联苯-2,4,4′,6-四甲酸银、咪唑-菲啰啉-苯氧乙酸镉配位聚合物的合成、结构及与DNA的相互作用
2017-11-01吕天喜胡未极吴小勇赵国良
鲁 雅 吕天喜 胡未极 吴小勇 赵国良*,,
联苯-2,4,4′,6-四甲酸银、咪唑-菲啰啉-苯氧乙酸镉配位聚合物的合成、结构及与DNA的相互作用
鲁 雅1吕天喜2胡未极2吴小勇2赵国良*,1,2
(1浙江师范大学行知学院,金华 321004)
(2浙江师范大学化学与生命科学学院,金华 321004)
由联苯-2,4,4′,6-四甲酸(H4BPTC,C16H10O8)和 2-4-(1H-咪唑-2-[4,5-f][1,10]菲啰啉基)苯氧乙酸(HPIMPHC,C21H14N4O3),水热合成了2种新型配位聚合物[Ag4(BPTC)]n(1),[Cd(PIMPHC)2]n(2)。用元素分析、红外光谱、热重分析和X射线单晶衍射对配合物进行了表征和结构分析。配合物 1属三斜晶系,空间群为P1;Ag的配位数分别为4、3、2,配位构型为变形四面体、平面三角形和V型。配合物2属正交晶系,空间群为Pbcn;Cd的配位数为6,配位构型为变形的八面体,Cd离子间通过三齿配体PIMPHC-桥连,形成2D网状结构。用EtBr荧光探针法研究了配体及配合物与ct-DNA的相互作用。
联苯-2,4,4′,6-四甲酸; 2-4-(1H-咪唑-2-[4,5-f][1,10]菲啰啉基)苯氧乙酸; Ag; Cd; DNA
0 Introduction
Coordination polymers are widely involved with many fields,which has the characteristics of a wide variety of compounds,novel structure and excellent performance.To carry out a systematic and in-depth study of them,it is not only helpful in promoting the development of basic concepts and basic theories of synthetic chemistry,materials chemistry and structural chemistry,but also has a very important practical significance for the development and application of such materials. It has been found that the coordination polymers can be widely used in the fields of gas storage[1-2]and separation[3-5],catalysis[6-7],chemical sensors[8-9],proton conductivity[10-11],bio-pharmaceutical[12-13],fluorescence[14-15],nonlinear optics[16-17],magnetic properties[18-20]and many other areas,thereby being attracted great concern and attention by the majority of chemists and materials scientists.However,it is necessary to obtain a novel and well-functioning coordination polymer,which is the central metal ion and bridging ligand regulation,that is,the central metal ions as a node,organic ligand as a link,the use of central metal ion-changing geometries and the symmetry of organic ligands to construct diverse structures.
Organic carboxylic acid compounds are the preferred ligands for the study of coordination compounds due to the diversity of their coordination modes and their strong coordination ability with metal ions.Carboxyl group can not only coordinate with metal ions in a variety of flexible coordination ways,but also can form multinuclear metal ion complexes or secondary building units(SBUs)in combination with metal ions,so as to construct more novel structures and properties.In addition,since the carboxyl group can be totally or partially deprotonated,it can be used as a donor or acceptor of hydrogen bonds,coordination bonds and metal bonds to participate in the supramolecular self-assembly.Therefore,the use of organic carboxylic acids as ligands to construct coordination polymers has attracted great interest.
In recent decades,the synthesis and properties of various carboxylic acids and their corresponding complexes have been studied continuously.Among them,multicomponent aromatic carboxylic acids,biphenyl polybasic carboxylic acids and heterocyclic polycarboxylic acids are the most favored because they are easy to form binuclear or multinuclear complexes with metal ions,thereby forming a two or threedimensional network of coordination polymers[21-23].In this paper,biphenyl-2,4,4′,6-tetracarboxylic acid and 2-(4-(1H-imidazo-2-[4,5-f]-[1,10]phenanthrolinyl)phenoxy)acetic acid were used as ligands to synthesize two kinds of coordination polymers.The complexes were characterized by elemental analysis,TGA,IR spectra and X-ray single crystal diffraction.
1 Experimental
1.1 Materials and measurements
2-(4-(1H-imidazo-2-[4,5-f]-[1,10]phenanthrolinyl)phenoxy)acetic acid was prepared according to literature[24],biphenyl-2,4,4′,6-tetracarboxylic acid and other reagents used were of commercially available quality and without purified before using. FTIR spectra were recorded on a Nicolet NEXUS 670 FTIR spectrophotometer using KBr discs in the range of 4 000~400 cm-1.Elemental analyses of C,H,N were performed on elemental analyzer,Elementar Vario ELⅢ.Crystallographic data were collected on a Bruker Smart ApexⅡCCD diffractometer.A Mettler Toledo thermal analyzer TGA/SDTA 851ewas used to carry out the thermoanalytical analysis with a heating rate of 10℃·min-1from 30 to 800℃ in air atmosphere.Fluorescence spectra were measured at room temperature with an Edinburgh FL-FS 920 TCSPC system.
1.2 Synthesis of[Ag4(BPTC)]n(1)
A mixture of H4BPTC(0.100 g,0.3 mmol),AgNO3(0.204 g,1.2 mmol)and H2O(18 mL)was sealed into a Teflon-lined stainless steel autoclave(25 mL),which was heated to 160℃for 72 h.After the sample was cooled to room temperature at a rate of 10℃·h-1,the colorless block crystals suitable for single-crystal analysis and physical measurements were obtained,washed with distilled water,and dried in air.Yield:36% (based on AgNO3).Anal.Calcd.for C16H6O8Ag4(%):C,25.34;H,0.79.Found(%):C,25.49;H,0.77.IR(KBr,cm-1):1 602(m),1 582(m),1 538(s),1 452(m),1 426(s),1 394(m),1 360(s),1 204(m),1 109(m),786(m),761(s),735(s),709(m).
1.3 Synthesis of[Cd(PIMPHC)2]n(2)
The synthetic method and procedure of 2 are similar to 1 except that the metal salt and ligand are replaced by CdCl2·2.5H2O and HPIMPHC,while the molar ratio of metal to ligand is adjusted to 1∶2.Yield:42%(based on CdCl2·2.5H2O).Anal.Calcd.for C42H26N8O6Cd(%):C,59.22;H,3.05;N,13.16.Found(%):C,59.11;H,3.02;N,13.08.IR(KBr,cm-1):3 074(w),2 361(w),1 608(s),1 521(m),1 482(s),1 448(m),1 398(m),1 361(m),1 257(m),1 227(m),1 072(m),831(m),812(m),736(m),694(m),641(m).
1.4 X-ray diffraction analysis
The single crystals of the two complexes with approximate dimensions(0.239 mm×0.204 mm×0.138 mm,1;0.219 mm ×0.159 mm ×0.086 mm,2)were selected and mounted on a Bruker Smart ApexⅡCCD diffractometer.The diffraction data were collected using a graphite monochromated Mo Kα radiation(λ=0.071 073 nm)at 296(2)K.The employed single crystals exhibit no detectable decay during the data collection.Absorption corrections were applied using SADABS[25].The structure was solved by direct methods with the SHELXS-97[26]program package and refined with the full-matrix least-squares technique based on F2using the SHELXTL-97[27]program package.Hydrogen atoms were added theoretically.Detail information about the crystal data is summarized in Table 1.Selected interatomic distances and bond angles are given in Table 2 and 3.
CCDC:952961,1;980891,2.
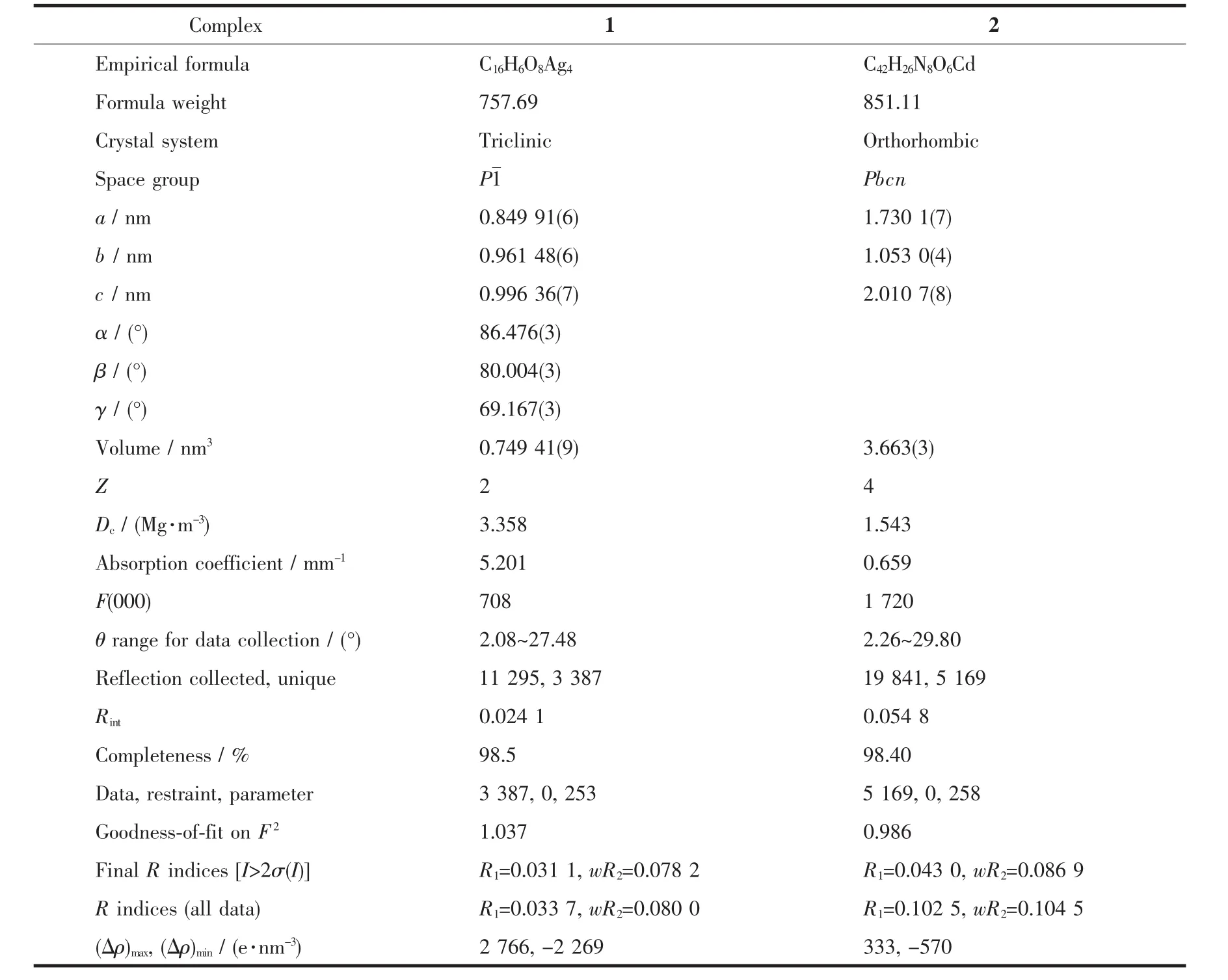
Table 1 Crystallographic data for the complexes

Table 2 Selected bond lengths(nm)and angles(°)of the complex 1
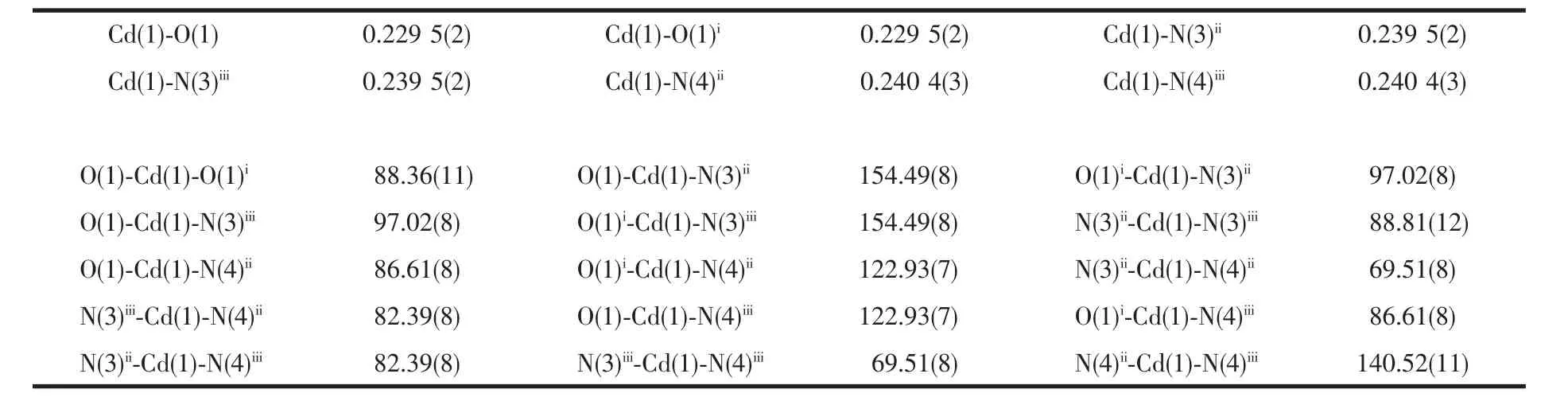
Table 3 Selected bond lengths(nm)and angles(°)of the complex 2
2 Results and discussion
2.1 Crystal structure analysis
2.1.1 Crystal structure of complex[Ag4(BPTC)]n(1)Fig.1 is a structural diagram of complex 1.Its asymmetric unit containing four Ag+ions and one ligand BPTC4-anion,which is a complex with tetranuclear structure.The coordination environment of silver ions in the complex is different.The coordination numbers of Ag1 and Ag4 are four,Ag2 is two,and Ag3 is three.Ag1 coordinates with the four oxygen atoms of the carboxyl group in the ligand BPTC4-.Ag1,O1,O5iv,O4viiare almost in a plane:∠O1-Ag1-O4vii=110(12)°, ∠O(4)vii-Ag(1)-O(5)iv=108.15(12)°,∠O(5)iv-Ag(1)-O(1)=141.75(11)°,and the sum of the three is 359.90°.Similarly,Ag4 is also coordinated with four oxygen atoms on the carboxyl group.Ag2 is coordinated with two oxygen atoms from two carboxyl groups of the ligand.Ag3 is coordinated with three oxygen atoms on the carboxyl group,it is almost on the same plane as the three coordinating oxygen atoms.The Ag-Obond lengths and bond angles(Table 2)in the complex are all within the reasonable range[28].One-dimensional ladder-type metal chain was formed by the central metal Ag+connection through the carboxyl oxygen.The distances of neighbor silver atoms(Ag1x…Ag3x0.315 6 nm,Ag1…Ag4 0.299 4 nm,Ag2…Ag4ii0.316 1 nm,Fig.2a)does not exceed the van der Waals radius between them (0.344 nm),indicating that Ag-Ag metal bonds exist in the complex.While the dihedral angle between two benzene rings on the same ligand is 55.738(1)°.

Fig.1 Molecular structure of complex 1 with 50%probability ellipsoids
It is worth noting that the carboxyl groups on the ligand have four coordination modes (Fig.2b),including cis-cis-bridged bidentate,bidentate plus single-atom bridge,bidentate plus diatomic bridge,chelate plus diatomic bridge.
2.1.2 Crystal structureof complex[Cd(PIMPHC)2]n(2)
X-ray diffraction analysis shows that complex 2 belongs to orthorhombic system with Pbcn space group.The molecular structure diagram is shown in Fig.3.The asymmetric unit contains a half Cd2+ion and one PIMPHC-anion ligand.The central metal ion is six-coordinated which six coordination atoms are from four ligand PIMPHC-anions.Two PIMPHC-anions provide two oxygen atoms (O1,O1ii,dCd-O1=0.229 5(2)nm),and the other two PIMPHC-anions provide four nitrogen atoms (N3,N4,N3iii,N4iii,dCd-N=0.239 5(2)~0.240 4(3)nm),forming a distorted octahedral geometry type.These bond lengths are close to the cadmium complexes of carboxylate reported in the literature[29-30].Benzene ring and phenanthroline ring on the same ligand PIMPHC-are not in the same plane,their dihedral angle is 22.317(9)°.As shown in Fig.4,the PIMPHC-anions are coordinated with metal ions in O-monodentate and N,N′-bidentate chelates.The metal ions form a two-dimensional lattice structure by bridging the PIMPHC-in the ac plane.
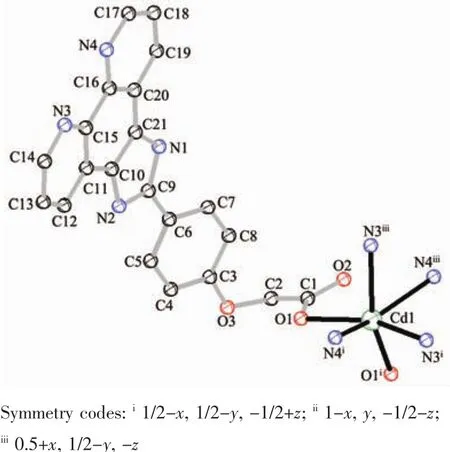
Fig.3 Molecular structure of complex 2 with 50%probability ellipsoids
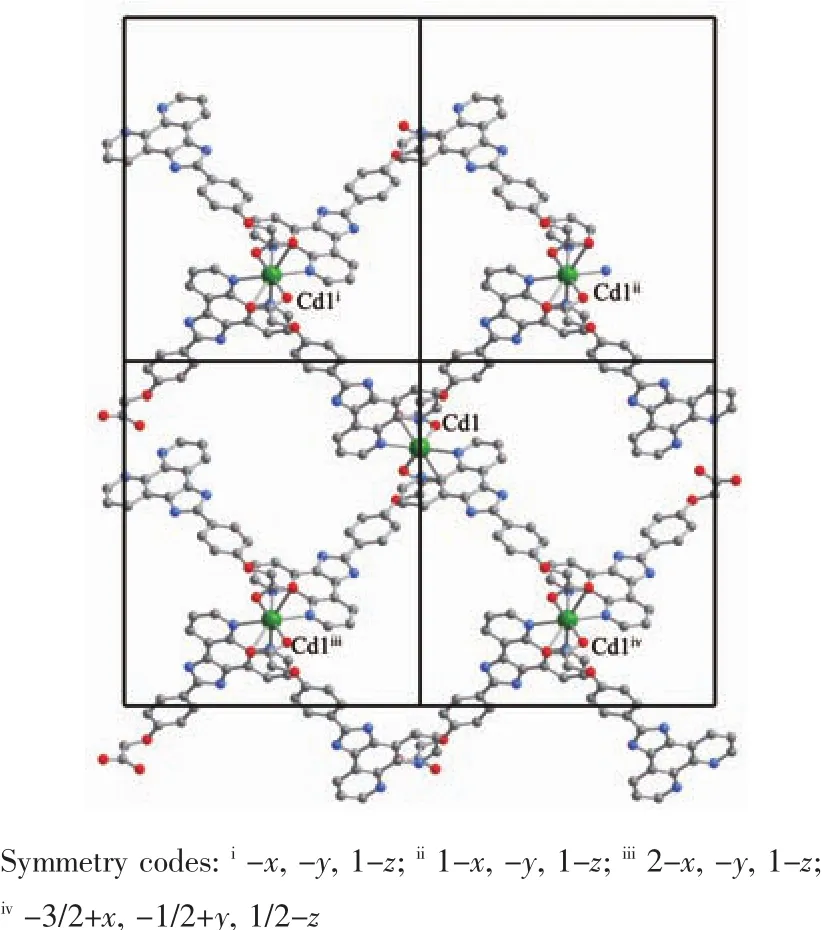
Fig.4 Two dimensional structure of complex 2 in ac plane
2.2 IR spestra
From the FT-IR spectra of the two complexes and the two corresponding ligands,the characteristic absorption peak of carboxyl group of free ligand H4BPTC appeared at 1 698 cm-1,This peak disappeared after formation of complex 1,thereby appeared asymmetric stretching vibration and symmetrical stretching vibration of the carboxyl group.The asymmetric stretching vibrationνas(COO-)appears at 1 602 cm-1and the symmetrical stretching vibration νs(COO-)appears at 1 426 cm-1,1 394 cm-1and 1 360 cm-1respectively.Carboxyl groups participate in the coordination,and there are different coordination forms of monodentate,chelate and bridging.For the complex 2,the characteristic absorption peak of the HPIMPHCat 1 720 cm-1disappeared and the asymmetric stretching vibrationνas(COO-)and symmetric stretching vibrationνs(COO-)at 1 608 cm-1and 1 448 cm-1appeared,respectively.TheΔνvalue was 160 cm-1,indicating that the carboxyl group existed in monodentate coordination.The absorption peak at 2 361 cm-1corresponds to the ligand imidazole ring stretching vibration of the N-H,and 736 cm-1is attributed tothe C-Nstretchingvibration[29,31].These results are good in agreement with single crystal structure analysis of complexes.
2.3 TG analysis
Thermal behaviours of two complexes were studied by thermal gravimetric analysis(TGA)(Fig.5).For complex 1,the weight loss between 235 and 417℃is ascribed to the decomposition of organic ligand and 57.05%residue may be Ag(Calcd.56.95%).For complex 2,the TG curve shows that the weight loss in the range of 252~455 ℃ corresponds to the decomposition of organic ligands and 15.22%residue may be CdO(Calcd.15.09%).

Fig.5 TGcurves of 1 and 2
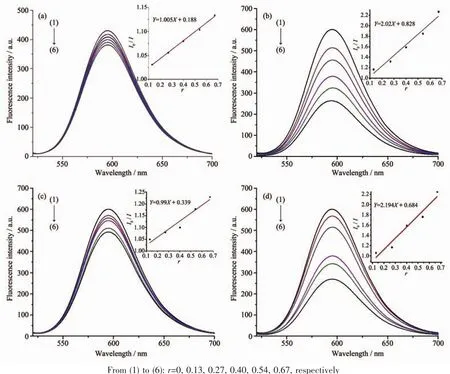
Fig.6 Emission spectra of EB-DNA system in the absence and presence of compounds:(a)H4BPTC,(b)complex 1,(c)HPIMPHC,(d)complex 2
2.4 DNA-binding
Fig.6 shows the fluorescence quenching curves of EB-DNA complex system with different concentrations of two ligands and their corresponding complexes.It can be seen that the EB-DNA complex system emits strong fluorescence at 592 nm.The fluorescence of EB-DNA complex system was quenched to different extents.It was inferred that all the compounds had different degree of insertion with DNA.In order to quantitatively study the binding capacity of DNA complexes.The fluorescence quenching constants of EB-DNA complexes were obtained according to Stem-Volmer formula[32]:I0/I=1+Ksqr,where I0and I were the fluorescence intensity of EB-DNA complex system and EB-DNA complex system with different concentrations,r is the ratio of compound to DNA concentration,and Ksqis the linear Stem-Volmer quenching constant.The binding constants Ksqof the compounds were calculated to be 1.005,2.02,0.99 and 2.194,respectively (Fig.6,Inset),and the size of the compounds was quantitatively determined by their ability to insert into the DNA.Compared with the binding constants of the ligands,the insertion of the corresponding complexes was stronger than that of the ligands.
[1]He Y B,Zhou W,Qian G D,et al.Chem.Soc.Rev.,2014,43(16):5657-5678
[2]Mason JA,Veenstra M,Long JR.Chem.Sci.,2014,5(1):32-51
[3]Li J R,Sculley J,Zhou H C.Chem.Rev.,2011,112(2):869-932
[4]Herm Z R,Bloch E D,Long J R.Chem.Mater.,2014,26(1):323-338
[5]Wang Q,Bai J,Lu Z,et al.Chem.Commun.,2016,52(3):443-452
[6]Yoon M,Srirambalaji R,Kim K.Chem.Rev.,2011,112(2):1196-1231
[7]Zhao M,Ou S,Wu CD.Acc.Chem.Res.,2014,47(4):1199-1207
[8]Kreno L E,Leong K,Farha O K,et al.Chem.Rev.,2011,112(2):1105-1125
[9]Hu Z,Deibert B J,Li J.Chem.Soc.Rev.,2014,43(16):5818-5840
[10]Hurd J A,Vaidhyanathan R,Thangadurai V,et al.Nat.Chem.,2009,1(9):705-710
[11]Sahoo SC,Kundu T,Banerjee R.J.Am.Chem.Soc.,2011,133(44):17950-17958
[12]Ma ZB,Moulton B.Coord.Chem.Rev.,2011,255:1623-1641[13]Efthimiadou EK,Karaliota A,Psomas G.J.Inorg.Biochem.,2010,104:455-466
[14]Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2011,112(2):1126-1162
[15]Zhao J,Wang X L,Shi X,et al.Inorg.Chem.,2011,50(11):3198-3205
[16]Yu WH,Wang X Z,Ren X M,et al.Inorg.Chem.Commun.,2008,11:799-801
[17]Shan X C,Zhou Y F,Zhang H B,et al.Inorg.Chem.Commun.,2012,22:149-153
[18]Chen P K,Che Y X,Zheng J M,et al.CrystEngComm,2010,12:720-724
[19]Chang X H,Qin J H,Ma L F,et al.Cryst.Growth Des.,2012,12(6):4649-4657
[20]Mereacre V,Baniodeh A,Powell A K,et al.J.Am.Chem.Soc.,2011,133(6):15335-15337
[21]Li B,Zang SQ,Ji C,et al.Cryst.Growth Des.,2012,12(3):1443-1451
[22]Lin X,Jia J H,Zhao X B,et al.Angew.Chem.Int.Ed.,2006,45(44):7358-7364
[23]Liu T F,Lu J,Guo Z G,et al.Cryst.Growth Des.,2012,10(4):1489-1491
[24]SHEN Wei(沈伟),HU Wei-Ji(胡未极),WU Xiao-Yong(吴小勇),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(6):1101-1110
[25]Sheldrick G M.SADABS,University of Göttingen,Germany,1996.
[26]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structure,University of Göttingen,Germany,1997.
[27]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[28]WANG Ji-Jiang(王记江),HOU Xiang-Yang(侯向阳),CAO Pei-Xiang(曹培香),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(4):829-832
[29]YU Yu-Ye(余玉叶),SHI Pei(石沛),SHENG Xiu-Dong(盛秀冬),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30(10):2332-2340
[30]Guo H D,Guo X M,Zhang H J,et al.Cryst.Growth Des.,2009,9(3):1394-1401
[31]Nakamoto K,Translated by HUANGDe-Ru(黄德如),WANG Ren-Qing(汪仁庆).Infrared and Raman Spectra of Inorganic and Coordination Compounds(无机和配位化合物的红外和拉曼光谱).Beijing:Chemistry Industry Press,1986.
[32]Lakowicz J R,Webber G.Biochemistry,1973,12(21):4161-4170
Syntheses,Structures,DNA Interaction of Agand CdCoordination Polymers Based on Biphenyl-2,4,4′,6-tetracarboxylic Acid and Imidazo-phenanthrolin-phenoxy Acetic Acid
Two novel coordination polymers[Ag4(BPTC)]n(1)and[Cd(PIMPHC)2]n(2)were synthesized under hydrothermal reactions by using biphenyl-2,4,4′,6-tetracarboxylic acid(H4BPTC,C16H10O8)and 2-(4-(1H-imidazo-2-[4,5-f][1,10]phenanthrolinyl)phenoxy)acetic acid(HPIMPHC,C21H14N4O3).They were characterized by elemental analysis,IR spectra,TGA,single crystal X-ray diffraction.Complex 1 crystallizes in triclinic system with space group P1,the coordination numbers of Agare four,three and two,forming distorted tetrahedron,flat triangular and V-type coordination geometry.Complex 2 crystallizes in orthorhombic system with space group Pbcn,Each Cdis bridged by the PIMPHC-anions,forming a distorted octahedral coordination geometry.The interaction of complexes,ligands and ct-DNA were studied by EtBr fluorescence probe.CCDC:952961,1;980891,2.
biphenyl-2,4,4′,6-tetracarboxylic acid;2-(4-(1H-imidazo-2-[4,5-f]-[1,10]phen-anthrolinyl)phenoxy)acetic acid;Ag;Cd;DNA
O614.122;O614.24+2
A
1001-4861(2017)10-1869-07
10.11862/CJIC.2017.215
LU Ya1LÜ Tian-Xi2HU Wei-Ji2WU Xiao-Yong2ZHAOGuo-Liang*,1,2
(1Xingzhi College,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
(2College of Chemistry and Life Science,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
2017-05-11。收修改稿日期:2017-07-19。
浙江省自然科学基金(No.LY12B01003)资助项目。
*通信联系人。 E-mail:sky53@zjnu.cn
