基于N-3-吡啶磺酰化氨基乙酸配体的Co配位聚合物的合成、晶体结构和磁性分析
2017-11-01廖蓓玲杨鸽鸽李石雄蒋毅民银秀菊
廖蓓玲 杨鸽鸽 李石雄*,,3 蒋毅民*, 银秀菊
廖蓓玲1,2杨鸽鸽2李石雄*,1,2,3蒋毅民*,2银秀菊1,2
(1河池学院化学与生物工程学院,宜州 546300)
(2广西师范大学化学与药学学院,桂林 541004)
(3华南理工大学环境与能源学院,广州 510006)
Co(OAc)2·2H2O,4,4′-偶氮吡啶(4,4′-azpy)和 N-3-吡啶磺酰化氨基乙酸(H2L)在乙醇和水混合溶液中反应生成了一个单核钴配位聚合物{[Co(L)(4,4′-azpy)(H2O)]·2H2O}n(1)。利用元素分析、IR、热重和单晶X射线衍射对其结构进行分析。结果表明该配位聚合物属于单斜晶系,P21/n空间群。热重分析结果表明,配位聚合物在失去配位水分子和游离水分子后结构仍然保持不变,并在238℃开始分解,具有良好的稳定性。磁性测试表明在配合物的中心离子Co之间存在反铁磁相互作用。
聚合物;N-3-吡啶磺酰化氨基乙酸;结构;磁性
0 Introduction
In recent years,the field of single-molecule magnets(SMMs)is developing very fast.This area mainly focuses on investigation of polynuclear 3d metal complexes[1-4],4f metal complexes(both monoand polynuclear)[5],as well as polynuclear 3d-4f metal complexes[6].In our previous work[7-9],we reported andstudied antiferromagnetic (AF)coupling interaction between magnetic centers.We found that N-((3-pyridine)-sulfonyl)aspartate can act as excellent building blocks with charge and multi-connecting ability in the construction of functional coordination polymers with porosity,photoluminescent or magnetic properties[10-11].Generally,there are many factors that affect self-assembly of coordination assemblies:such as chemical structure of the ligands chosen,coordination geometry preferred by metals ions,reaction temperature and solvent system,counter ions and methods of crystallization[12-18].Compared with the previously investigated N-((3-pyridine)-sulfonyl)aspartate ligand,herein,we chose N-(pyridine-3-sulfonyl amino)acetate(H2L)and 4,4′-azopyridine(4,4′-azpy)mixed ligands as reagent,and obtained one mononuclear cobalt polymer{[Co(L)(4,4′-azpy)(H2O)]·2H2O}n(1).Its thermogravimetric and magnetic properties were analyzed.
1 Experimental
All solvents and chemicals were commercial reagents and used without further purification.N-(pyridine-3-sulfonyl amino)acetate (H2L)was synthesized according to references[19].Elemental analyses(carbon,hydrogen,and nitrogen)were performed with a Perkin-Elmer 240 elemental analyzer.IR spectra were measured from KBr pellets on a Nicolet 5DX FT-IR spectrometer.The power X-ray diffraction(PXRD)studies were performed with a Bruker AXSD8 Discover instrument(Cu Kα radiation,λ=0.154 184 nm,U=40 kV,I=40 mA)over the 2θrange of 5°~50°at room temperature.The TGA was performed by Perkinelmer Pyris Diamond TG-DTA.The magnetic measurements were carried out with a Quantum Design MPMS-XL7 and a PPMS-9 ACMSmagnetometer.

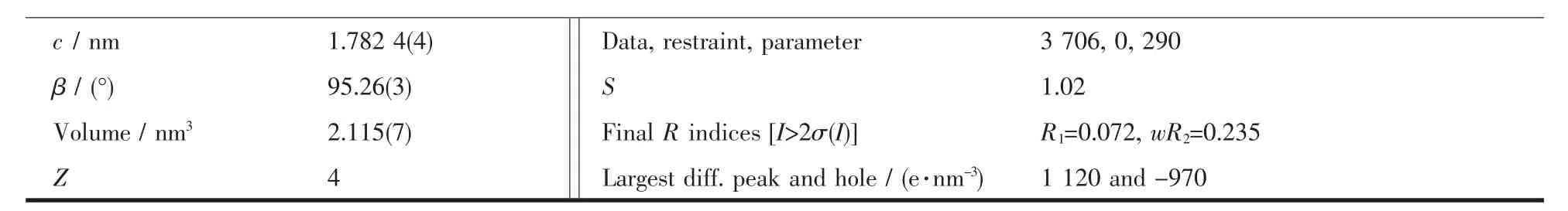
Table 1 Crystal data and structure parameters for polymer 1
1.1 Synthesis of{[Co(L)(4,4′-azpy)(H2O)]·2H2O}n(1)
Co(OAc)2·2H2O(0.062 2 g,0.25 mmol)and H2L(0.037 23 g,0.17 mmol)were mixed in 10 mL H2O and 10 mL ethanol.The mixture was stirred at 40℃for 10 min,and the pH value was adjusted to 6 with 1.0 mol·L-1NaOH.Ligand 4,4′-azpy(0.018 4 g,0.1 mmol)was added.After reaction for 4 h,the mixture was filtered,and a dark red solution was obtained.After 15 days,red massive crystals of 1 were obtained by evaporation at room temperature.Yield:65%(based on H2L).Anal.Calcd.for C17H20CoN6O7S(%):C,39.89;H,3.91;N,16.43.Found(%):C,39.35;H,3.85;N,37.40.IR(cm-1):3 410s,2 357w,1 599s,1 572s,1 418s,1 309m,1 249s,1 150s,1 086m,954w,854m,705w,595m,572w.
1.2 X-ray diffraction
Red block single crystal of polymer 1 having approximate dimension of 0.200 mm×0.180 mm×0.150 mm was mounted on the top of a glass fiber.The diffraction data for polymer 1 were collected on an Agilent Super NOVA diffractometer equipped with a graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm)at 293(2)K.The structure was solved by direct methods using SHELXL-97[20]and refined by full matrix least-squares on F2using SHELXS-97[20].An empirical absorption correction was applied with the program SADABS[21].The hydrogen atoms were placed at calculated positions and refined as riding atoms with isotropic displacement parameters.Crystallographic crystal data and structure processing parameters for polymer 1 are summarized in Table 1.Selected bond lengths and bond angles for polymer 1 are listed in Table 2,and hydrogen bond parameters for polymer 1 are listed in Table 3.
CCDC:1541464,1.
Continued Table 1
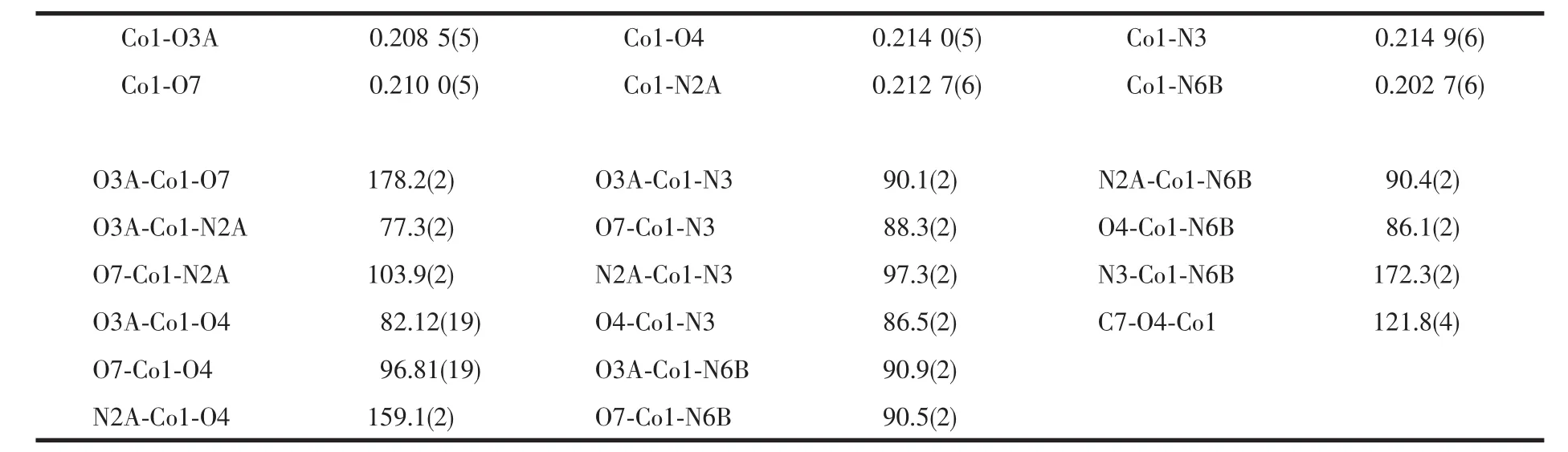
Table 2 Selected bond lengths(nm)and bond angles(°)for polymer 1
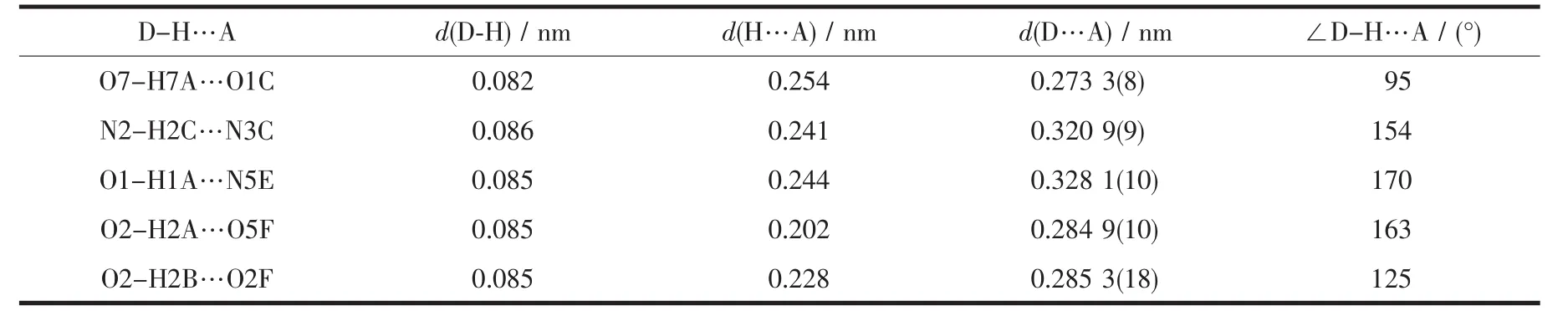
Table 3 Hydrogen bonds for polymer 1
2 Results and discussion
2.1 Crystal structure of{[Co(L)(4,4′-azpy)(H2O)]·2H2O}n(1)
Single-crystal X-ray diffraction analysis reveals that polymer 1 crystallizes in monoclinic system,space group P21/n.The coordination environment of Coin 1 is shown in Fig.1a.The asymmetric unit of 1 consists of one Coion,one deprotonated L2-,one 4,4′-azpy,one coordinated water molecule,and two free water molecules.The Coion in 1 is coordinated with three nitrogen atoms(N3 and N6A from two 4,4′-azpy ligands,N2A from deprotonated L2-)and three oxygen atoms(O4 and O4A from two deprotonated L2-ligands,O7 from coordinated water molecule).So,the center Coions in 1 is six-coordinated.It is worth noting that the coordination of L2-involves in different coordination modes.The oxygen on the carboxyl group of a ligand L2-takes a monodentate coordination;but the nitrogen atom from amino group and oxygen atom on the carboxyl group of another ligand L2-form a chelated five-membered ring with the central Coions.For polymer 1,the Co-Odistancesfall in the range of 0.208 5(5)~0.214 0(5)nm(Co1-O3A 0.208 5(5)nm,Co1-O4 0.214 0(5)nm,Co1-O7 0.210 0(5)nm),and the Co-N distances fall in the range of 0.202 7(6)~0.214 9(6)nm(Co1-N2A 0.212 7(6)nm,Co1-N3 0.214 9(6)nm,Co1-N6B 0.202 7(6)nm).The bond anglesof O3A-Co1-O7 and O3A-Co1-N2Aare178.2(2)°and 77.3(2)°,respectively.The bond lengths and bond angles indicate that the coordination environment of the central Coion is a slightly distorted octahedral geometry.

Fig.1 (a)Coordination environment of Coions in 1 with thermal ellipsoids at 50%level;(b)One-dimensional chain structure of 1;(c)Two-dimensional network structure formed by 4,4′-azpy ligands bridging Co ions in ab plane;(d)Three dimensional structure formed by hydrogen bonds
In 1,the oxygen atoms of carboxyl groups in the L2-are coordinated by a monodentate mode,and two oxygen atoms on each carboxyl group bridge two different Coions,thus forming a one-dimensional dendritic structure by cross-bridging the oxygen through the carboxylate of L2-,and the pyridine ring of ligand separated on both sides (Fig.1b).The 4,4′-azpy molecule takeμ2-bridging mode to bridge two different Coions between the chains,forming a two-dimensional network structurein ab plane(Fig.1c).
Owing to the introduction of water molecules,polymer 1 had a large number of hydrogen bonds.The polymer 1 has not only intramolecular,but also intermolecular hydrogen bonds.The free water molecules are present in 1 by forming O-H…O and O-H…N hydrogen bonds with the O5 atom of sulfonyl and the N5 atom of 4,4′-azpy ligand,respectively.These hydrogen bonds finally generate a three-dimensional network structure along c-axis by intermolecular hydrogen bonds bridging two 2D planes(Fig.1d).
2.2 XRD analysis
In order to check the purity of polymer 1,powder X-ray diffraction of the as-synthesized sample was measured at room temperature.The peak positions of experimental patterns are in good agreement with the simulated ones,which clearly indicates good purity of the polymer 1(Fig.2).

Fig.2 PXRD patterns for 1
2.3 Thermal gravimetric analysis of polymer 1
The thermal stability of polymer 1 was tested in the temperature range of 45~1 000 ℃ under a nitrogen atmosphere at a heating rate of 5 ℃·min-1for Pyris Diamond TG-DTG Analyzer.The TGA curves of polymer 1 (Fig.3)showed that 1 first loses two free water molecules and one coordination water molecule(Obsd.9.76%,Calcd.10.56%)in the range of 20~117℃.In the range of 117~282℃,the structure of the polymer 1 is still very stable.Then there is weight loss,responsible for the decomposition of 4,4′-azpy(Obsd.40.10%,Calcd.37.54%)in the range of 283~330℃.Further weight loss,responsible for the decomposition of all organic components(Obsd.42.89%,Calcd.42.24%)happens in the range of 330~725 ℃,and the last remaining amount of 18.83%might be Co2O3(Calcd.16.23%).
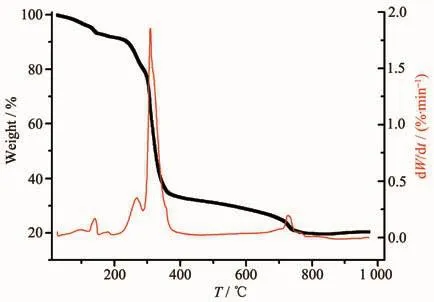
Fig.3 TGA curve for 1
2.4 Magnetic analysis for polymer 1
When T=2 K,it can be seen from the M-H curve(Fig.4b)that the magnetization(M)increases rapidly with the field strength(H)when the field strength is less than 2.5 T,showing antiferromagnetic interaction in 1.In the DCfield,the reciprocal 1/χmof the magnetic susceptibility linearly changes with the temperature in the 1 000 Oe and 2~300 K,and Curie-Weiss law is consistent with the Curie constant C=3.356 6 cm3·mol-1·Kand Weisstemperature=-17.05 K.Thenegative Weiss temperature indicates that there is an antiferromagnetic exchangein 1(Fig.4c).This issimilar to the magnetic properties of the cobalt-related complexes reported in the literature[24-27].The magnetic properties of this type are found by the presence of anti-ferromagnetic exchange between the onedimensional chains formed by the cationic bridging metal ions[24-27], and there is a weak ferroelectric exchange between the ligands connected by azobisilide[28],which is more unique.
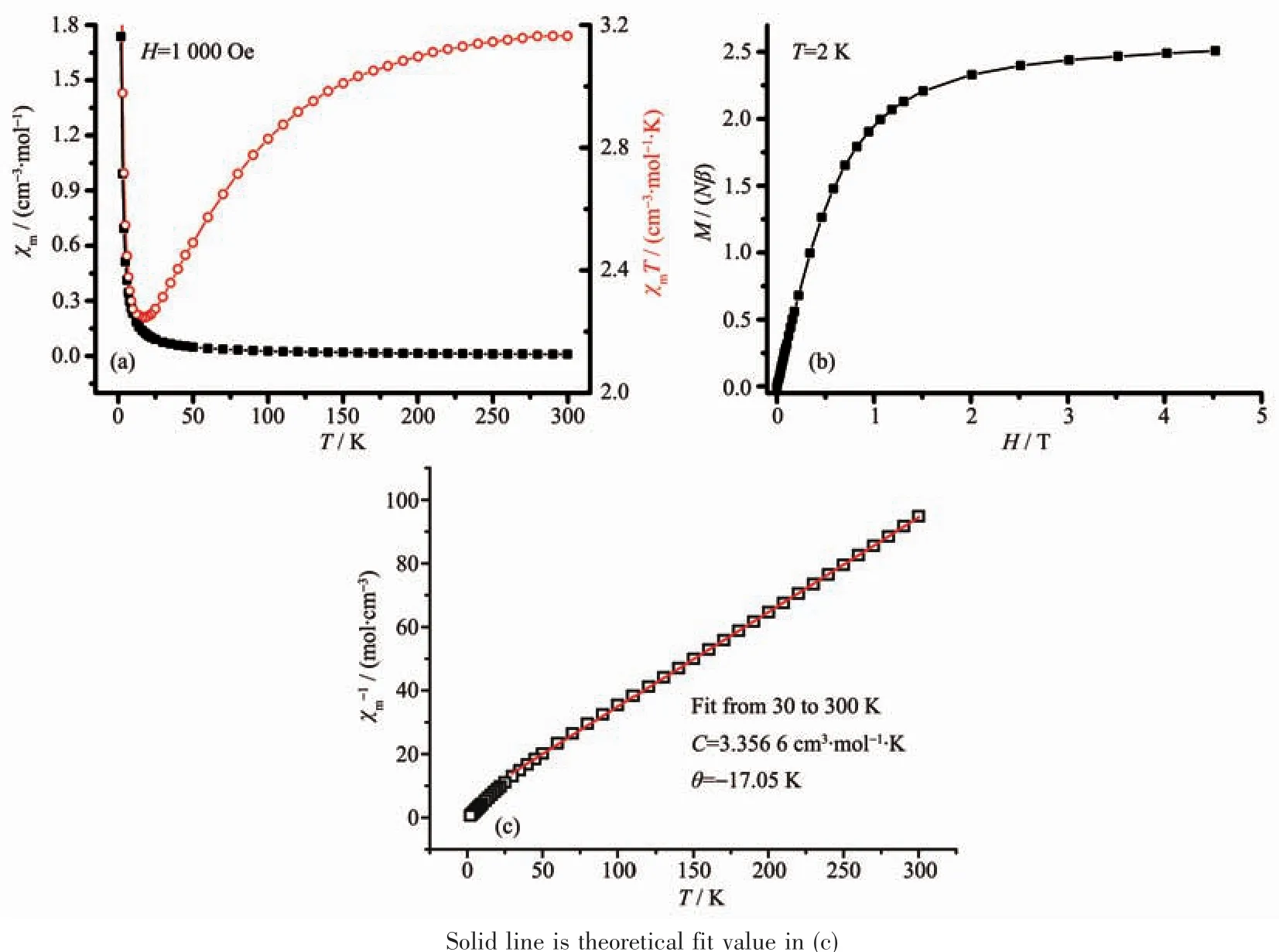
Fig.4 (a)χm or χm T vs T plot of polymer 1;(b)M-H curve of 1 at 2 K;(c)1/χm-T curve of 1 at 1 000 Oe
3 Conclusions
In summary,one mononuclear cobalt polymer{[Co(L)(4,4′-azpy)(H2O)]·2H2O}n(1) based on N-(pyridine-3-sulfonyl amino)acetate had been synthesized and characterized by elemental analysis and single crystal X-ray diffraction analysis. X-ray diffraction study reveals that the polymer 1 belongs to monoclinic system and P21/n space groups,and it is a polymer with 2D structure.The 3D structure of 1 is formed by hydrogen bonds.The magnetic investigation shows that there is antiferromagnetic interactions between Coions in polymer 1.
[1]Zhang SH,Zhang Y D,Zou H H,et al.Inorg.Chim.Acta,2013,396:119-125
[2]Zhang S H,Zhao R X,Li G,et al.RSC Adv.,2014,4(97):54837-54846
[3]Xiao Y,Liu Y Q,Li G,et al.Supramol.Chem.,2015,27(3):161-166
[4]Wang J,Feng C,Ge C M,et al.J.Clust.Sci.,2016,27(6):2001-2011
[5]Woodruff D N,Winpenny R E P,Layfield R A.Chem.Rev.,2013,113:5110-5129
[6]Zhang SH,Huang Q P,Zhang H Y,et al.J.Coord.Chem.,2014,67(19):3155-3166
[7]Liao B L,Li S X,Guo J J,et al.Russ.J.Coord.Chem.,2016,42(4):285-291
[8]LIAO Bei-Ling(廖蓓玲),LI Shi-Xiong(李石雄),YIN Xiu-Ju(银秀菊),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(7):1255-1260
[9]Jia J J,Zhang L K,Li S X,et al.Chin.J.Struct.Chem.,2017,36(2):266-272
[10]Limas N G,Manz T A.RSCAdv.,2016,6(51):45727-45747
[11]Liddle ST,van Slageren J.Chem.Soc.Rev.,2015,44(19):6655-6669
[12]Aubin SM J,Wemple M W,Adams DM,et al.J.Am.Chem.Soc.,1996,118(33):7746-7754
[13]Zhang SH,Wang J,Zhang H,et al.Dalton Trans.,2017,46(2):410-419
[14]Wang J M,Zhang C,Zhang X Q,et al.Inorg.Nano-Metal.Chem.,2017,47(6):893-896
[15]LI Huan(李欢),CHEN Yun-Zhou(陈云舟),WANG Yan-Jun(王艳君),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(12):2198-2204
[16]Miyasaka H,Yamashita M.Dalton Trans.,2007,4:399-406
[17]Li SX,Liao B L,Yang G G,et al.Synth.React.Inorganic Met.-Org.Chem.,2015,45(6):926-929
[18]Jia JJ,Li SX,Yin X J,et al.Russ.J.Inorg.Chem.,2017,62(1):63-71
[19]SHI Li-Li(史黎黎),QIN Fan(秦凡).J.Jianghan Univ.:Nat.Sci.Ed.(江汉大学学报:自然科学版),2007,35:44-46
[20]Sheldrick G M.SHELXL-97,University of Göttingen,Germany,1997.
[21]Sheldrick G M.Acta Crystallogr.Sect.A,2008,A64:112-122
[22]Zhao R X,Hai H,Li G,et al.J.Clust.Sci.,2014,25(6):1541-1552
[23]Li G,Wang W,Zhang SH,et al.J.Clust.Sci.,2014,25(6):1589-1597
[24]Xu X X,Liu X X,Zhang X,et al.Polyhedron,2009,28(14):2997-3004
[25]Hu B W,Zhao J P,Yang Q,et al.J.Solid State Chem.,2009,182(10):2918-2923
[26]Han Y F,Li M,Wang T W,et al.Inorg.Chem.Commun.,2008,11(9):945-947
[27]Sengupta O,Song Y,Mukherjee P S.Dalton Trans.,2009(46):10343-10352
[28]Zhu L N,Liang M,Wang Q L,et al.J.Mol.Struct.,2003,657(1):157-163
Syntheses,Structures and Magnetic Analysis of CoCoordination Polymer Based on N-(Pyridine-3-sulfonyl amino)Acetate
The reaction of Co(OAc)2·2H2O,4,4′-azopyridine(4,4′-azpy)with N-(pyridine-3-sulfonyl amino)acetate(H2L)in ethanol and water leadstotheformation of onemononuclear cobalt polymer{[Co(L)(4,4 ′-azpy)(H2O)]·2H2O}n(1).The structure of 1 was analyzed by elemental analysis,IR,TG and single crystal X-ray diffraction.The results show that complex 1 belongs to monoclinic system and P21/n space groups.The TGA shows that the structure of 1 remains unchanged during the loss of coordination water molecules and free water molecules,and begins to decompose at 238℃,indicating 1 has good stability.The magnetic investigation shows that there is antiferromagnetic interactions between Coions in polymer 1.CCDC:1541464,1.
polymer;N-(pyridine-3-sulfonyl amino)acetate;structure;magnetic
O614.81+2
A
1001-4861(2017)10-1843-06
10.11862/CJIC.2017.189
LIAOBei-Ling1,2YANG Ge-Ge2LI Shi-Xiong*,1,2,3JIANG Yi-Min*,2YIN Xiu-Ju1,2
(1College of Chemistry and Biological Engineering,Hechi University,Yizhou,Guangxi 546300,China)
(2School of Chemistry and Pharmacy,Guangxi Normal University,Guilin,Guangxi 541004,China)
(3School of Environment and Energy,South China University of Technology,Guangzhou 510006,China)
2017-04-01。收修改稿日期:2017-05-26。
广西高校科研项目(No.YB2014331)和广西自然科学基金(No.2014GXNSFAA118035)资助项目。*
。 E-mail:lsx1324@163.com,790223684@qq.com
