金原子簇催化碳-碳偶联反应的研究进展
2017-11-01周洋李杲
周 洋 李 杲
(1中国科学院大连化学物理研究所,催化基础国家重点实验室,大连 116023;2中国科学院大学,北京 100049)
金原子簇催化碳-碳偶联反应的研究进展
周 洋1,2李 杲1,*
(1中国科学院大连化学物理研究所,催化基础国家重点实验室,大连 116023;2中国科学院大学,北京 100049)
近十几年来,金原子簇(尺寸1−2 nm)逐渐发展成为一种新型的纳米材料。特别在近几年中,金原子簇催化剂广泛地应用于纳米催化中,例如选择性氧化还原以及碳-碳偶联等反应。与传统的金纳米颗粒(> 2 nm)显著不同,金原子簇具有独特的电子性质和结构,能很好地关联金原子簇催化剂的结构与其催化性能,特别是对金原子簇的催化反应机理的研究。在本综述中,我们阐明了金原子簇催化剂在碳-碳偶联反应中的应用,其中包括Ullmann、Sonogashira、Suzuki和A3-偶联等反应。并进一步揭示了金原子簇表面有机配体(例如芳香烃硫醇vs脂肪烃硫醇)对催化反应的影响,以及其它金属在金核内部的掺杂(例如铜、银、铂、钯等)改变原子簇的电子结构从而来调控其催化性能。最后,在原子层面上关联金原子簇结构和催化性能,并初步探讨催化反应机制。金原子簇催化剂的深入研究将为高效金纳米催化剂的设计提供一些建设性的思路和策略。
金原子簇;碳-碳偶联;配体效应;金属掺杂效应;Ullmann偶联;Sonogashira交叉偶联;Suzuki交叉偶联;A3-偶联
1 Introduction
Since it was discovered by Haruta et al.1, gold nanoparticles(Au NPs, size from 3 to 100 nm) occupy an important niche in heterogeneous catalysis, which have been widely applied in selective oxidation2−5and hydrogenation6−8, a few to name.These conventional gold nanocatalysts are polydisperse, which become a major issue in fundamental catalysis9. For example,the size dependent of nanoparticles are often averaged out in polydisperse catalysts, and it also is difficult to relate the catalytic properties to the structure and intrinsic properties of nanoparticles. To overcome these obstacles, nanoparticle catalysts of well-defined are highly desirable, leading to the development of bulk single crystal model catalysts with only one facet and the shape-controlled nanocrystal catalysts (e.g.,nanorod, cube, octahedron, etc)10−12.
In recent years, remarkable advances have been achieved in solution phase synthesis of gold nanoclusters, which represents a burgeoning area with increasing application in nanotechnology and nanoscience13−16. The size of these gold nanoclusters is ultra-small, usually about 1−2 nm, with a few dozen to a few hundred gold atoms. The structure of these gold nanoclusters can be well solved by STEM or by X-ray crystallography, which may offer an opportunity to achieve atomic level understanding of the precise relationship between the active-site structure and the catalytic properties17.
These gold nanoclusters of 1−2 nm exhibit excellent catalytic performance (e.g., in the selective oxidation and hydrogenation), sometime better than the corresponding gold complexes and gold nanoparticles18. It is mainly attributed to their high surface-to-volume ratio, surface geometric effect(e.g., precise surface atom arrangement and low-coordinated gold atoms, Auδ+(0 < δ < 1)), the distinct electronic properties,as well as the quantum size effect. Recently, these gold nanoclusters showed good catalytic behaviors in the carbon-carbon coupling reactions19. Traditionally, these carbon-carbon coupling reactions are catalyzed by copper,palladium, platinum complexes and nanoparticles in the previous literatures20,21.
In this review, we only focus on the Au nanoclustercatalyzed carbon-carbon coupling reaction (Scheme 1),including Ullmann coupling of aromatic halides, Suzuki cross-coupling of phenylboronic acid and phenyl halides,Sonogashira cross-coupling of phenyl halides and phenylacetylene, and even A3-coupling reaction of phenylacetylene, amines and aldehydes. These carbon-carbon bond formations are one of the most important protocols in organic synthesis. These formed C―C bonds are very often observed in the natural products, such as alkaloids as well as in numerous biologically active parts of pharmaceutical and agrochemical specialities. Moreover, the catalytic mechanisms of these four carbon-carbon coupling reactions, using gold nanoclusters as calculated mode, were thorough discussed in this reviews.
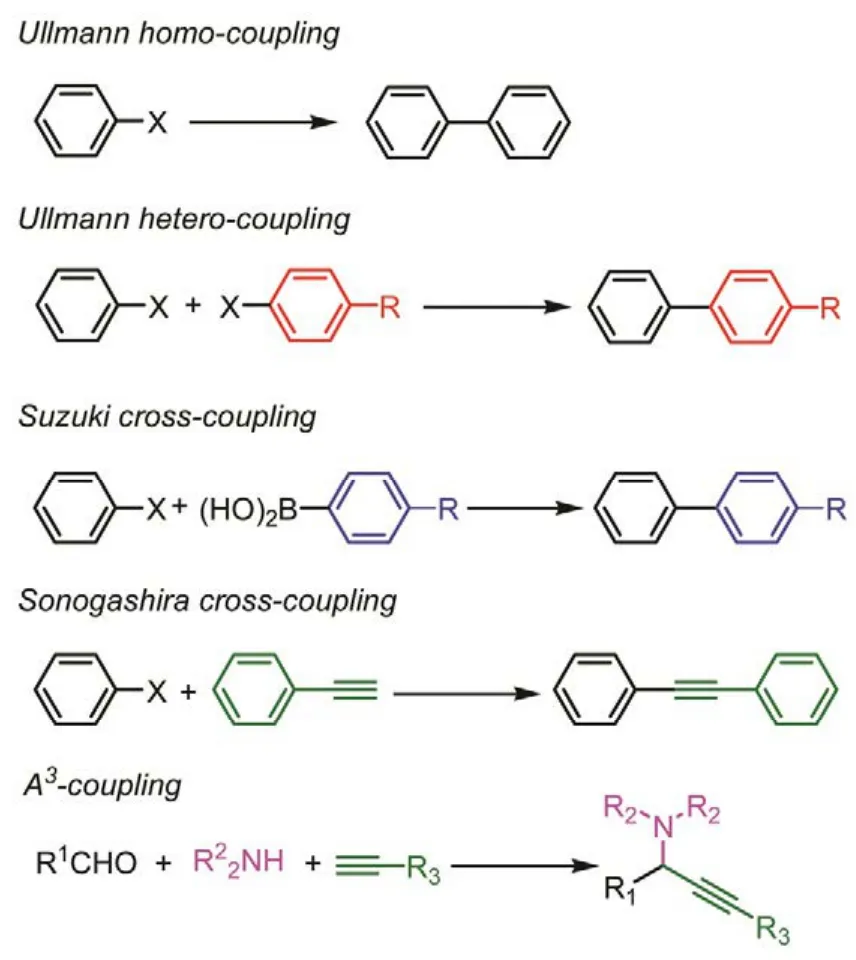
Scheme 1 Gold cluster-catalyzed C-C coupling reaction discussed in this review.


2 Activation of Ph−B(OH)2, Ph−I, and C≡C−H bond on naked Au38 clusters: DFT simulation
The activation of both the Ph―B(OH)2bond and C―I bond of iodobenzene (IB) on the gold clusters is the two key steps in the Suzuki cross-coupling reactions. Meanwhile, the activation of the C―I bond is the key point in the Ullmann coupling. On the other hand, in the Sonogashira cross-coupling reaction, the activation of the C≡C―H bond of Phenylacetylene (PA) also play very important role. The energy of the adsorption and activation process on the different well-defined facet and sites are distinct22−25. To detailed study on the catalytic mechanism of the activation of Ph―B(OH)2, C―I, and C≡C―H bond by gold clusters, the density functional theory calculations should be an easy and fast pathway.
Corma et al. firstly investigated the tentative mechanism and the active sites of the activation of Ph―B(OH)2, Ph―I, and C≡C―H bond, using naked Au38cluster and partially oxidized Au38O2cluster as model in the DFT simulation26. Of note, the Au38and Au38O2nanocluster model used in the computational analysis is rather different from the real catalysts in terms of size and structure. Naked Au38cuboctahedral cluster model contain low coordinated metallic Au0atoms, Scheme 2(a). The Au38O2cluster model included low coordinated metallic Au0and cationic Auδ+species. In the Au38O2cluster model, two O atoms bonded to an Au atom adopting an oxide-like structure,and four Au atoms bonded to one O atom, Scheme 2(b). The possible role of the cationic gold species and the adsorbed oxygen on the coupling was investigated, as oxygen is one of the intriguing facts in the coupling reactions.
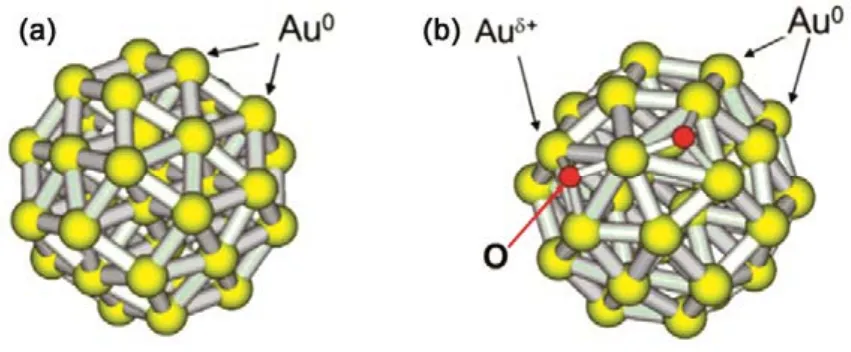
Scheme 2 Optimized structures of the nanogold catalyst models used in the DFT simulation.
Frist, Corma et al. examined the homo-coupling reactions of organoboronates, using naked Au38or partially oxidized Au38O2clusters as the quasi-molecular catalyst27. The possible role of the cationic gold species and the adsorbed oxygen on the coupling was investigated, as oxygen is one of the intriguing facts in the homo-coupling reactions. The DFT calculation suggested that the phenylboronate should be preferentially adsorbed on neutral gold atoms (naked Au38), rather than on positive Auδ+sites (Au38O2), Fig.1(a). After adsorption on gold,the Ph―B bond was dissociated with a low energy (ca. 6.5 kcal·mol−1), leading to phenylboronic acid. And, the phenyl group (Ph) strongly bonded to low-coordinated gold atom (2.46 Å, Fig.1(a), down profile). And then, the all geometries and energy prof i les of the various states have been considered(Fig.1). Two possibilities with similar high activation energy were considered for the transition state leading to biphenyl, i.e.,the two coupling phenyl groups should be ligated either on the same gold atom at a corner of the Au38cluster or on two neighbor gold atoms, Fig.1(b). However, the energy of the two transition states is some high (> 30 kcal·mol−1), and the charged gold atoms can lower the barrier at this stage. Take all together,the DFT results gave a 6.5 kcal·mol−1energy on the partially oxidized Au38O2clusters, which make the activation of Ph―B(OH)2feasible.
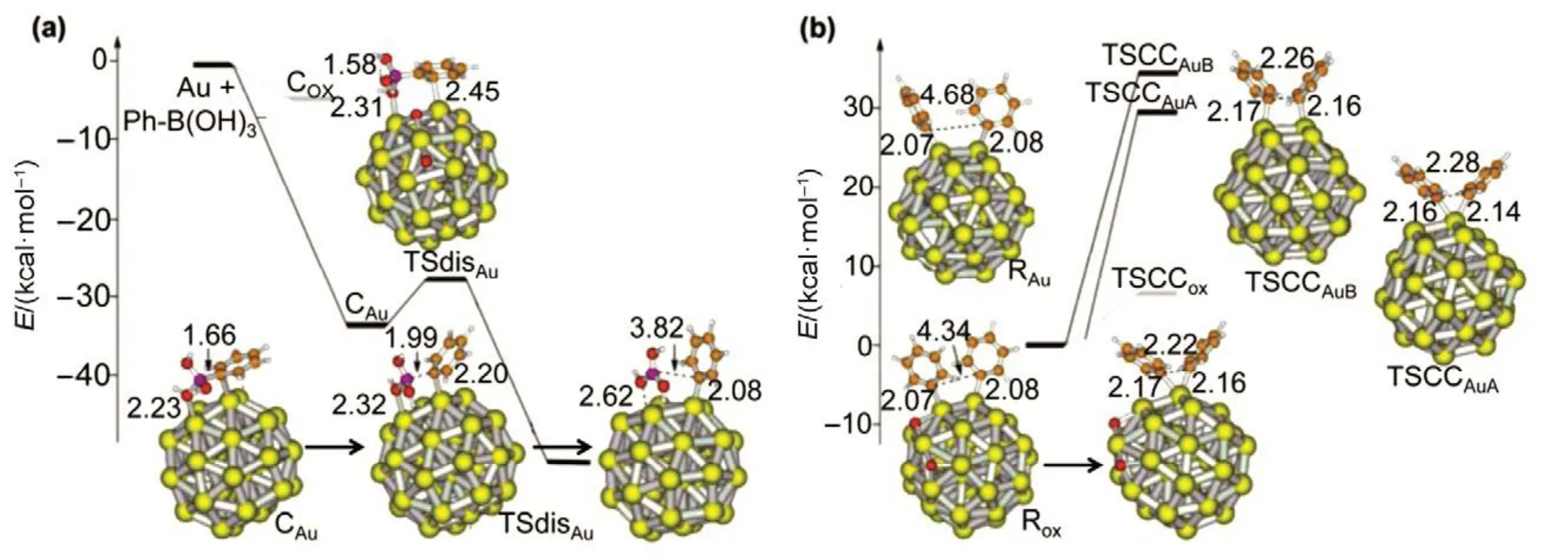
Fig.1 Calculated energy prof i les for (a) dissociation of PhB(OH)3– anion and (b) coupling of two phenyl fragments giving rise to biphenyl on a naked Au38 nanoparticle and on a partially oxidized Au38O2 nanoparticle.

Fig.2 Optimized geometries of the species involved in the Sonogashira cross-coupling reaction on a naked Au38 cluster (neutral Au0 atoms).
At the same time, Corma and co-worker also calculated the adsorption process and possible IB dissociation to yield diphenyl (DP), using the same naked Au38cluster as the DFT model28. The DFT calculation results indicated that the iodobenzene molecule interacted strongly with the Au38clusters.The iodine atom was directly bonded to an Au atom of 2.77 nm–1, Fig.2(a). In the activation process, the C―I bond length increases to 2.48 Å. Meanwhile, the iodine atom and the phenyl group interact with two neighbor gold atoms; the optimized Au―I and Au―C distances are 2.78 and 2.21 Å, respectively,Fig.2. The activation energy of 11.3 kcal·mol–1implied that the rupture of Ph―I bond on gold cluster is also feasible.
Further, the two catalyst models, i.e., Au38and Au38O2cluster,also have been applied to study the catalytic mechanism and the exactly the active sites for the gold cluster catalyzed Sonogashira cross-coupling reaction and the competitive homo-coupling reactions [100]. The breakage energy of the C―H bond of PA on the Au38model is some high (40 kcal·mol−1). However, the gold cluster catalyzed Sonogashira coupling is carried out in the presence of base (e.g., K2CO3),and the alkyne deprotonation is assisted by the base. Therefore,the energy of the PA deprotonation on the Au38cluster decreased to 8.9 and 7.8 kcal·mol−1at the DFT and DFT-D levels (Table 1), when a carbonate CO32−fragment was introduced. It indicated that the presence of a base can enhances the PA deprotonation.
When the IB was approached to the Au38O2cluster, the interaction with the Auδ+sites is weaker than with neutral Au0atoms, and its dissociation energy is larger than on Au0sites(Table 1), indicating that cationic gold will not favorably compete with Au0for IB dissociation. On the other hand, PA adsorption on Auδ+atom is relatively strong, with an adsorption energy of −9.0 kcal·mol–1(Table 1). And then the proton was transferred to O atom in the deprotonation step (Fig.3), with a low barrier of 6.7 kcal·mol–1. So, it is interesting that it required low activation energy for the surface reaction when the cross-coupling reaction occurred on the Au38O2cluster. The phenyl fragment attached to an Au0atom, and the phenylacetylenyl fragment bonded to an Auδ+site, facilitating the formation of diphenylacetylene (DPA). Of note, the cross-coupling step on the cluster surface is the rate-determining step. The activation energy on the Au38O2cluster is much lower than that on Au38(Table 1).
3 Physical property of the gold clusters
3.1 Framework
Recently, lots of gold clusters’ structures can be determined and resolved by X-ray crystallography, which can be applied as the practical simulation model. In this review, the framework of the Au25(PPh3)10(SR)5X2(X = Cl/Br) and Au25(SR)18cluster are used as the catalytic model for mechanism study (vide infra). Thus, we here briefly discuss the two clusters’ structures.As shown in the Fig.4(A), the Au25(SR)18cluster comprises an icosahedral 13-Au-atom core and six Au2(SR)3staples29. Of note, there are two types of thiolate ligands on the staple motifs of Au25(SR)18= Au25(SR1)6(SR2)12, i.e., the “―SR1” ligandsbridge two exterior Au atoms and the “―SR2” bridges an exterior Au atom and one on the Au13icosahedral core.Meanwhile, the Au25(PPh3)10(SR)5X2cluster is composed of two icosahedral Au13units by sharing one common vertex, i.e.,the waist sites (Fig.(4B))30,31. Ten phosphine ligands directly bind the two Au5pentagonal rings at the end of the rod. And two halides (Cl/Br) bind to two apical Au atoms. The two Au13icosahedrons are connected by the thiolate ligands (―SR) or deprotonated alkyne (―C≡CR)32. Of note, the Au3sites and the waist sites are deemed as the catalytic active sites, and the surface atom on the Au13kernel of the Au25(PPh3)10(SR)5X2and Au25(SR)18clusters also play an important role in the catalysis33.

Table 1 Adsorption (Eads), activation (Eact), and reaction energies (in kcal·mol–1) calculated at the DFT and dispersion corrected DFT-D levels for the three elementary steps in the Sonogashira reaction over the Au38 and Au38O2 models.
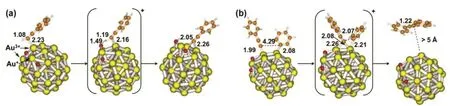
Fig.3 Optimized geometries of the species involved in the Sonogashira cross-coupling reaction on a partially oxidized Au38O2 cluster.
3.2 Thermal stability
The free Au25(SR)18cluster is super stable after the 150 °C annealing treatment in a vacuum oven, evidenced by the UV-Vis, NMR, and MALDI-MS analyses32. Next respected to the oxide-supported Au25(SR)18, UV-Vis and STEM shows that the Au25clusters are still intact after the annealing process(150 °C)34, Fig.5. Further, the XPS analysis shows that the chemical state of surface Au atoms in the Au25(SR)18/oxide catalyst is positively charged (Auδ+)35, in which 0 < δ < 1.
4 Catalytic properties of gold nanoclusters
4.1 Ullmann homo-coupling
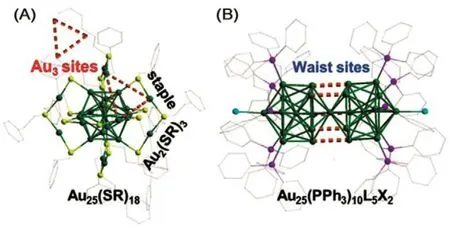
Fig.4 Frameworks of (A) Au25(SR)18 and (B) Au25(PPh3)10L5X2 nanoclusters (where, L = ―SR and PA, X = Cl/Br). (A) There are two non-equivalent types of thiolate ligands on the staple motif“―(SR2)―Au―(SR1)―Au―(SR2)―”.
Since the first 70 years of the 20th century, copper were almost the only metal usable for the aryl-aryl bond formation,initially as copper metal in the reductive symmetrical coupling of aryl halides36(called as the Ullmann homo-coupling reaction). And in recent decade, the gold nanoclusters were conducted in the Ullmann homo-coupling reaction, as the C―I bond of iodide can be activated by the gold nanoclusters via a C―Aun―I intermediate.

Fig.5 (A) UV-Vis spectra of TiO2, Au25(SR)18, and Au25(SR)18/TiO2. (B) STEM image of Au25(SR)18/TiO2 catalyst.
Monopoli et al. reported that the gold nanoclusters of ~1 nm,with a large surface area, showed high catalytic activity in the Ullmann homo-coupling reaction of aryl iodides in the presence of ionic liquids (ILs)37. Two different reaction conditions were investigated: (i) H2O/TBAOH/glucose at 90 °C and (ii) molten TBAA/glucose at 90 °C. The ILs,tetrabutylammonium acetate (TBAA) and tetrabutylammonium hydroxide (TBAOH), played a dual role (surfactant and base species) during the coupling reactions. This reaction also can be expanded to aryl iodides, and the yield of the symmetrical biaryl products was from 40% to 98% (Scheme 3). Of noted, it was almost no reactive, when using bromobenzene as reactant.Bigger size gold nanoparticles (~20 nm in size) showed slightly lower catalytic activity than smaller gold nanoclusters due to the reduced surface area.
Dhital et al.38described that the bimetallic Au/Pd alloy nanoclusters exhibited unique catalytic activity in Ullmann homo-coupling of chloroarenes at low temperatures (27−45 °C).It is worth note that there is unreactive when using the monometallic Au:PVP or Pd:PVP (PVP: poly(N-vinyl-2-pyrrolidone) as catalyst. Next, Au0.5Pd0.5:PVP catalyst showed much better activity than corresponding catalysts-Au0.8Pd0.2:PVP and Au0.2Pd0.8:PVP. Further,quantum chemical calculations were performed using the,as computational model for the monometallic Au:PVP nanocluster and Au0.5Pd0.5:PVP and Au0.8Pd0.2:PVP nanoclusters, respectively (Fig.6(A)). It is worthy to note that theare only computational model and are not realistic catalysts. The computational results suggested that the involvement of Au as a nearest heteroatom is crucial to initiate the reaction. On the other hand, its composition up to 50% in bimetallic catalyst enhances the catalytic activities. Thus, alloy effects39, such as“ligand effect” and “ensemble effect”, enhance the catalytic performance in the Au/Pd bimetallic system (Fig.6(B)).
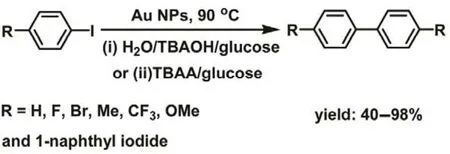
Scheme 3 Ullmann homo-coupling reaction catalyzed by Au NPs in ILs.
Further, Li et al.40examined the catalytic activity of the oxide-supported Au25(SR)18nanoclusters in the Ullmann homo-coupling reactions of aryl iodides. The optimized conditions for the nanocluster catalyzed homo-coupling reaction were carried out at 130 °C in DMF solvent under a N2atmosphere. Under the optimized reaction conditions, the CeO2-supported Au25(SR)18clusters give the best catalytic performance, a 99.8% iodobenzene conversion. Further, the Au25(SR)18-catalyzed homo-coupling reactions was expanded to a variety of substituents with different functional groups (e.g.,nitro, methoxy, and aldehyde). It was observed that the electron-rich substrate performed better than these electron-def i cient substrates in the catalysis. No distinct oxide support ef f ect was found (e.g., CeO2, SiO2, TiO2, and Al2O3).The reactant is speculated to be adsorbed and activated on the Au3sites of the Au25(SR)18clusters, Fig.4(A). And then, these Au cluster of sub-nano size (0.8−2 nm) are explored in the another carbon-carbon coupling reactions.
4.2 Ullmann Hetero-Coupling
Next, the gold nanoclusters were expanded to Ullmann hetero-coupling. Jin and coworkers41investigated the catalytic property of Au25capped by different thiolate ligands (e.g.,aromatic thiolate: naphthalenethiolate (―SNap) and benzenethiolate (―SPh), and aliphatic ones: hexanethiolate and phenylethanethiolate (PET)) in the Ullmann hetero-coupling reactions. The catalytic reactions are same with the Au25-catalyzed Ullmann homo-coupling, except using 4-methyl-iodobenzene (CH3―C6H4―I) and 4-nitroiodobenzene (NO2―C6H4―I) as reactant. The catalytic results are compiled in the Table 2. Intriguingly, the aromatic thiolate ligated Au25clusters (e.g., Au25(SPh)18and Au25(SNap)18) show much better catalytic behaviors (including conversion and selectivity) than these protect ed by alkyl thiolate ligands (e.g.,Au25(SC6H13)18and Au25(PET)18). The Au25(SNap)18cluster give a very high selectivity for the hetero-coupling product (82%),which is much higher than the corresponding Cu, Pd, and Au complexes (the selectivity is < 30%). Unfortunately, the conversion and selectivity dropped to 79% and 47% (2nd cycle)and 50% and 15% (3rd cycle), due to the gradual removal of the protecting “―SNap” ligands and thus decomposition of the gold nanoclusters. These results strongly indicate that the chemical nature of the protecting ligands exerts a major influence on the catalytic properties of the gold clusters.

Fig.6 (A) Energy profile diagram of oxidative addition of chlorobenzene (Ph-Cl) on Au20− and Au10Pd−10 clusters; (B) Tentative mechanism for Ullmann homo-coupling of chloroarenes (Ar-Cl) catalyzed by bimetallic Au-Pd catalyst.
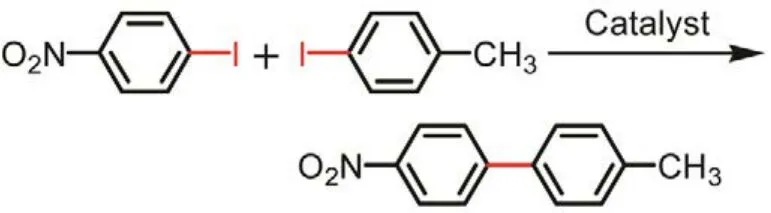

Table 2 The catalytic performance of Au25(SR)18/CeO2 catalysts for Ullmann hetero-coupling between 4-methyl-iodobenzene and 4-nitro-iodobenzene. Of note, the conversion (Conv.) is based on the 4-nitro-iodobenzene, and selectivity (Select.) towards the hetero-coupling product.
Furthermore, DFT simulations were carried out to explain the catalytic results. Here, one thiolate ligand on the Au25(SR)18model is proposed to be removed under the thermal conditions,and then two gold atoms on the staple motif can expose to reactants and are deemed as the catalytic active sites41. DFT calculations also show that the interaction energy of 4-nitroiodobenzene with the exposed gold atoms of Au25(SCH3)2(SH)15and Au25(SNap)2(SH)15model is −4.3 and−7.2 kcal·mol−1with Au―I bond length of 2.87 and 2.82 Å(Fig.7(A, B)), respectively. In the nudged elastic band (NEB)approach, the activation energy for the homo- and hetero-coupling reactions on the Au25capped by -SCH3ligands are comparable (Fig.7(C)). While, in the case of Au25capped by ―SNap ligands, intriguingly, the activation energy of hetero-coupling reaction is 2.7 kcal·mol−1less than that of homo-coupling (Fig.7(D)). It suggests that the gold cluster catalysts protected by aromatic thiolate not only can improve the conversion rate of the reaction but also can favor the formation of hetero-coupled product over the homo-coupled one.
4.3 Suzuki cross-coupling
Au25clusters are expanded to the Suzuki cross-coupling reaction in the presence of imidazolium-based ILs42. The Suzuki cross-coupling was carried out at 90 °C. The most salient feature is that imidazolium-based ILs exerted a major influence on the conversion of p-iodoanisole to the desire products. The Au25(SR)18/TiO2exhibits very low catalytic activity in the common organic solvents such as ethanol,toluene, o-xylene, and dimethylformamide, Table 3.Interestingly, the catalytic activity of the Au25cluster drastically increase to 89%–99% when BMIM·X is added to the reaction system, Table 3. The imidazolium-based ILs acts as an excellent catalytic promoter for the coupling reactions. Of note,anions of BMIM·X only have a minor effect, and the BMIM cation play a key role in the catalysis. No activity is found when using BDiMIM·BF4as solvent, indicating that the acidic proton at position 2 of the imidazolium ions plays an important role.

Fig.7 Interaction of 4-nitro-iodobenzene with the exposed gold atoms of (A) Au25(SCH3)2(SH)15 and (B) Au25(SNap)2(SH)15.Energy vs reaction coordinate of the hetero- and homo-coupling reactions with (C) “―SCH3” and (D) “―SNap” ligands.The “―SNap” and “―SCH3” stand for the aromatic and alkyl thiolate.
To gain insight into the possible active species during the catalysis, the free [Au25(PET)18]−reacted with the BMIM·BF4IL under the reaction conditions41. Some new mass peaks are found in the MALDI mass spectrum, and these peaks are assigned to Au25−n(SR)18−n(n = 1−4) species, Fig.8. Of note,these new formed species are not the fragments caused by MALDI method, and these species were detected by ESI-MS in the case of Au25(PET)18with Lewis acid (e.g., Cu+, Co2+, etc)43.It implies that the imidazolium-based ILs may assist the formation of Au21(SR)14species during the catalysis, and these species maybe associated with the catalytic sites for the reactions.
Further, the DFT calculations are performed to study and simulate the possible mechanism of the consecutive removal of the “Au-SR” units with the help of imidazolium-based ILs. Two mechanisms were proposed42, Fig.9. In mechanism 1, the removal of thiolate fragments of Au25cluster and “SR-Au” units via imidazolium cations, thereby producing low-coordinated gold atoms for iodobenzene adsorption. The first step is the DMI-H2+adsorption on ―SR2 through the interaction between H2 of the former and S of the latter. And then the thiolate group was protonated and detached from the nanoclusters to give a gold atom site (G1, Fig.9), which can be deemed as an active site for the coupling reactions. Au24(SCH3)species (Im4) was formed, when the –SR2 on the same staple motif was attacked by a hydroxide (e.g., CO). On the other hand, the surface ligand removal via N-heterocyclic carbine was also considered.The carbine directly bond with gold atoms, and one“DMI-Au-SCH3” unit eventually detaches from the nanoclusters to yield active site G2. The “-SR-Au” units were sequentially removed with aid of DMI-H2+. The Au24(SCH3)−17species can undergo a structural change via ligand exchange to yield active site G3. Thus, the low-coordinated gold atoms (G1, G2, G3, and G4) on the gold clusters are proposed to be the catalytic sites for the coupling reactions42.

Table 3 Catalytic performance of thiolate-capped Au25 cluster supported on TiO2 as catalysts for Suzuki cross-coupling reaction of iodoanisole and phenylboronic acid in the imidazoliumbased ionic liquids.
4.4 Sonogashira cross-coupling
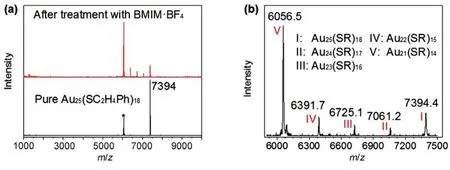
Fig.8 (a) Positive mode MALDI mass spectra of the initial [Au25(PET)18]− (in dichloromethane) and samples after the treatment with BMIM·BF4 or with BMIM·BF4 and K2CO3; (b) Zoom-in of the MALDI mass spectrum of the sample after the treatment of[Au25(PET)18]− with BMIM·BF4.
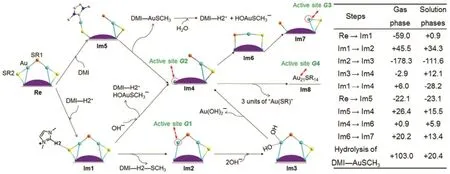
Fig.9 (Left panel) Proposed reaction Mechanism 1 for formation of catalytic active site on Au25 cluster promoted by imidazolium-based ionic liquids.
As the gold clusters can activate both the iodobenzene and alkyne molecules, thus, the catalytic application of the cluster may expand to Sonogashira cross-coupling reactions. Li et al.44investigated the catalytic application of the oxide-supported Au25(PET)18cluster to the Sonogashira cross-coupling reaction of iodobenzene and phenylacetylene. The supported catalyst was made by impregnation of oxide powders (including CeO2,TiO2, MgO, and SiO2) in a CH2Cl2solution of Au25(PET)18(~1% (w) loading), and then annealed at 150 °C (1 h) in a vacuum oven. STEM and TG analysis confirmed that the protecting thiolate ligands were remained on the surface of gold clusters after the annealing. The Au25(PET)18/oxide catalysts after thermal treatment was applied in the Sonogashira cross-coupling reaction of iodobenzene and phenylacetylene;the optimized condition was carried out under N2atmosphere at 160 °C using DMF as solvent and K2CO3as base. A high conversionof p-iodoanisole (up to 96.1%) with excellent selectivity for cross-coupling product (up to 88.1%) was obtained when it was catalyzed by Au25(PET)18/CeO2catalyst(Fig.10). The smaller size Au25(PET)18cluster catalyst performed much better than the larger sized 2−3 nm Au clusters and CeO2-supported Au nanoparticles (bare, ~20 nm). Support effects were also investigated in the coupling, and no distinct effect of the oxide supports was observed (i.e., CeO2, SiO2,TiO2, and MgO). The conversion of p-iodoanisole exhibited no significant loss, while the selectivity was decreased from 88.1% to 64.5% after 5 cycles. Of note, the TEM analysis showed that the Au cluster grew up to big nanoparticles of > 3 nm, meaning that the organic ligand capped gold nanoclusters cannot be intact under the harsh reaction conditions. The drop in selectivity should be caused the gradual degradation of clusters in the multiple recycling tests, as larger Au clusters gave a much lower selectivity.
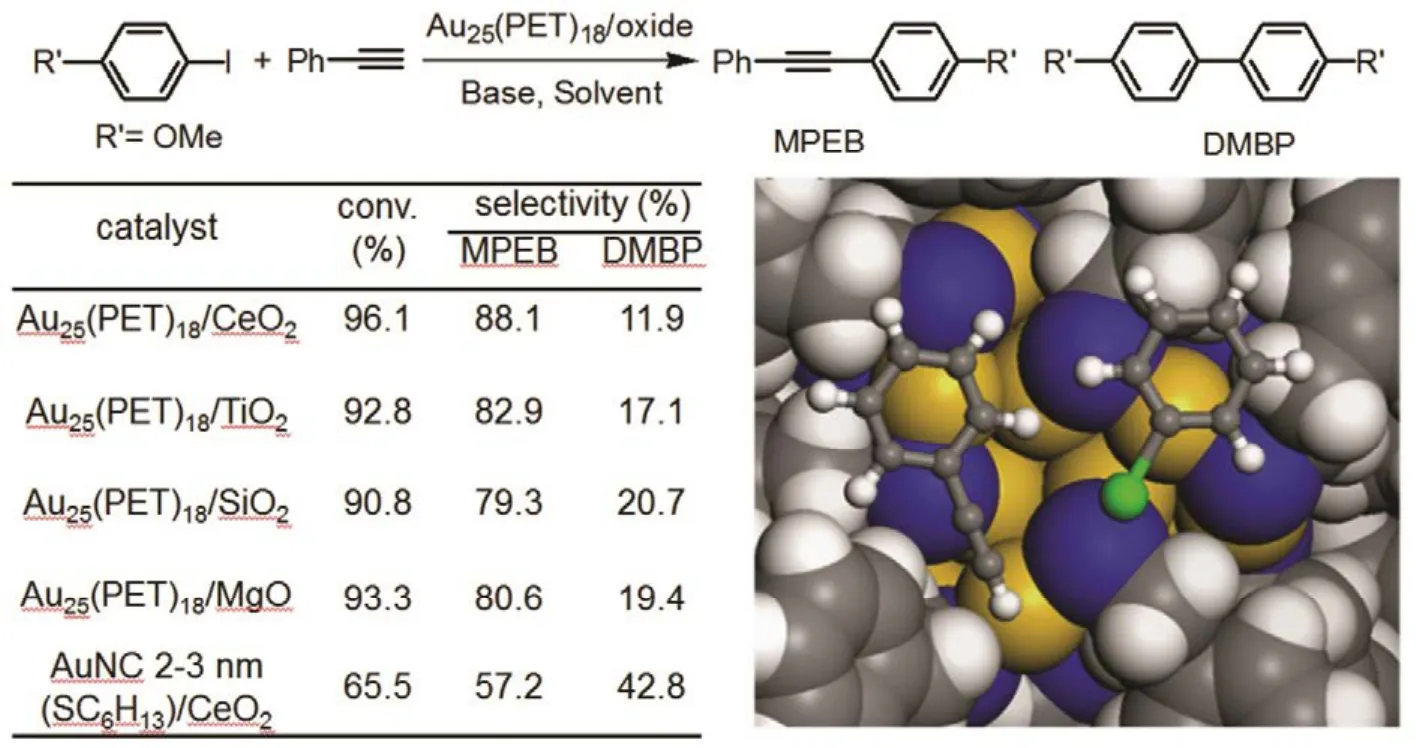
Fig.10 (Left panel) The catalytic performance of oxide-supported Au25(PET)18 cluster catalysts for Sonogashira cross-coupling reaction of p-iodoanisole and phenylacetylene. (Right panel) Top view of the co-adsorption of phenylacetylene and iodobenzene on the Au3 site of the Au25(PET)18 nanoclusters.
Moreover, density functional theory (DFT) calculation of the reactant adsorption shows that both reactants (i.e., iodobenzene and phenylacetylene) prefer to adsorb on the open facet with the phenyl ring facing a surface gold atom (Fig.10). A total adsorption energy reaches −0.90 eV when the two reactants co-adsorb on the Au25(SR)18catalyst44. The DFT results suggest that the catalytic active sites is associated with the Au25(SR)18,which is consistent with the experimental results.
Although the framework of the 25-atom cluster is similar45,46,the electronic property and the catalytic behavior of the bimetallic clusters can be largely modified by the foreign dopants47−51. Very recently, Li et al.52investigated the doping effects of the Au25(SR)18nanoclusters in Sonogashira cross-coupling reactions. The experiment and DFT simulations suggest that the copper and silver dopants are preferentially located occupy sites at the cl uster kernel instead of the staple motif of nanoclusters. While, the Pt atom only can singly dopes and locates at the center of the cluster. The AgxAu25−x(SR)18gave an overall performance comparable to Au25(SR)18, and Pt1Au24(SR)18causes a drop in catalytic activity. The catalytic activity of the titania-supported catalysts follows a decreasing order: AgxAu25−x(SR)18≈ Au25(SR)18> CuxAu25−x(SR)18>Pt1Au24(SR)18. Intriguingly, the CuxAu25−x(SR)18prefer the Ullmann homo-coupling pathway and give rise to homo-coupling product, which is in opposite to the other three cluster catalysts. Overall, the catalytic activity is largely affected by the electronic effect in the bimetallic clusters’ core(i.e., Pt1Au12, CuxAu13−x, and Au13), and the product selectivity is primarily determined by the type of atoms on the MxAu12−xshell47.
4.5 A3-coupling
One-pot multicomponent coupling reactions (MCRs), where several reactants come together in one vessel to yield one product, have gained overwhelming interest over the last decade due to the environmental and economic perspectives;they are more efficient, cost effective and less wasteful than traditional methods53−55. The three-component coupling of aldehydes, amines and alkynes, commonly known as A3-coupling, is an example of MCRs to yield propargylamine56.This reaction involves the formation of new C―C and C―N bonds in a one-pot procedure. The activation of the terminal alkynes is the key step for the A3-coupling. Thus the gold clusters should have capability in this reaction, as the cluster catalyst has exhibited good activity in the semi-hydrogenation of terminal alkynes32.
First, the catalytic efficiency of the Au25(PPh3)10(PA)5X2/TiO2was evaluated in different solvents and reaction temperatures57. The most salient feature is that the conversion highly affected by the solvent polarity; the catalyst showed better activity in the polar solvents (e.g., water). The catalyst also showed good recyclability. It is interesting that the cluster catalyst gave no conversion when the reactant aldehyde changed to ketone (Scheme 4), which is totally different with the catalytic behaviors of the Au complexes and naked gold nanoparticles. It strongly suggests that the electronic factors and steric hindrance increase from the substituents significantly influence the conversion rate of the reaction.
It was found that the conversion slightly increased during the induction period (0 to 3 h), and then it dramatically improved in the time evolution of the reaction conversion for the gold cluster catalyzed A3-coupling57. It meant that some phosphine ligands are removed during induction period and the gold atoms are exposed to the reactants. The phosphine removal was also observed in the case of Au11(PPh3)7X3with the aid of base(e.g., pyridine), evidenced by UV-Vis and ESI-MS analyses58.
Finally, the catalytic mechanism was explored. One phenylacetylene was adsorbed onto the M1 site, Fig.11. And then a deprotonation of the terminal H was detached in the presence of amine (e.g., HN(CH3)2). The formed iminium ion(H++ HCHO + HN(CH3)2H2C=N(CH3)2++ H2O) may interact with the “PhC≡C―” on site M1 and gave the propargylamine product.
Further, Jin and coworkers also reported the A3-coupling reaction catalyzed by Au38(PET)24nanoclusters59. They argued that the synergistic effect of the ligand protected Auδ+surface(0 < δ < 1) and the electron-rich 23-Au-atom core were responsible for for high catalytic performance. And very recently, Li et al. reported that cadmium doped gold nanocluster ([Au13Cd2(PPh3)6(PET)6(NO3)2]2Cd(NO3)4)showed good catalytic behaviors in the A3-coupling60. The active sites are associated to the cooperation between the exerted cadmium atoms and the neighbor gold atoms on the surface of Au13icosahedron.
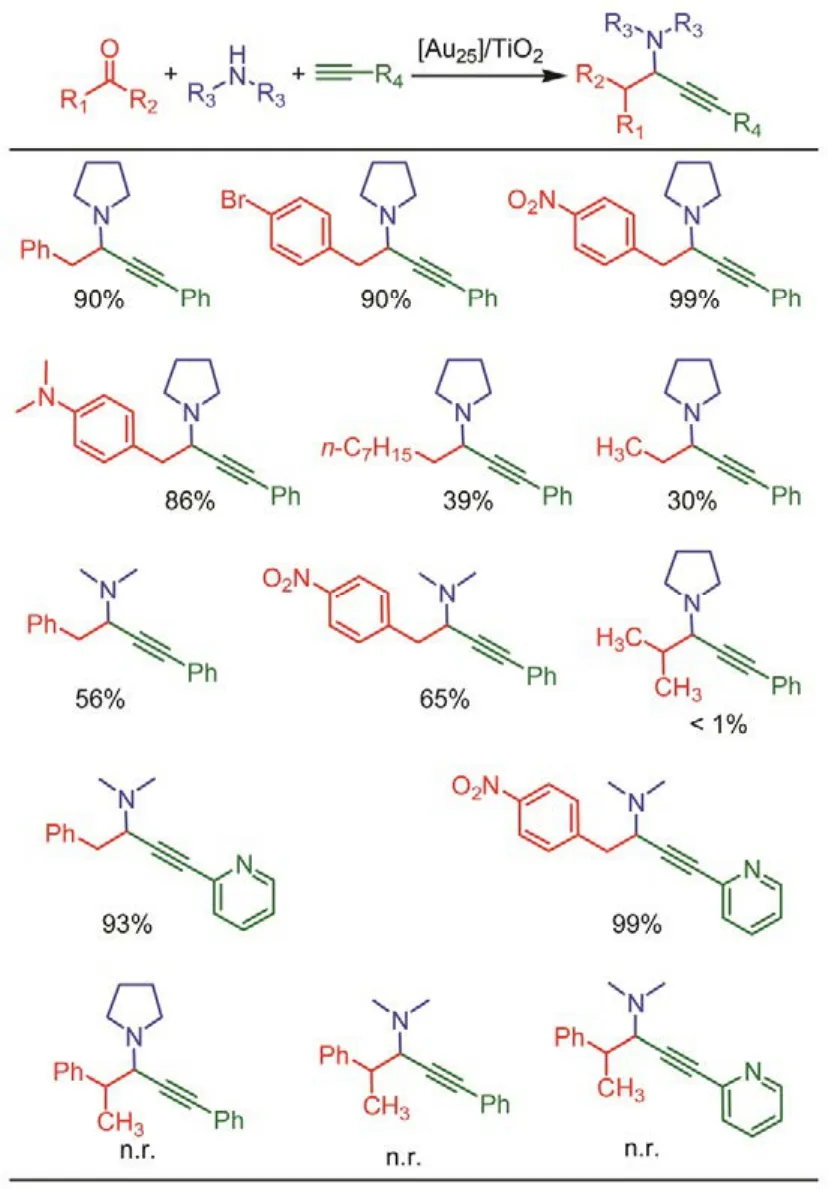
Scheme 4 The catalytic performances of the TiO2-supported Au25(PPh3)10(PA)5X2 ([Au25]/TiO2) catalyst for A3-coupling reaction.n.r. = no reaction
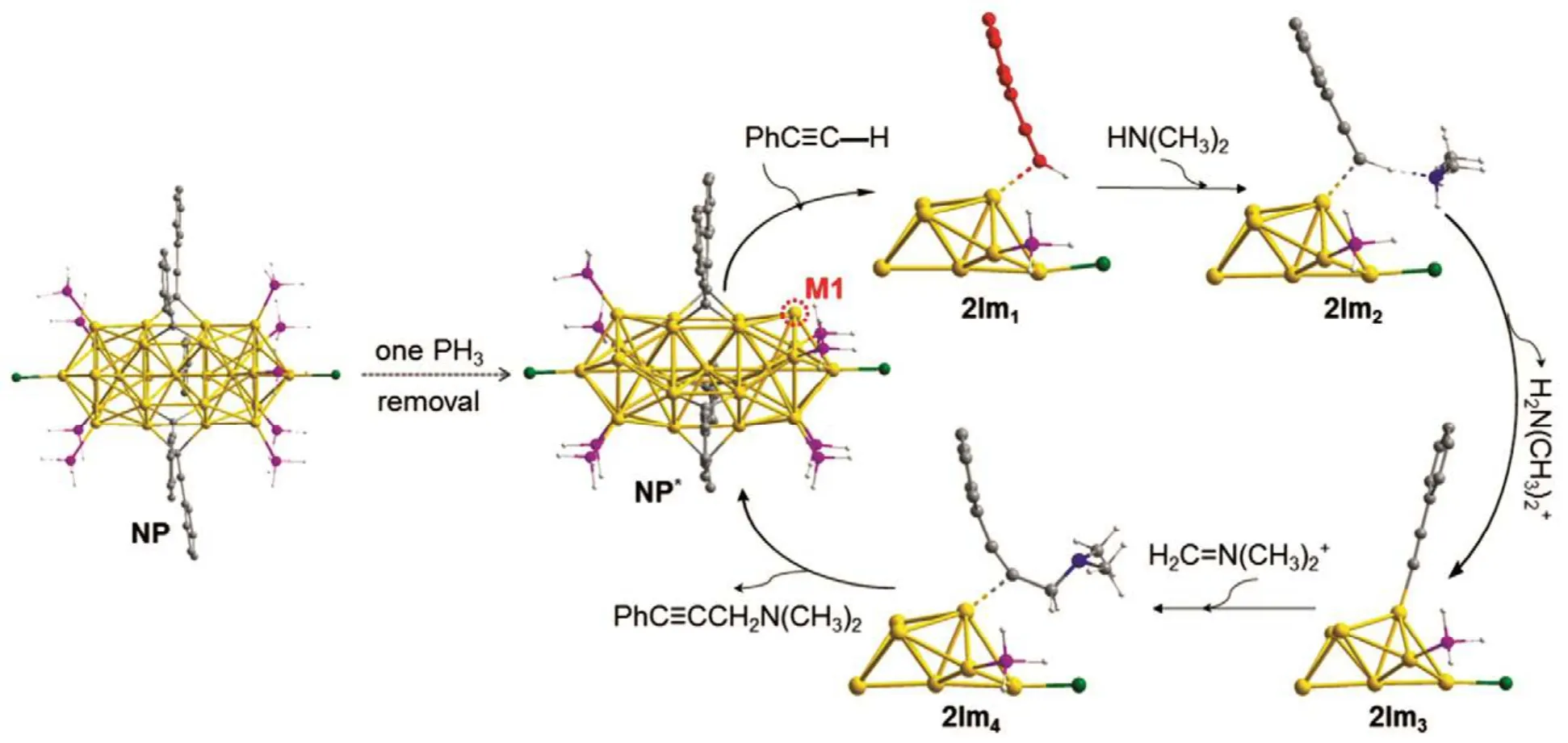
Fig.11 Possible removal of one phosphine ligand provides a gold atom (M1) from the [Au25(PH3)10(PA)5Cl2]2+ cluster, and proposed mechanism for the catalyzed A3-coupling reaction over the Au25(PH3)10(PA)5Cl2 cluster.Color code: Au, yellow; P, purple; X, green; N, blue; C, grey; H, white.
5 Summary and outlook
Over the last decade years, the outstanding catalytic application s of the gold nanoclusters have been observed,leading to a novelty perspective for gold nanocatalysis,especially in the carbon-carbon coupling reactions, such as Ullmann coupling of halides, Suzuki cross-coupling of phenylboronic acid and iodobenzene, Sonogashira cross-coupling of iodobenzene and phenylacetylene, and A3-coupling reaction of alkynes, amines, and aldehydes. These gold nanoclusters, with well-defined atomic frameworks and the sizes of from 1 to 2 nm, performed more efficient in the carbon-carbon coupling reaction in this review. The high catalytic activity, especially the excellent, is mainly due to the unique framework and electronic property of the gold nanoclusters and the functional protecting ligands. However,these thiolate/phosphine protected Au nanoclusters cannot keep intact during the catalysis processes, and the catalytic performance decrease or disappear with the increasing size of formed particles. Thus, how to maintain the gold nanoclusters becomes a big challenge in the further study.
The oxide-supports in this review are mainly focused on the ceria and titanium oxide (P25). CeO2-supported Au clusters gave rise to better performance than these supported on the TiO2, which may be owing to its basic nature and redox property (CeO2with Ce3+and Ce4+species). The other metal oxides (e.g., SiO2, Al2O3, Fe2O3, MgO, BaO, ZrO2, etc) are few examined. Expansion of new type supports also is another important direction, such as about high surface area materials(e.g., zeolite, activated carbon, graphene or graphite oxide and MOFs61). For example, the porous materials, e.g., MOFs and zeolite, exhibit regular tunnels and cages, which can hold the Au clusters and enhance their stability during the catalysis processes. The concentration enrichment of the reactants in the tunnels and cages of MOFs and zeolite also can increase the catalytic activity. And steric effects of organic linker of MOFs may improve the product selectivity. Furthermore, the Lewis and Bronsted acid sites of zeolite can turn the catalytic performance.
Finally, we expect gold cluster catalysts can be further developed to carbon-heteratom coupling reactions to form C―O62and C―N63bonds, which are very useful drug intermediates. These carbon-heteratom coupling reactions are found to be easy catalyzed by homogeneous AuIand goldIIIcomplex catalysts. Beyond the carbon-carbon coupling, these well-defined gold nanoclusters can be explored as a good model in the selective oxidation and hydrogenation and electrocatalysis64−66, as the reaction condition is milder and the Au cluster can be intact during the catalysis. Future research on the gold nanoclusters will contribute to the fundamental catalysis and give cue to the new design of highly efficient catalysts for other specific chemical processes.
(1) Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. J. Catal. 1989,115, 301. doi: 10.1016/0021-9517(89)90034-1
(2) Tsukuda, T.; Tsunoyama, H.; Sakurai, H. Chem. Asian J. 2011, 6, 736.doi: 10.1002/asia.201000611
(3) Della, P. C.; Falletta, E.; Rossi, M. Chem. Soc. Rev. 2012, 41, 350.doi: 10.1039/c1cs15089h
(4) Li, G.; Qian, H.; Jin, R. Nanoscale 2012, 4, 6714.doi: 10.1039/c2nr32171h
(5) Liu, C.; Yan, C.; Lin, J.; Yu, C.; Huang, J.; Li, G. J. Mater. Chem. A 2015, 3, 20167. doi: 10.1039/c5ta05747g
(6) Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem. Int. Ed. 2006, 45,7896. doi: 10.1002/anie.200602454
(7) Li, G.; Zeng, C. Jin, R. J. Am. Chem. Soc. 2014, 136, 3673.doi: 10.1021/ja500121v
(8) Li, G.; Jiang, D.; Kumar, S.; Chen, Y.; Jin, R. ACS Catal. 2014, 4,2463. doi: 10.1021/cs500533h
(9) Taketoshi, A.; Haruta, M. Chem. Lett. 2014, 43, 380.doi: 10.1246/cl.131232
(10) Alonso, F.; Beletskaya, I. P.; Yus, M. Tetrahedron 2008, 64, 3047.doi: 10.1016/j.tet.2007.12.036
(11) Seechurn, C.; Kitching, M. O.; Colacot, T. J.; Snieckus, V. Angew.Chem. Int. Ed. 2012, 51, 5062. doi: 10.1002/anie.201107017
(12) Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Polshettiwar, V. Chem.Soc. Rev. 2011, 40, 5181. doi: 10.1039/c1cs15079k
(13) Chen, Y.; Wang, J.; Liu, C.; Li, Z.; Li, G. Nanoscale 2016, 8, 10059.doi: 10.1039/c5nr08338a
(14) Goswami, N.; Yao, Q.; Chen, T.; Xie, J. Coord. Chem. Rev. 2016,329, 1. doi: 10.1016/j.ccr.2016.09.001
(15) Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. X. Chem. Rev. 2016, 116,10346. doi: 10.1021/acs.chemrev.5b00703
(16) Liu, C.; Li, T.; Li, G.; Nobusada, K.; Zeng, C.; Pang, G.; Rosi, N. L.;Jin, R. Angew. Chem. Int. Ed. 2015, 127, 9826.doi: 10.1002/anie.201502667
(17) Li, G.; Jin, R. Acc. Chem. Res. 2013, 46, 1749.doi: 10.1021/ar300213z
(18) Li, Z.; Abroshan, H.; Liu, C.; Li, G. Curr. Org. Chem. 2017, 21, 476.doi: 10.2174/1385272820666161020152707
(19) Li, G.; Jin, R. Nanotechnol. Rev. 2013, 5, 529.doi: 10.1515/ntrev-2013-0020.
(20) Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem.Rev. 2002, 102, 1359. doi: 10.1021/cr000664r
(21) Daugulis, O.; Do, H. -Q.; Shabashov, D. Acc. Chem. Res. 2009, 42,1074. doi: 10.1021/ar9000058
(22) Kanuru, V. K.; Kyriakou, G.; Beaumont, S. K.; Papageorgiou, A. C.;Watson, D. J.; Lambert, R. M. J. Am. Chem. Soc. 2010, 132, 8081.doi: 10.1021/ja1011542
(23) González-Arellano, C.; Abad, A.; Corma, A.; García, H.; Iglesias, M.;Sánchez, F. Angew. Chem., Int. Ed. 2007, 46, 1536.doi: 10.1002/anie.200604746
(24) Lin, J.; Abroshan, H.; Liu, C.; Zhu, M.; Li, G.; Haruta, M. J. Catal.2015, 330, 354. doi: 10.1016/j.jcat.2015.07.020
(25) Li, G.; Zeng, C.; Jin, R. J. Phys. Chem. C 2015, 119, 11143.doi: 10.1021/jp511930n
(26) Boronat, M.; Combita, D.; Concepción, P.; Corma, A.; García, H.;Juárez, R.; Laursen, S.; López-Castro, J. D. J. Phys. Chem. C 2012,116, 24855. doi: 10.1021/jp3071585
(27) Boronat, M.; Corma, A. J. Catal. 2011, 284, 138.doi: 10.1016/j.jcat.2011.09.010
(28) Corma, A.; Juárez, R.; Boronat, M.; Sánchez, F.; Iglesiasc, M.;García, H. Chem. Commun. 2011, 47, 1446.doi: 10.1039/c0cc04564k
(29) Zhu, M.; Aikens, C. M.; Hollander, F. J.; Schatz, G. C.; Jin, R. J. Am.Chem. Soc. 2008, 130, 5883. doi: 10.1021/ja801173r
(30) Qian, H.; Eckenhoff, W. T.; Bier, M. E.; Pintauer, T.; Jin, R. Inorg.Chem. 2011, 50, 10735. doi: 10.1021/ic2012292
(31) Shichibu, Y.; Negishi, Y.; Watanabe, T.; Chaki, N. K.; Kawaguchi, H.;Tsukuda, T. J. Phys. Chem. C. 2007, 111, 7845.doi: 10.1021/jp073101t
(32) Li, G.; Jin, R. J. Am. Chem. Soc. 2014, 136, 11347.doi: 10.1021/ja503724j
(33) Kauffman, D. R.; Alfonso, D.; Matranga, C.; Li, G.; Jin, R. J. Phys.Chem. Lett. 2013, 4, 195. doi: 10.1021/jz302056q
(34) Yu, C.; Li, G.; Kumar, S.; Kawasaki, H.; Jin, R. J. Phys. Chem. Lett.2013, 4, 2847. doi: 10.1021/jz401447w
(35) Chen, H.; Liu, C.; Wang, M.; Zhang, C.; Li, G.; Wang, F. Chin. J.Catal. 2016, 37, 1787. doi: 10.1016/S1872-2067(16)62478-6
(36) Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2008, 47, 3096.doi: 10.1002/anie.200703209
(37) Monopoli, A.; Cotugno, P.; Palazzo, G.; Ditaranto, N.; Mariano, B.;Cioffi, N.; Ciminale, F.; Nacci, A. Adv. Synth. Catal. 2012, 354,2777. doi: 10.1002/adsc.201200422
(38) Dhital, R. N.; Kamonsatikul, C.; Somsook, E.; Bobuatong, K.; Ehara,M.; Karanjit, S.; Sakurai, H. J. Am. Chem. Soc. 2012, 134, 20250.doi: 10.1021/ja309606k
(39) Gao, F.; Goodman, D. W. Chem. Soc. Rev. 2012, 41, 8009.doi: 10.1039/c2cs35160a
(40) Li, G.; Liu, C.; Lei, Y.; Jin, R. Chem. Commun. 2012, 48, 12005.doi: 10.1039/c2cc34765b
(41) Li, G.; Abroshan, H.; Liu, C.; Zhuo, S.; Li, Z.; Xie, Y.; Kim, H. J.;Rosi, N. L.; Jin, R. ACS Nano 2016, 10, 7998.doi: 10.1021/acsnano.6b03964
(42) Abroshan, H.; Li, G.; Lin, J.; Kim, H. J.; Jin, R. J. Catal. 2016, 337,72. doi: 10.1016/j.jcat.2016.01.011
(43) Li, G.; Abroshan, H.; Chen, Y.; Jin, R.; Kim, H. J. J. Am. Chem. Soc.2015, 137, 14295. doi: 10.1021/jacs.5b07716
(44) Li, G.; Jiang, D.; Liu, C.; Yu, C.; Jin, R. J. Catal. 2013, 306, 177.doi: 10.1016/j.jcat.2013.06.017
(45) Kumara, C.; Aikens, C. M.; Dass, A. J. Phys. Chem. Lett. 2014, 5,461. doi: 10.1021/jz402441d
(46) Jin, R.; Nobusada, K. Nano Res. 2014, 7, 285.doi: 10.1007/s12274-014-0403-5
(47) Li, W.; Liu, C.; Abroshan, H.; Ge, Q.; Yang, X.; Xu, H.; Li, G.J. Phys. Chem. C 2016, 120, 10261. doi: 10.1021/acs.jpcc.6b00793
(48) Jiang, D.; Whetten, R. L. Phys. Rev. B 2009, 80, 115402.doi: 10.1103/PhysRevB.80.115402
(49) Qian, H.; Jiang, D.; Li, G.; Gayathri, C.; Das, A.; Gil, R. R.; Jin, R.J. Am. Chem. Soc. 2012, 134, 16159. doi: 10.1021/jacs.5b07716
(50) Xie, S.; Tsunoyama, H.; Kurashige, W.; Negishi, Y.; Tsukuda, T.ACS Catal. 2012, 2, 1519. doi: 10.1021/cs300252g
(51) Li, G.; Jin, R. Catal. Today 2016, 278, 187.doi: 10.1016/j.cattod.2015.11.019
(52) Li, Z.; Yang, X.; Liu, C.; Wang, J.; Li, G. Prog. Nat. Sci.: Mater. Int.2016, 26, 477. doi: 10.1016/j.pnsc.2016.09.007
(54) Ganem, B. Acc. Chem. Res. 2009, 42, 463. doi: 10.1021/ar800214s
(55) Climent, M. J.; Corma, A.; Iborra, S. RSC Adv. 2012, 2, 16.doi: 10.1039/c1ra00807b
(56) Wei, C.; Li, C. -J. J. Am. Chem. Soc. 2003, 125, 9584.doi: 10.1021/ja0359299
(57) Chen, Y.; Liu, C.; Abroshan, H.; Wang, J.; Li, Z.; Li, G.; Haruta, M.J. Catal. 2016, 337, 287. doi: 10.1016/j.jcat.2016.05.023
(58) Liu, C.; Abroshan, H.; Yan, C.; Li, G.; Haruta, M. ACS Catal. 2016,6, 92. doi: 10.1021/acscatal.5b02116
(59) Li, Q.; Das, A.; Wang, S.; Chen, Y.; Jin, R. Chem. Commun. 2016,52, 14298. doi: 10.1039/c6cc07825g
(60) Li, M.; Tian, S.; Wu, Z. Chin. J. Chem. 2017,doi: 10.1002/cjoc.201600526 60
(61) Vilhelmsen, L. B.; Walton, K. S.; Sholl, D. S. J. Am. Chem. Soc.2012, 134, 12807. doi: 10.1021/ja305004a
(62) Aguilar, D.; Contel, M.; Navarro, R.; Soler, T.; Urriolabeitia, E. P.J. Organomet. Chem. 2009, 694, 486.doi: 10.1016/j.jorganchem.2008.09.058
(63) Ciobanu, M.; Cojocaru, B.; Teodorescu, C.; Vasiliu, F.; Coman, S.M.; Leitner, W.; Parvulescu, V. I. J. Catal. 2012, 296, 43.doi: 10.1016/j.jcat.2012.09.002
(64) Yan, C.; Liu, C.; Abroshan, H.; Li, Z.; Qiu, R.; Li, G. Phys. Chem.Chem. Phys. 2016, 18, 23358. doi: 10.1039/c6cp04569c
(65) Kawasaki, H.; Kumar, S.; Li, G.; Zeng, C.; Kauffman, D. R.;Yoshimoto, J.; Iwasaki, Y.; Jin, R. Chem. Mater. 2014, 26, 2777.doi: 10.1021/cm500260z
(66) Liu, C.; Zhang, J.; Huang, J.; Zhang, C.; Hong, F.; Zhou, Y.; Li, G.;Haruta, M. ChemSusChem 2017, doi: 10.1002/cssc.20170040
A Critical Review on Carbon-Carbon Coupling over Ultra-Small Gold Nanoclusters
ZHOU Yang1,2LI Gao1,*
(1Gold Catalysis Research Center, State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China;2University of Chinese Academy of Sciences, Beijing 100049, P. R. China)
Well-defined gold nanoclusters have been documented as new and promising materials in the field of nanoscience. They have been well explored for the nanocatalysis of reactions like selective oxidation and hydrogenation, carbon-carbon coupling, etc. These Au nanoclusters possess unique electronic properties and crystal structure, which provide an excellent opportunity to correlate atomic structure with intrinsic catalytic property and to investigate the mechanisms of reactions over Au nanoclusters. In this review, we generalize the catalytic application of gold nanoclusters in carbon−carbon coupling reactions, including Ullmann, Sonogashira, Suzuki, and A3-coupling reactions. Herein, we have discussed ligand engineering (e.g., aromatic and aliphatic thiolate) as well as the effect of metal dopants(e.g., Cu, Ag, Pt, and Pd). Finally, the tentative catalytic mechanisms and the structure−performance relationships were discussed at the atomic level, which will give some clue for the design of efficient gold cluster catalysts.
Gold cluster; Carbon-carbon coupling; Ligand engineering; Doping effect; Ullmanncoupling; Sonogashira cross-coupling; Suzuki cross-coupling; A3-coupling
March 3, 2017; Revised: March 29, 2017; Published online: April 10, 2017.
her B.S. from Hebei Normal University in 2015. She is a graduate student at State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences.Her current research interests are preparation and catalytic application of gold nanoclusters.
LI Gao, received his B.S. from Hunan Normal University in 2004, and Ph.D.(2011) from Shanghai Jiao Tong University. After his postdoctoral research in Carnegie Mellon University(2011−2014), he joined State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences as a professor in 2014. His current research interests focus on the preparation and catalytic application of metal nanoclusters.
O643
10.1021/cr0505728
[Feature Article]
10.3866/PKU.WHXB201704101 www.whxb.pku.edu.cn
*Corresponding author. Email: gaoli@dicp.ac.cn; Tel: +86-411-8246-3017.
© Editorial office of Acta Physico-Chimica Sinica
ZHOU Yang,
