有研究发现miR-519d不仅具有癌基因的功能,还与肿瘤的侵袭和转移功能密切相关[21]。有研究表明磷酸化的XIAP能发挥泛素连接酶的功能,通过直接结合Mdm2,最终导致细胞质中p53蛋白水平的升高,介导Mdm2的快速降解,以此来抑制细胞自噬[21-22]。本研究发现转染miR-519d mimics后,细胞转移能力没有明显下降(P>0.05),表明miR-519d对于肿瘤细胞的生长调控作用可能与侵袭及转移过程无关。Western实验结果显示处理后实验组的XIAP蛋白相对表达量为(0.98±0.34),阴性对照组为(7.54±1.22),空白对照组为(7.67±1.32),对比差异有统计学意义(P<0.05),表明miR-519d的应用能抑制XIAP的表达。
总之,增加miR-519d的表达能对卵巢癌细胞的增殖起抑制作用,并促进其凋亡,而对卵巢癌细胞的转移没有影响,能抑制XIAP的表达,表明miR-519d在卵巢癌的生长中起到一定的抑制作用。
[1] Hua Y,Larsen N,Kalyana-Sundaram S,et al.miRConnect 2.0:identification of oncogenic,antagonistic miRNA families in three human cancers〔J〕.BMC Genomics,2013,15(14):179-182.
[2] Nymoen DA,Slipicevic A,Holth A,et al.MiR-29a is a candidate biomarker of better survival in metastatic high-grade serous carcinoma〔J〕.Hum Pathol,2016,54(13):74-81.
[3] Li Y,Liang C,Wong KC,et al.Inferring probabilistic miRNA-mRNA interaction signatures in cancers:a role-switch approach〔J〕.Nucleic Acids Res,2014,42(9):76-81.
[4] Gadducci A,Sergiampietri C,Lanfredini N,et al.Micro-R-
NAs and ovarian cancer:the state of art and perspectives of clinical research〔J〕.Gynecol Endocrinol,2014,30(4):266-271.
[5] Weng S,Wang W,Li Y,et al.Continuous cadmium exposure from weaning to maturity induces downregulation of ovarian follicle development-related SCF/c-kit gene expression and the corresponding changes of DNA methylation/microRNA pattern〔J〕.Toxicol Lett,2014,225(3):367-377.
[6] Nam EJ,Kim S,Lee TS,et al.Primary and recurrent ovarian high-grade serous carcinomas display similar microRNA expression patterns relative to those of normal ovarian tissue〔J〕.Oncotarget,2016,9(15):640-647.
[7] Sulaiman SA,Ab Mutalib NS,Jamal R.miR-200c Regulation of Metastases in Ovarian Cancer:Potential Role in Epithelial and Mesenchymal Transition〔J〕.Front Pharmacol,2016,23(7):271-279.
[8] Liu J,Dou Y,Sheng M.Inhibition of microRNA-383 has tumor suppressive effect in human epithelial ovarian cancer through the action on caspase-2 gene〔J〕.Biomed Pharmacother,2016,24(83):1286-1294.
[9] Yang Z,Wang XL,Bai R,et al.miR-23a promotes IKKα expression but suppresses ST7L expression to contribute to the malignancy of epithelial ovarian cancer cells〔J〕.Br J Cancer,2016,115(6):731-740.
[10] Hong F,Li Y,Xu Y,et al.Prognostic significance of serum microRNA-221 expression in human epithelial ovarian cancer〔J〕.J Int Med Res,2013,41(1):64-71.
[11] Xu B,Lefringhouse J,Liu Z,et al.Inhibition of the integrin/FAK signaling axis and c-Myc synergistically disrupts ovarian cancer malignancy〔J〕.Oncogenesis,2017,6(1):e295.
[12] Ayyagari VN,Hsieh TJ,Diaz-Sylvester PL,et al.Evaluation of the cytotoxicity of the Bithionol - cisplatin combination in a panel of human ovarian cancer cell lines〔J〕.BMC Cancer,2017,17(1):49.
[13] Li X,Chen W,Zeng et al.microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAP〔J〕.Br J Cancer,2017,116(1):66-76.
[14] Xie S,Jiang H,Zhai XW,et al.Antitumor action of CDK inhibitor LS-007 as a single agent and in combination with ABT-199 against human acute leukemia cells〔J〕.Acta Pharmacol Sin,2016,37(11):1481-1489.
[15] Li S,Yang L,Wang J,et al.Analysis of the chemotherapeutic effects of a propadiene compound on malignant ovarian cancer cells〔J〕.Oncotarget,2016,7(35):57145-57159.
[16] Chen W,Zeng W,Li X,et al.MicroRNA-509-3p increases the sensitivity of epithelial ovarian cancer cells to cisplatin-induced apoptosis〔J〕.Pharmacogenomics,2016,17(3):187-197.
[17] Chen W,Huang L,Hao C,et al.MicroRNA-155 promotes apoptosis in SKOV3,A2780,and primary cultured ovarian cancer cells〔J〕.Tumour Biol,2016,37(7):9289-9299.
[18] Chen X,Gong L,Ou R,et al.Sequential combination therapy of ovarian cancer with cisplatin and γ-secretase inhibitor MK-0752.〔J〕.Gynecol Oncol,2016,140(3):537-544.
[19] Einbond LS,Wu HA,Sandu C,et al.Digitoxin enhances the growth inhibitory effects of thapsigargin and simvastatin on ER negative human breast cancer cells〔J〕.Fitoterapia,2016,109(2):146-154.
[20] Ge G,Zhang W,Niu L,et al.miR-215 functions as a tumor suppressor in epithelial ovarian cancer through regulation of the X-chromosome-linked inhibitor of apoptosis〔J〕.Oncol Rep,2016,35(3):1816-1822.
[21] Chen XX,Xie FF,Zhu XJ,et al.Cyclin-dependent kinase inhibitor dinaciclib potently synergizes with cisplatin in preclinical models of ovarian cancer〔J〕.Oncotarget,2015,6(17):14926-14939.
[22] Zhao WJ,Deng BY,Wang XM,et al.XIAP associated factor 1 (XAF1) represses expression of X-linked inhibitor of apoptosis protein (XIAP) and regulates invasion,cell cycle,apoptosis,and cisplatin sensitivity of ovarian carcinoma cells〔J〕.Asian Pac J Cancer Prev,2015,16(6):2453-2458.
EffectsofMicroRNA-519dontheGrowthandExpressionofXIAPofOvarianCancerCellLine
YANGTing,ZHANGWeixia.
TheSecondAffiliatedHospitalofXi’anMedicalUniversity,Xi’an,710038
ObjectiveTo study the effects of MicroRNA-519d (miR-519d) on the the growth and expression of XIAP of ovarian cancer cell line.MethodsThe human ovarian cancer cell lineOVCAR3 were transfected to miR-519d mimic (experimental group) and NC (negative control group),no transfection were the blank control group,after transfection of 48 h were given MTT assay,flow cytometry assay and Transwell invasion experiments,observed the growth of ovarian cancer cell.The expression of XIAP was determined by WB assay.Resultsquantitative PCR Real-time test showed that the expression level of miR-519 dwere significantly higher than that of negative group and blank control group (P<0.05).MTT showed that the cell proliferation inhibition rate in the experimental group was (29.81±8.24)%,and the negative control group and blank control group were (2.49±0.28)% and (1.98±0.34)%,compared were statistically significant difference (P<0.05).Flow cytometry showed that the apoptosis rate of experimental group was (8.33±1.39)%,while the negative group and the blank control group were (2.13±2.11)% and (1.89±1.08)% respectively.The Transwell invasion experiment showed that there were no significant difference compared among the three groups(P>0.05).The results of Western showed that the relative expression of XIAP protein in the experimental group was (0.98±0.34),and the negative control group was (7.52±1.22),with a blank control group of (7.67±1.32),and the difference was statistically significant (P<0.05).ConclusionTransient transfection of mimics miR-519d in the ovarian cancer cell line can inhibit the cell growth and promote its apoptosis but has no effect on the metastasis,it can inhibit the expression of XIAP,which indicates that miR-519d might inhibit the proliferation of ovarian cancer cells at the post transcriptional level.
MicroRNA-519d;Ovarian cancer;Cell proliferation;Cell invasion;XIAP
710038 西安医学院第二附属医院
10.3969/j.issn.1001-5930.2017.10.009
R737.31
A
1001-5930(2017)10-1596-04
2017-04-06
2017-06-19)
(编辑吴小红)
