Study on the Design and Performance of Double-base Propellant/AmmoniumPerchlorate Based Incendiary Agent
2017-11-01WANGHaipengYANGHongtaoZHUXuqiangYANGYueCHENGYiSONGDongming
WANG Hai-peng, YANG Hong-tao, ZHU Xu-qiang, YANG Yue, CHENG Yi, SONG Dong-ming
(1. Nanjing Research Institute on Simulation Technique, Nanjing 210094, China;2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China;3. School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China)
StudyontheDesignandPerformanceofDouble-basePropellant/AmmoniumPerchlorateBasedIncendiaryAgent
WANG Hai-peng1, YANG Hong-tao2, ZHU Xu-qiang3, YANG Yue3, CHENG Yi3, SONG Dong-ming3
(1. Nanjing Research Institute on Simulation Technique, Nanjing 210094, China;2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China;3. School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China)
Double-base (DB) propellant/ammonium perchlorate (AP) as the main components of the incendiary agent were designed and the combustion performance of incendiary agent was adjusted by adding metal (boron, magnesium, aluminum) combustible agent. The combustion heat, burning rate, flame temperature and thermal performance of incendiary agent were measured by automatic calorimeter, digital camera, thermocouple and TG-DSC.The results show that adding the metal powders can increase the combustion heat, burning rate and flame temperature of the incendiary agent, and change their flame structure. For long-distance ignition of liquid fuel with high boiling point, the effect of boron powder is the best in three kinds of metal powders, the flame temperature of DB/AP/B can reach 1070℃, the flame length reaches 25cm, the combustion process is more stable, while DB/AP/Mg and DB/AP/Al produce a large number of sparks in the combustion process. AP and metal powder have no effect on the thermal decomposition of DB propellant.
double-base propellant; incendiary agent; combustible agent; long-distance ignition; ammonium perchlorate
Incendiary agent, a pyrotechnic composition using the heat energy of burning to ignite target[1], can be divided into nonmetal-containing incendiary agents and metal-containing incendiary agents. Nonmetal-containing incendiary agents mainly include napalm, phosphorus and triethyl aluminum, and it is difficult to ignite high boiling fuel for the low heat energy[2]. A great quantity of work has been done by many researchers on metal-containing incendiary agents which can release great heat. It may be divided into three categories, thermite, pyrotechnic incendiary agents and metal-containing incendiary agents according to performance. For thermites, a metal oxide acts as the oxidizer, and aluminum act as the fuel[3-5]. Despite the thermite combustion can release great heat, the application is greatly limited because of high ignition temperature[6]. Pyrotechnic incendiary agents compose of oxidant, binding and fuel. It has the advantages of low ignition temperature and high burning temperature, these advantages make pyrotechnic incendiary agents widely apply in firebomb[7]. Metal-containing incendiary agents mainly consist of rare earth metals.
The combustion residue level of existing incendiary agents containing oxidant is high and the flame length is short, such as thermite, metal-containing incendiary agents. High temperature solid residual and short flame are not satisfactory for ignition of liquid fuel with high boiling point[8]. The character of DB propellant and AP incendiary agents is long flame but low combustion residue. In this study, the metals (boron, magnesium, aluminum ) were added into DB/AP incendiary agents to increase the combustion heat and residue, so that it can improve the ignition performance for long-distance ignition of liquid fuel with high boiling point.
1 Experimental
1.1Materialsandmethods
AP, a category of analytical grade chemicals, which was obtained from Sinopharm Chemical Reagent Co., Ltd., had a purity of above 99%, and the particle size was about 149μm.
Boron (B, amorphous, purity>97%), which was purchased from Yingkou Pengda Fine Chemicals Co., Ltd., had an average particle size of 2 to 4μm as is specified by the supplier.
Aluminum (Al) powder, which was obtained from Nanjing Emperor Nano Material Co., Ltd., had an average primary particle diameter of 70μm as is specified by the supplier.
Magnesium (Mg) powder, which was obtained from Shanghai Longxin Science and Technology development Co., Ltd., had an average particle size of 70 to 150μm as is specified by the supplier.
The composition (mass fraction)of the double-base propellant (DB) was: NC 63%, NG 33%, Vaseline 2.8%, PbO 1.2%.
In our previous study, it was found that the heat of combustion of the sample reached a maximum value when the mass ratio of AP/DB was 1∶1[9]. In this study, addition of 10% (mass fraction) metal powder at constant DB/AP ratio was used to increase flame temperature, five different samples were given in Table 1. The compositions were dry mixtures of two or three components after double-base propellant was ground into powder.
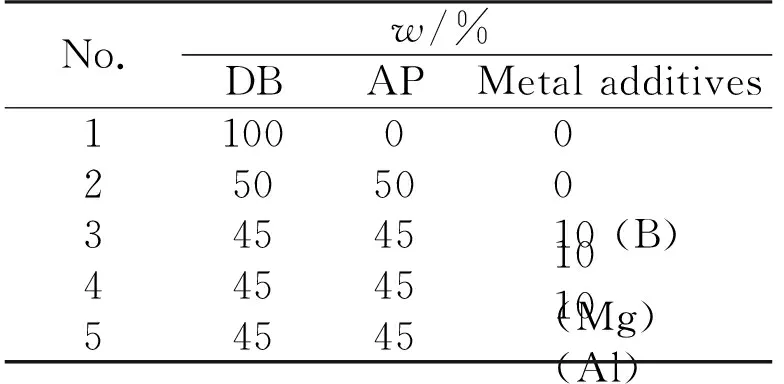
Table 1 Chemical composition of five incendiary agents
1.2Measurementofcombustionheat
The heat of combustion was measured by using Hunan Youxin YX-ZR Type automatic calorimeter. About 1g of incendiary agent, 0.1g of transition composition (the mass ratio of incendiary agent/igniter is 3/7) and 0.1g of igniter (Si/Pb3O4) were compressed at approximately 8.0MPa to produce a cylindrical strand. The height of the cylinder was about 10mm and the diameter was 9mm. The structure of the cylinder was given in Fig.1. Samples were ignited by a nickel chrome ignition wire directly. Each of the samples was tested two times, and the average value was taken.
1.3Measurementofburningcharacteristics
About 1g of incendiary agent, 0.1g of transition composition and 0.1g of igniter were compressed at approximately 8.0MPa in an aluminum cylindrical tube-shell. Samples were ignited by a nickel chrome ignition wire directly in air at the standard atmospheric pressure. Burning time and combustion condition were recorded by Nikon camera. Flame temperature was measured by a 1mm diameter WRe5/WRe26 thermocouple, which was placed above the top of the tube-shell about 1cm and connected to a dynamic signal analyzer (Industrialacs Computer 2000). The thermocouple is a sensor used to measure temperature. It consists of two wire legs made from different metals. The wires legs are welded together at one end, creating a junction. This junction is where the temperature is measured. When the junction experiences a change in combustion temperature of incendiary agent, a voltage is created and recorded by signal analyzer. The voltage can then be interpreted using thermocouple reference tables to calculate the temperature. The data collecting frequency was set to 12.5kHz.
1.4Thermalperformanceanalysis
The thermal performances of the samples were investigated by means of TG-DSC (NETZSCH STA449C)-MS (NETZSCH-QMS403C). Approximately 2mg of sample was heated over a temperature range of ambient to 500℃. The heating rate was 10K/min, and the samples were carried in Al2O3crucibles (80μL). High-purity argon with a gas flow rate of 20mL/min was used as purge gas.
2 Results and discussion
2.1Combustionheat
The combustion heats of samples 1, 2, 3, 4, 5 are 2.79, 5.94, 8.02, 7.81, 7.62kJ/g, respectively, which shows that combustion heat is significantly improved after the addtion of metals. This is due to the fact that the metals have high combustion heat and the AP provides more oxygen. This is consistent with Xiangyu Li’s work[9]which stated that the combustion heat increases with the increase of oxygen balance of sample, when the oxygen balance is below zero. Furthermore, DB/AP/B has the highest combustion heat. But as a result of incomplete combustion there is minor difference for samples 3, 4, 5.
2.2Burningrate
The burning rate is affected by various-factors (such as ingredients heterogeneity, initial temperature and density). In order to reduce the error, the experiment was carried out for three times, burning rates were obtained by dividing the height of cylinder in the tube-shell by burning time, and took the average values.
The burning rates of samples 1, 2, 3, 4, 5 are 1.06, 1.22, 2.09, 1.77, 1.56mm/s, respectively. This result shows that the burning rate is not significantly improved after the sole oxidizer AP was added. The value is significantly improved after AP/metal composite was added, especially metal powder boron (sample 3), which improves the burning rate by 0.97 times compared with the DB. Magnesium metal (sample 4) improves 0.67 times due to the heat effect of the metals. And B and Mg have a relatively high propensity to react while on the surface and in the condensed phase of the propellant, thus producing a significant increase in burning rate through condensed phase heat release. Aluminum is less prone to react in the condensed phase and on the surface[10].
2.3Combustionflameandflametemperature
Images of the flame which the length reaches the maximum were captured to study the combustion process of each sample. Fig.2 shows the images of the longest flame that represent combustion condition and the length of the flame (in the background the interval of the white stripes is 2cm).
The results indicate that all samples exhibit the difference type of flame structure, incendiary agents adding metal powder produce much sparks, especially Mg and Al, which is due to the fact that metals burning in air. The DB/AP/B flame is surrounded by green radiation, which can be interpreted as BO2emissions from boron combustion products[11]. The DB and DB/AP exhibit a low-intensity flame, metal fuel additives significantly improve combustion performance, thus result in heavily burning, and increase flame temperature.
The flame temperatures of DB and DB/AP are 636℃, 863℃, respectively. That of DB/AP/B and DB/AP/Mg are higher than 1000℃. And the flame temperature of DB/AP/Mg is the highest, which can reach 1260℃. The flame temperature of DB/AP/B can reach 1070℃. As for DB/AP/B, the heat of combustion for boron is much higher than that of aluminum and magnesium, the flame temperature is also higher than that of other samples, but that heat is not fully released in the combustion process due to lower combustion efficiency[12]. However, the combustion process for boron is relatively stable, and the length of the flame of DB/AP/B (25cm) is much longer than that of DB/AP/Mg (11cm) and DB/AP/Al (12cm). Liu[8]found that the sparks could not ignite the liquid fuel of high boiling point, the ignition of liquid fuel depended mainly on high temperature flame. Boron is a better metal additive than magnesium and aluminum to long-distance ignition of liquid fuel of high boiling point.
2.4Thermalperformanceandgasproductsanalysis
In thermal analysis, by linear heating, the onset temperature of reaction can be observed, the weight decrease in TG, the heat release in DSC and the gaseous products from thermal decomposition in MS can also be observed.
Fig.3 shows the TG and DSC curves of pure AP and samples 1-5. As can be seen from the TG curve of the sample AP shows a two-step decomposition process, which were respectively known as the low-temperature decomposition (LTD) and the high-temperature decomposition (HTD)[13-14]. However, its DSC curve has an endothermic peak and two exothermic peaks. The endothermic peak at 246°C is the phase transition of AP from the orthorhombic to cubic phase, which can also be observed for the samples 2, 3, 4 and 5 at about 246°C, this result shows that DB and DB/metal composite have no effect on phase transition of AP. The two exothermic peaks correspond to the low-temperature and high-temperature decomposition of AP. Ion current of mass to charge ratio 37 is detected (Fig.4), it is certain that there is HCl in the gas products of AP decomposition which mainly happens in the high-temperature decomposition stage.
For the sample DB (sample 1), the TG curve shows two-step decomposition. The first step of the TG curve corresponds to the volatilization and decomposition of NG, inducing the invisible peak in DSC curve. The second step is mainly the decomposition of NG and NC, corresponding to a visible exothermic peak in DSC curve. The reason of the only one visible exothermic peak appears in the DSC curve is that the two decomposition stages occur in succession and the temperature ranges are near together, the decomposition heats of the two processes overlap each other in DSC curve, inducing the only one exothermic peak to appear in the temperature range.
Four-step decomposition can be observed from the TG and DSC curves of the sample 2, the first two steps correspond to the decomposition of DB, the last two steps correspond to the decomposition of AP, the results show that AP additive has no effect on the decomposition of DB, but the decomposition of AP mainly happens in the low-temperature stage as a result of that the solid residual produced by DB decomposition has effect on the decomposition of AP.
For the samples 3, 4 and 5, the first two steps of TG curves correspond to the decomposition of DB. After adding AP/B or AP/Al composite, the decomposition of AP shifts two-step to one, but four-step decomposition of AP can be observed after adding AP/Mg composite.
Furthermore, no HCl is detected in the gas products for the samples 1, 2, 3, 4 and 5. This result shows that HCl reacts with the solid residual after DB decomposition, to produce solid chloride.
3 Conclusions
(1) AP and AP/metal composite improve the combustion properties of the DB, such as increase the combustion heat, the burning rate and the flame temperature, and make the flame structure change very greatly. After AP/B composite was added, the flame temperature can reach 1070℃, the flame length is much longer than that of adding AP/Mg and AP/Al, and the combustion process of DB/AP/B is relatively stable. For long-distance ignition of liquid fuel of high boiling point, boron is a better metal additive than magnesium and aluminum.
(2) Thermal analysis results show that AP and AP/metal composite have no effect on the thermal decomposition of DB. But DB and metal additives greatly change the thermal decomposition of AP and the gas products of AP decomposition.
[1] Pan Gong-pei, Yang Shuo. Chemistry of Pyrotechnics[M]. Beijing: Beijing Institute of Technology Press, 1997.
[2] Lehikoinen U. Tracer incendiary materials including liquid alkylaluminium and compatible inorganic oxidizer:US, 3788907[P].1972.
[3] Gao Kun, Luo Yun-jun, Li Guo-ping, et al. Effects of preparation method on the properties of Al/Fe2O3nano-thermites[J]. Chinese Journal of Explosives & Propellants(Huozhayao Xuebao), 2012, 35(3):11-14.
[4] Zhao Ning-ning, He Cui-cui, Liu Jian-bing, et al. Preparation and characterization of superthermite Al/MnO2and its Compatibilities with the propellant components[J]. Chinese Journal of Explosives & Propellants(Huozhayao Xuebao), 2012, 35(6):32-36.
[5] Piercey D G., Klapötke T M. Nanoscale aluminum-metal oxide (thermite) reactions for application in energetic materials[J]. Central European Journal of Energetic Materials, 2010, 7(2):115-129.
[6] Waite H R. Incendiary composition including zinc-mischmetal alloy:US, 3809586[P]. 1974.
[7] Selleck E G. Incendiary composition:US, 3653995[P]. 1972.
[8] Xiang-ling Liu. Preparation and properties of DB/AP/Al incendiary agent[D]. Nanjing: Nanjing University of Science and Technology, 2013.
[9] Li Xiang-yu, Liu Xiang-ling. Thermal decomposition properties of double-base propellant and ammonium perchlorate[J]. Journal of Thermal Analysis and Calorimetry, 2014,115(1):887-894.
[10] Iishihar A, Brewster M Q. Combustion studies of boron, magnesium, and aluminum composite propellants[J]. Combustion Science and Technology, 1992,87:75-290.
[11] Spalding M J, Krier H, Burton R L. Boron suboxides measured during ignition and combustion of boron in shocked Ar/F/O2and Ar/N2/O2mixtures[J]. Combustion and Flame, 2000,120:200-210.
[12] Wu Xiong-gang, Yan Qi-long, Guo Xin, et al. Combustion efficiency and pyrochemical properties of micron-sized metal particles as the components of modified double-base propellant[J]. Acta Astronautica, 2011,68:1098-1112.
[13] Jacobs P W M C, Whitehead H M. Decomposition and combustion of ammonium perchlorate[J]. Chemical Reviews, 1969,69(4):551-559.
[14] Bircumshaw L L, Newman B H. The thermal decomposition of ammonium perchlorate. introduction, experimental, analysis of gaseous products, and thermal decomposition experiments[J]. Mathematical and Physical Sciences, 1954,227(1168):115-117.
DB/AP系燃烧剂的设计与性能研究
王海朋1,杨洪涛2,朱绪强3,杨 月3,成 一3,宋东明3
(1. 南京模拟技术研究所,江苏 南京 210094;2. 西安近代化学研究所, 陕西 西安 710065;3. 南京理工大学化工学院,江苏 南京 210094)
设计了以双基(DB)推进剂、高氯酸铵(AP)为主要组分的燃烧剂,并加入金属可燃剂B、Mg、Al来调整燃烧剂的燃烧性能,采用全自动量热仪、数码摄像机、热电偶和TG-DSC测试了燃烧剂的燃烧热、燃速、火焰温度和热性能。结果表明,金属粉的加入可以提高燃烧剂的燃烧热、燃速和火焰温度,并可以改变其火焰结构;对于长距离、高沸点物质的引燃,3种金属粉中B粉的效果最佳,DB /AP/B的火焰温度可达1070℃,火焰长度达25cm,其燃烧过程也更稳定,而DB /AP/Mg和DB /AP/Al在燃烧过程中产生大量的火星;AP和金属粉对DB推进剂的热分解没有影响。
双基药;燃烧剂;可燃剂;长距离点火;高氯酸铵
TJ55;TQ567DocumentCodeAArticleID1007-7812(2017)05-0015-04
10.14077/j.issn.1007-7812.2017.05.003
date:2017-05-24;Reviseddate2017-08-15
Foundation:The National Natural Science Foundation of China (No.51202113)
Biography:WANG Hai-peng (1980-), male, master, senior engineer, research field: unmanned aerial vehicle motor. E-mail: whappy1999@yeah.net
Introduction
