Use of prostate-specific antigen testing in Medicare beneficiaries:Association with previous evaluation
2017-10-25GregoryCooperTzuyungDougKouMarkSchluchterAviDorSiranKoroukianSimonKim
Gregory S. Cooper, Tzuyung Doug Kou, Mark D. Schluchter, Avi Dor, Siran M. Koroukian, Simon P. Kim
Use of prostate-specific antigen testing in Medicare beneficiaries:Association with previous evaluation
Gregory S. Cooper1,3, Tzuyung Doug Kou1, Mark D. Schluchter3,4, Avi Dor5, Siran M. Koroukian3,4, Simon P. Kim2,3
Objective: Determine uptake of prostate-specific antigen (PSA) testing in Medicare beneficiaries according to previous receipt of PSA testing.
Methods:A 5% random sample of men aged 67 years or older without a previous diagnosis of prostate cancer was identified through 2009—2012 Medicare claims. We measured the annualized frequency of PSA screening among men due for PSA testing, stratified by PSA testing use in the previous 2 years, and clustered by ordering provider.
Conclusion:Receipt of PSA testing is highly dependent on whether an individual was tested in the recent past. In previously unscreened men, the largest decrease occurred in 2012, which may reflect in part the publication of US Preventive Services Task Force guidelines, but there was much less impact among men already being screened.
Prostate-specific antigen; Medicare; mass screening; clinical practice patterns
Introduction
Prostate cancer is among the most frequently diagnosed cancers in the United States, both overall and in the Medicare-eligible population (aged 65 years or older) [1]. Despite the high incidence and mortality associated with prostate cancer [1], the merits of prostate-specific antigen (PSA) screening in the general population are controversial. In 2008, the US Preventive Services Task Force (USPSTF) initially determined that there was insufficient evidence to recommend or not recommend routine prostate cancer screening with either PSA testing or digital rectal examination in men younger than 75 years [2]. In contrast, it concluded that the potential harms of screening would outweigh the benefits in men aged 75 years or older. In May 2012, the revised USPSTF guidelines recommended that prostate cancer screening no longer be performed by either method in men who are of average risk of having prostate cancer[3]. Other guidelines, including those of the American Cancer Society [4], the American Urological Association [5], and most recently, the National Comprehensive Cancer Network [6] recommended that men who are aged 50—74 years, 55—69 years, and 45—75 years respectively and have at least a 10-year life expectancy should have an opportunity to make an informed decision with their health care provider about whether to be screened for prostate cancer. In addition, the American Urological Association guidelines recommend every other year testing among men who elect to have PSA screening [5].
Previous studies have used administrative data, including Medicare claims and Veterans Administration files, to examine the use of PSA testing according to age and publication of practice guidelines and clinical trial data [7—12]. Studies have documented variability in the use of screening according to geographic region [12] and physician characteristics [11], as well as a modest decline in screening following the publication of the 2008 [8, 11, 13] and 2012 [14—17] USPSTF guidelines [2,3] and screening trial publications [18, 19]. Three recently published studies used data from the National Health Interview Survey [20—22] and reported declines in PSA testing use following publication of the USPSTF guidelines.However, despite the consensus that if PSA testing is offered,it should be performed on a regular (i.e., annual or biannual)basis, all studies have used a cross-sectional approach to measure screening. In these studies, PSA testing was considered as a one-time event and patients were not stratified according to previous use of screening or whether they were up to date with screening.
We therefore performed a population-based analysis with Medicare claims data to determine the use of PSA testing according to receipt of previous screening. In addition to measures of previous PSA testing, our analyses also considered factors such as sociodemographics, comorbidity, and physician supply. We hypothesized that the frequency of screening did not change among men who were already undergoing testing but declined in men who were previously not screened.
Methods
Data sources
The study cohort included claims from a 5% random sample of Medicare beneficiaries from 2004 to 2012. On the basis of the selection criteria for the 5% sample, the same beneficiaries were contained in the sample from year to year. To measure the use of PSA testing, we included files from 2009 to 2012,with the 2004—2008 data used to exclude previous prostate cancer diagnoses and determine previous use of PSA testing.The relevant files included the Medicare Carrier Files, the Medicare Outpatient Files, and the Medicare Beneficiary Summary Files.
In addition, we used the 2010 US Census data, which provided ZIP-code level information of socioeconomic status.These data were used as ecological measures in the patientlevel regression analyses. The 2010 American Medical Association Masterfile, which contains information on both American Medical Association members and nonmembers,was used to categorize physician density per 100,000 population at the county level.
The sample was limited to men aged 67 years or older who were contained in the 5% random sample of Medicare beneficiaries and continuously enrolled. Because Medicare enrollment typically begins at age 65 years, this age restriction was used so as to have at least a 2-year look-back period to exclude men with a previous diagnosis of prostate cancer or prostate carcinoma in situ [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)185, 233.4, 602.3, V10.46], which have different guidelines, as well as to measure PSA testing use in the preceding 2 years.Also, because of the high likelihood of incomplete claims,we excluded beneficiaries who were enrolled in Medicaremanaged care plans during the look-back period as well as those who were not enrolled in Medicare Part A and Part B.
在某车道分隔断面设计工作中,设计人员分别提出了两种不同的设计方案,第一种设计方案在对车行道进行分隔的过程中,主要运用中央分隔带,而第二种设计方案在对车行道进行分隔时,则主要运用栏杆分隔的方式。通过结合实际情况以及以往车道分隔断面设计经验可知,通过采用中央分隔带对车行道进行分隔设计,一方面可以有效增加道路的绿化面积,形成一种天然的绿色景观。另一方面其也可以充分发挥分隔作用,避免对向行驶车辆之间相互干扰。当车辆在夜间行驶时,交叉口同时被作为左转和调头车道,采用中央分隔带的设计形式能够在很大程度上保障车辆夜间行驶安全,因而相比第二种设计方案,前一种设计方案更加科学合理,应用价值更高。
To limit the analysis to PSA testing performed for probable screening indications as opposed to surveillance or symptom evaluation, we used a previously developed and validated algorithm to increase the specificity of PSA testing [6]. In addition to a prostate cancer diagnosis, this algorithm also excluded men with a history of prostatectomy, androgen deprivation therapy, or elevated PSA level, and also urinary symptoms within 3 months before the PSA test claim.
Measures
Demographic characteristics were obtained from Medicare claims, and included age and race. Ecological measures of socioeconomic status included median household income and proportion of high school graduates among adults aged 25 years or older. A previously validated, weighted comorbidity index that included both outpatient and inpatient diagnosis codes was included for the 12-month to 1-month period before the PSA test date or the end of the follow-up period [23]. As previously defined, to exclude “rule out” diagnoses, a comorbid condition had to appear more than once in outpatient files.The Beneficiary Summary File contained fields for state buyin and dual eligibility, which indicate lower socioeconomic status and/or with heightened vulnerability. The geographic region of residence was divided into Northeast, Midwest,South, and West.
During each calendar month, we considered the proportion of eligible men who received one or more PSA tests,divided by the number of men who were otherwise eligible for screening and who had not received a PSA test during the previous 24 months. Men were included in the numerator only if they actually received a PSA test during that month, and the denominator changed from month to month as new men became due for testing. PSA tests were identified through relevant procedure codes (CPT-4 84153, G0103).To account for delays in obtaining screening, a 90-day extension from the beneficiary’s due date for repeated screening was used to satisfy the criterion for screening. This approach was previously used in a study of the impact of health care reform on receipt of mammography and colonoscopy [24].Beneficiaries were censored at the month of death or disenrollment from fee-for-service Medicare plans on the basis of the Beneficiary Summary File. We also censored individuals at the time of prostate cancer diagnosis (ICD-9-CM 185) during 2009—2012.
Analysis
We first summarized PSA testing frequency by calendar month according to whether the patient was due for screening during that month (i.e., no PSA test in the previous 24 months). Because of the 90-day window to account for being up to date with testing, a cutoff for the due date of September 30, 2012, was used, and patients with due dates after that were excluded for calculation of frequencies for October through December 2012. The analyses were stratified according to whether the patient had no evidence of PSA testing during the previous 2-year period, or whether the patient had undergone testing during the previous 2 years and was due for repeated screening.
Univariate analysis was used to determine the association of calendar year with the use of PSA testing. Because individual patients were eligible for screening in more than 1 year,generalized estimating equation (GEE) logistic regression was used to account for within-patient correlation. In addition, as individual providers tend to have unique practice patterns with regard to PSA testing, we included physician clustering in the GEE regression models. We then used multivariate GEE models to determine the independent association of demographic,socioeconomic and clinical measures with receipt of PSA testing. As in the monthly frequencies, the analyses were stratified according to the presence of previous PSA testing. For men with previous PSA testing, we also added a covariate for the time since the most recent PSA test.
The Medicare claims data were obtained through a data use agreement with the Centers for Medicare and Medicaid Services, and approval was obtained from the University Hospitals Cleveland Medical Center Institutional Review Board.
Results
Using the 5% random sample of Medicare beneficiaries in 2009—2012, we identified 1,614,857 eligible beneficiaries. From this cohort, we excluded 1,201,421 for the following non-mutually exclusive indications: age younger than 70 years (n=696,971), enrollment in Medicare-managed care plans (n=442,615), lack of enrollment in Medicare Part B(n=435,579), prior prostate cancer diagnosis (n=5871), and enrollment because of end-stage renal disease or disability(n=23,188). The final sample consisted of 598,184 men, including 333,514 (55.8%) with at least one PSA test and 264,670(44.2%) with no evidence of PSA testing.
The characteristics of men with and without PSA testing are shown in Table 1. The age distribution of the group with PSA testing was somewhat in favor of older age compared with the group without PSA testing, whereas the racial and ethnic distribution was similar between the two groups. The PSA group had a higher proportion of men with at least one comorbid condition. Men with PSA testing were
more likely to live in the South and reside in regions with a higher median income and educational level as well as in regions with a greater density of primary care providers and urologists.
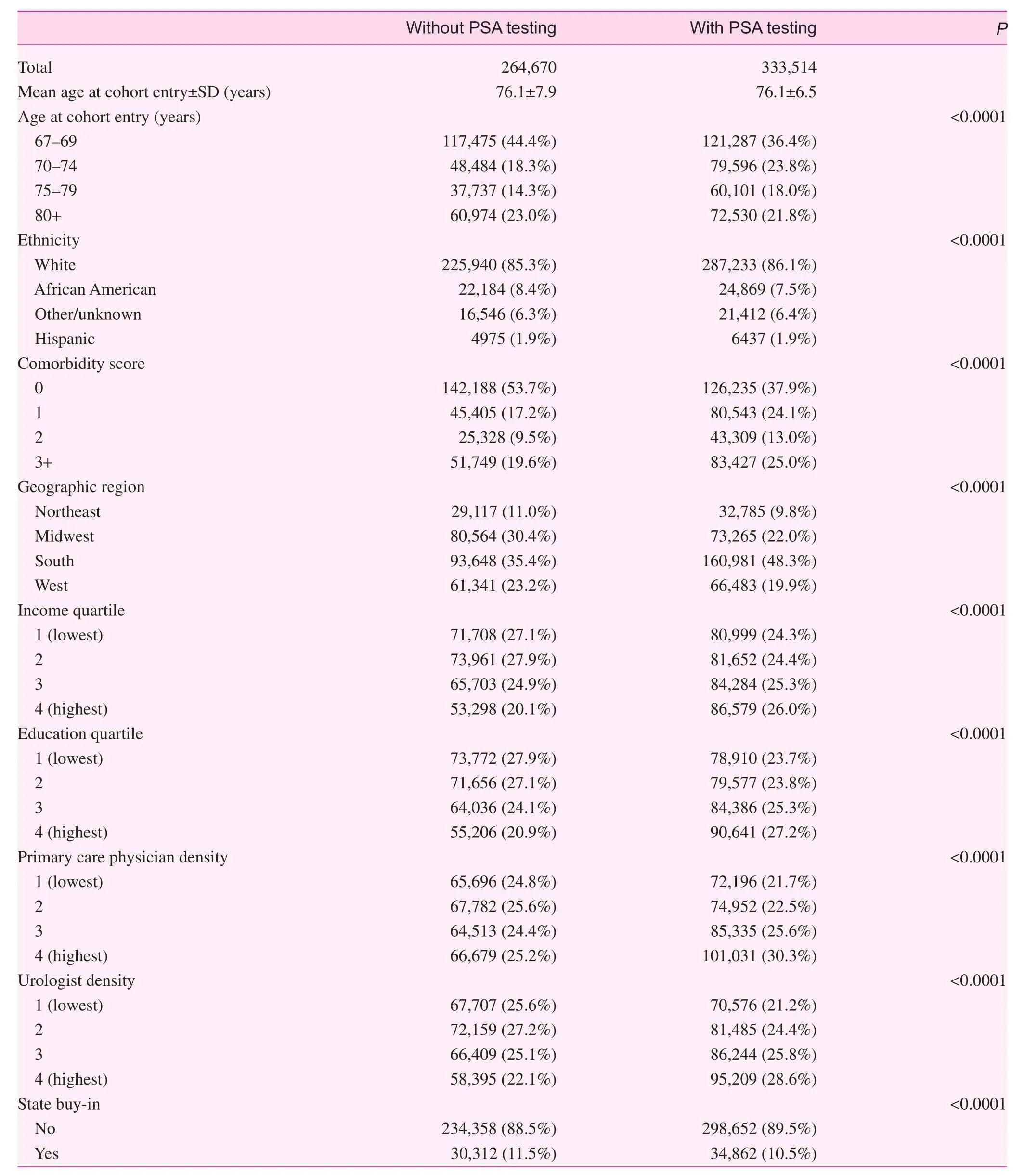
Table 1. Demographic characteristics of men according to prostate-specific antigen testing
The monthly frequencies of PSA testing according to receipt of previous screening are shown in Fig. 1. Within a given month, the screening rates were consistently higher for men with previous screening (typically 13%—16% of those due for screening) than for men without testing in the previous 2 years (typically 3%—4%). For men without previous screening, there was a decline in the frequency of testing according to calendar year, and this was most pronounced in 2012(Fig. 2). Compared with 2009, the corresponding odds ratios were 0.98 [95% confidence interval (CI) 0.96—1.00] in 2010,0.94 (95% CI 0.92—0.95) in 2011, and 0.66 (95% CI 0.65—0.68)in 2012. In contrast for men with previous screening, the testing frequencies were relatively constant from 2009 to 2011,and declined more modestly in 2012. The corresponding odds ratios were 0.98 (95% CI 0.97—0.99) in 2010, 0.99 (95% CI 0.98—1.00) in 2011, and 0.80 (95% CI 0.79—0.81) in 2012. In addition, for both groups and all study years, use of PSA testing tended to be higher in the earlier part of the calendar year than in the later part.
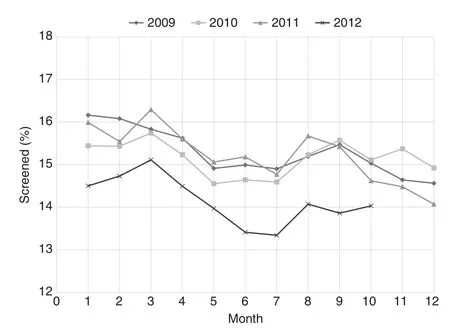
Fig. 1. Monthly rates of prostate-specific antigen testing in men with previous testing. The rates remained fairly constant until 2012, when they declined.
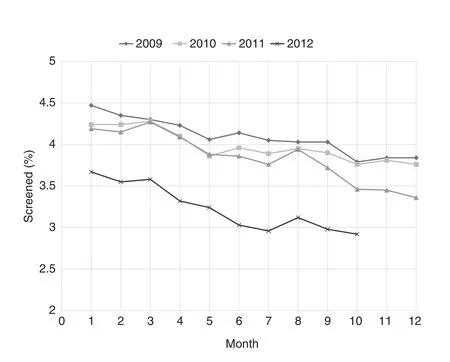
Fig. 2. Monthly rates of prostate-specific antigen testing in men without previous testing. The rates declined in each year of the analysis.
The results of the multivariate GEE analyses for men with previous PSA testing are shown in Table 2. PSA testing was less common in African Americans and men in the northeastern United States. There was minimal or no association of median income or density of primary care physicians, but screening tended to be more frequent in regions with higher median educational levels. However, PSA testing use was highest in regions with a greater density of urologists.There was no substantive association of calendar year through 2011 with PSA testing use, but use did decline in 2012.
In contrast, the multivariate results of PSA testing among men without previous screening differed across many parameters (Table 3). In this group, there was a pronounced decline with older ages, but an increase in PSA testing use with higher levels of comorbidity. African Americans were less likely but members of other racial groups were more likely to be tested.The highest use of testing was found in the southern United States, and PSA testing use tended to be highest in regions with higher median income and educational level. In contrast to men with previous screening, there was no consistent association with urologist density. Also, in contrast to the previously screened men, the frequency of PSA testing declined over time in men without recent screening, especially for 2012.
Discussion
Although prostate carcinoma is commonly diagnosed and is a leading cause of cancer-related death among men, the benefits and harms of PSA screening for this cancer in the general population are controversial. Consequently, given the concerns about false positive tests, overdiagnosis, and overtreatment,none of the current practice guidelines recommend universal screening [3—6]. Our study, which used a longitudinal design as opposed to the cross-sectional design from previous reports,found that testing patterns differed significantly depending on whether a patient had received PSA testing in the past.Whereas the rates of PSA testing declined over time among previously unscreened men, screening frequency remained more constant among men with evidence of prior PSA testing.In addition, although guidelines generally do not recommend screening in men aged 70—75 years or older [3—6], there was much less of a drop off in testing with age among men with previous PSA screening. The findings suggest that once a man is enrolled in a screening program, there is lower impact of changes in external practice guidelines.
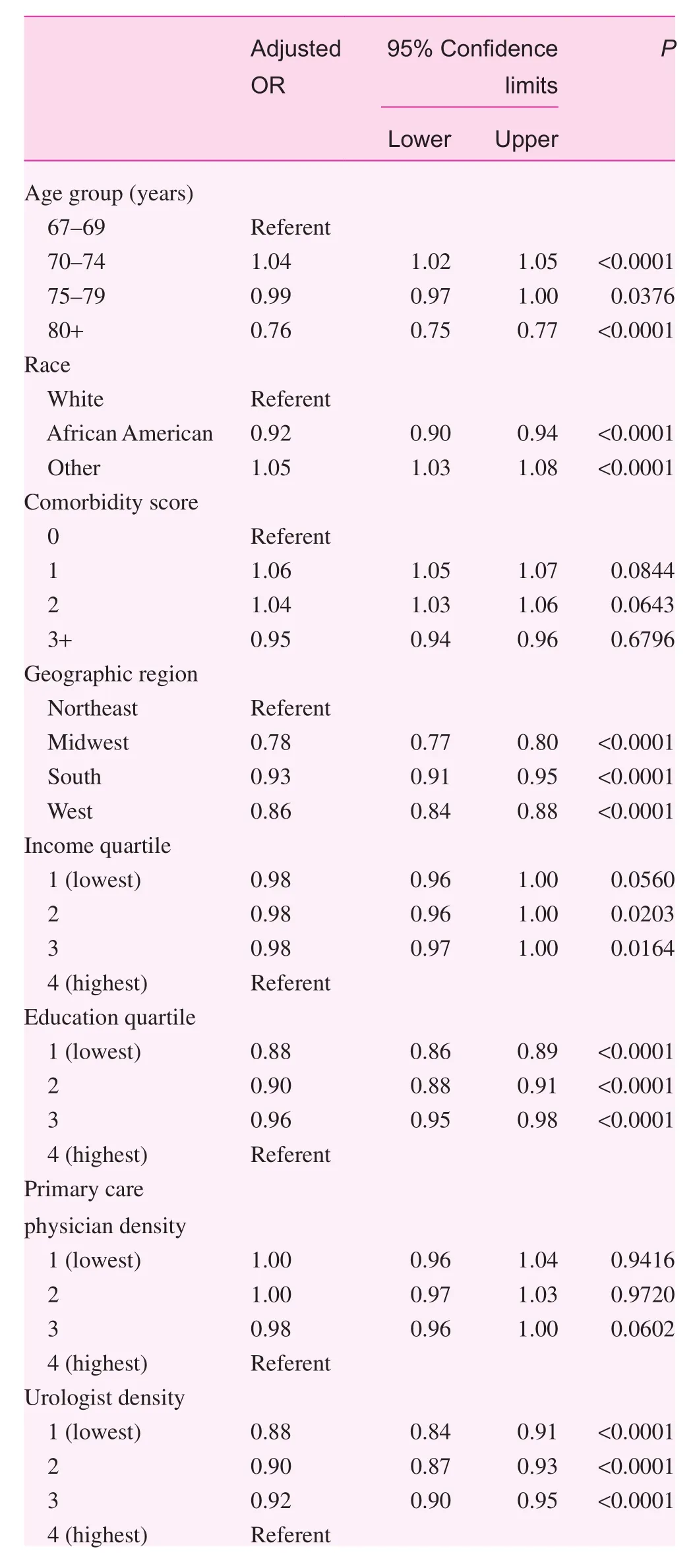
Table 2. Multivariate analysis of prostate-specific antigen testing among men with previous prostate-specific antigen testing and due for repeated testing

Table 2 (continued)
Our study also found that for men without previous screening, an increase in PSA testing was associated with increased comorbidity, and a decrease was associated with advancing age. Although the comorbidity findings appear counterintuitive [25], it may reflect more frequent contact with health care providers and hence a greater opportunity to order PSA testing. In addition, previous studies have documented aggressive treatment of low-risk prostate cancer among men with significant comorbidity [26], suggesting that in contrast to age, clinicians may have difficulty assessing competing risks of comorbid illnesses. We found that in both the previously screened individuals and the unscreened individuals, the rate of PSA testing was somewhat lower in African American men compared with white men. Although the prostate cancer incidence and mortality are higher in African Americans [1],screening guidelines that stratify recommendations by race [5]differentiate only the age to start screening. Previous studies in younger men [20, 22, 25] and Medicare patients [11] showed either no racial disparity in PSA testing use [11, 20, 22] or only a modest difference [25].

Table 3. Multivariate analysis of prostate-specific antigen testing among men with no prostate-specific antigen testing in the previous 2 years

Table 3 (continued)
Prior studies used administrative data from Medicare beneficiaries and the Department of Veterans Affairs as well as population-based surveys to examine the use of PSA testing according to patient characteristics, physician factors, geographic region, and changes in practice guidelines[7—12]. Using a cross-sectional approach, these studies found PSA rates of up to 40%—50% during a defined time period,with only a modest decline with advancing age and comorbidity. There was also significant variability in the rate of PSA testing among primary care providers [11], and it was more common in regions with greater total expenditures and end-of-life care [12]. Following publication of the 2008 USPSTF guidelines recommending routine screening not be performed in men older than 75 years, studies reported a modest decline in screening rates [8, 11, 13]. In addition,publication of clinical trial data was also associated with a small decrease in the use of screening [9]. Previous studies from single institutions also examined the potential impact of the 2012 USPSTF guidelines on screening. These studies documented a decline in the overall rate of PSA testing among primary care providers [16] and in prostate biopsies[15]. Three recently published articles used the National Health Interview Survey to examine PSA testing receipt before and after the publication of the 2012 USPSTF guidelines. One study found that although there was a significant decline in testing in men older than 50 years, there continued to be a high frequency of screening in men older than 75 years and/or with significant comorbidity [20]. Another study found that screening frequency increased from 2005 to 2008 but declined from 2010 to 2013, which correlated with a decrease in early-stage cancer incidence [21]. A third study found that the decrease in PSA testing from 2010 to 2013 was limited only to men younger than 75 years [22].In contrast, an analysis of the 2012 Behavioral Risk Factor Surveillance System data reported only a minimal decline in PSA testing receipt, with an estimate of 37.1% receiving PSA testing among men aged 50 years or older [16]. Because of differences in study design (cross-sectional vs. longitudinal)and use of monthly as opposed to yearly rates, the proportion of men with screening cannot be directly compared with our findings. However, the temporal trends that were observed were evident in most studies, including our own.
We recognize several important limitations with the use of Medicare claims data to measure PSA testing. First, the data were collected for billing purposes and not research,and thus lacked any clinical detail. For screening procedures,this includes the inability to differentiate screening versus surveillance or diagnostic indications, although we used a previously validated algorithm with a higher specificity for screening indications [6] and excluded men with a previous prostate cancer diagnosis. In addition, the accuracy of claims data for measuring PSA testing use is thought to be high [27].Our study design also could not measure patient and physician preferences regarding screening, both of which were likely associated with screening receipt. However, the results were clustered by provider, which accounts in part for physician practice patterns. Because of incomplete claims data,the study did not include men who were enrolled in Medicare Advantage Plans or those not enrolled in Medicare Part B,and it was not known if the trends of PSA testing use in these groups would be similar. The study was also limited to an older patient population, and thus the impact of guidelines and other factors in younger, privately insured individuals could not be measured. Moreover, despite the lack of USPSTF recommendations, PSA testing has remained a covered benefit under Medicare without any out-of-pocket expenses. Analyses in younger patient groups have found mixed results with regard to changes in PSA testing uptake after 2012 [20—22].Finally, because patient-level socioeconomic status was not available in claims data, we used small area measures, a commonly used approach in studies of Medicare data.
In summary, we found that receipt of PSA testing is highly dependent on whether an individual was tested in the recent past. Although overall rates of PSA testing use declined with time, the largest decrease occurred in both previously screened and unscreened men in 2012, which may reflect publication of the most recent USPSTF guidelines.
Acknowledgments
This research was supported by a Research Scholar Grant(RSGI-12-218-01-CPHPS) from the American Cancer Society (to G.S.C.), the Case Comprehensive Cancer Center(P30-CA43703-18), and the Case Clinical and Translational Science Collaborative (UL1 TR000439).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Significance statement
Universal screening for prostate cancer with prostate specific antigen (PSA) testing in men has not been recommended by the US Preventive Services Task Force since 2012 and had an indeterminate recommendation prior to that. However, previous studies that have shown changes following guidelines have not considered whether men were already undergoing screening. Using a population-based sample of Medicare beneficiaries, we found that among previously unscreened men, there was a significant decline in testing from 2009—2012 that was most pronounced in 2012. In contrast for men already screened, the decline was much less apparent. The findings suggest that receipt of PSA, including after the 2012 guidelines, is highly dependent on whether an individual was tested in the recent past.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7—30.
2. US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:185—91.
3. Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120—34.
4. Smith RA, Andrews K, Brooks D, DeSantis CE, Fedewa SA,Lortet-Tieulent J, et al. Cancer screening in the United States,2016: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2016;66:96—114.
5. Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ,Greene KL, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013;190:419—26.
6. Carroll PR, Parsons JK, Andriole G, Bahnson RR, Barocas DA,Castle EP, et al. Prostate cancer early detection, version 2.2015.J Natl Compr Canc Netw 2015;13:1534—61.
7. Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA 2006;296:2336—42.
8. Howard DH, Tangka FK, Guy GP, Ekwueme DU, Lipscomb J.Prostate cancer screening in men ages 75 and older fell by 8 percentage points after task force recommendation. Health Affairs 2013;32:596—602.
9. Zeliadt SB, Hoffman RM, Etzioni R, Gore JL, Kessler LG, Lin DW. influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst 2011;103:520—3.
10. Ma X, Wang R, Long JB, Ross JS, Soulos PR, Yu JB, et al. The cost implications of prostate cancer screening in the Medicare population. Cancer 2014;120:96—102.
11. Goodwin JS, Jaramillo E, Yang L, Kuo Y-F, Tan A. Is anyone listening? Variation in PSA screening among providers for men 75+ before and after United States Preventive Services Task Force recommendations against it: a retrospective cohort study.PLoS One 2014;9:e107352.
12. Bynum J, Song Y, Fisher E. Variation in prostate-specific antigen screening in men aged 80 and older in fee-for-service Medicare.J Am Geriatr Soc 2010;58:674—80.
13. Lee SY, Friderici J, Stefan MS, Rothberg MB. Impact of the 2008 U.S. Preventive Services Task Force recommendation of frequency of prostate-specific antigen screening in older men.J Am Geriatr Soc 2014;62:1912—5.
14. Bhindi B, Mamdani M, Kulkarni GS, Finelli A, Hamilton RJ,Trachtenberg J, et al. Impact of the U.S. Preventive Services Task Force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol 2015;193:1519—24.
15. Perez TY, Danzig MR, Ghandour RA, Badani KK, Benson MC, McKiernan JM. Impact of the 2012 United States Preventive Services Task Force statement on prostate-specific antigen screening: analysis of urologic and primary care practices.Urology 2015;85:85—91.
16. Cohn JA, Wang CE, Lakeman JC, Silverstein JC, Brendler CB, Novakovic KR, et al. Primary care physician PSA screening practices before and after the final U.S. Preventive Services Task Force recommendation. Urol Oncol 2014;32:41.e23—30.
17. Sammon JD, Pucheril D, Diaz M, Kibel AS, Kantoff PW,Menon M, et al. Contemporary nationwide patterns of selfreported prostate-specific antigen screening. JAMA Intern Med 2014;174:1839—41.
18. Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310—9.
19. Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S,Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320—8.
20. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate specific antigen base screening. J Clin Oncol 2015;33:2416—23.
21. Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O, et al.Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA 2015;314:2054—61.
22. Sammon JD, Abdollah F, Choueiri TK, Kantoff PW, Nguyen PL,Menon M, et al. Prostate specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA 2015;314:2077—9.
23. Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data. Med Care 2002;40 (Suppl):26—35.
24. Cooper GS, Kou TD, Schluchter MD, Dor A, Koroukian SM.Changes in receipt of cancer screening in Medicare beneficiaries following the Affordable Care Act. J Natl Cancer Inst 2015;108(5):djv374.
25. Royce TJ, Hendrix LH, Stokes WA, Allen IM, Chen RC. Cancer screening rates in individuals with different life expectancies.JAMA Intern Med 2014;174:1558—65.
26. Daskivich TJ, Chamie K, Kwan L, Labo J, Dash A, Greenfield S,et al. Comorbidity and competing risks for mortality in men with prostate cancer. Cancer 2011;117:4642—50.
27. Freeman JL, Klabunde CN, Schussler N, Warren JL, Virnig BA, Cooper GS. Measuring breast, colorectal and prostate cancer screening with Medicare claims data. Med Care 2002;40(Suppl):36—42.
1. Division of Gastroenterology,University Hospitals Cleveland Medical Center, 11100 Euclid Ave., Cleveland, OH 44106, USA
2. Department of Urology, University Hospitals Cleveland Medical Center, 11100 Euclid Ave.,Cleveland, OH 44106, USA
3. Case Comprehensive Cancer Center, Wearn Building, Case Western Reserve University,11100 Euclid Ave., Cleveland,OH 44106, USA
4. Department of Epidemiology and Biostatistics, School of Medicine, Wood Building, Case Western Reserve University,10900 Euclid Ave., Cleveland,OH 44106, USA
5. Department of Health Policy and Management, Milken Institute School of Public Health,George Washington University,950 New Hampshire Ave., NW,Washington, DC 20052, USA
Gregory S. Cooper,
Division of Gastroenterology,University Hospitals Cleveland Medical Center, 11100 Euclid Ave., Cleveland, OH 44106, USA Tel.: +1-216-8445385
Fax: +1-216-9830347
E-mail:
gregory.cooper@uhhospitals.org
9 March 2017;
Accepted 27 June 2017
猜你喜欢
杂志排行
Family Medicine and Community Health的其它文章
- Primary care and cancer
- The association of inherited variation in the CLOCK gene with breast cancer tumor grade
- Symptoms predicting health-related quality of life in prostate cancer patients treated with localized radiation therapy
- Complex multimorbidity and health outcomes in older adult cancer survivorsa
- The relationship between anxiety about prostate cancer among patients with biochemical cancer recurrence and the use of complementary and alternative medicines, diet, and exercise
- Short sleep duration as a contributor to racial disparities in breast cancer tumor grade
